Abstract
Background
Overexpression of inducible nitric oxide synthase (iNOS) and increased nitric oxide generation may be associated with the hyperdynamic circulation of patients with cirrhosis. We have, for the first time, used the highly selective iNOS inhibitor, 1400W, to determine whether iNOS activity contributes to the regulation of vascular tone in patients with cirrhosis and ascites.
Methods
Bilateral forearm blood flow was measured using strain gauge plethysmography in eight patients with cirrhosis and ascites, and eight matched healthy volunteers during intrabrachial infusion of 1400W (0.1–1 μmol/min), NG‐monomethyl‐L‐arginine (L‐NMMA, a non‐selective NOS inhibitor; 2–8 μmol), and norepinephrine (a control vasoconstrictor; 60–480 pmol/min).
Results
In patients with cirrhosis, 1400W, L‐NMMA, and norepinephrine caused dose dependent reductions in forearm blood flow: peak reductions of 11 (5)%, 37 (4)%, and 48 (5)%, respectively (p<0.05 for all). In contrast, 1400W had no effect on blood flow (+4 (8)%; NS) in healthy controls despite similar reductions in blood flow with L‐NMMA and norepinephrine (39 (5)% and 49 (5)%, respectively; p<0.05 for both).
Conclusions
We have, for the first time, demonstrated that 1400W causes peripheral vasoconstriction in patients with cirrhosis but not healthy matched controls. This suggests that iNOS contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites, and may contribute towards the hyperdynamic circulation associated with this condition.
Keywords: cirrhosis, endotoxin, forearm, inducible nitric oxide
Patients with advanced liver disease have a hyperdynamic circulation that is characterised by low arterial pressure, high cardiac output, splanchnic vasodilatation, and low systemic vascular resistance.1,2,3 This occurs despite activation of endogenous vasoconstrictor systems, and increased activity of endogenous vasodilators, such as nitric oxide,4 has been suggested as a possible cause. Plasma concentrations of nitric oxide and its metabolites nitrite and nitrate are increased in patients with cirrhosis and ascites.5,6 Exhaled breath of patients contains higher nitric oxide concentrations than normal subjects, and correlates with cardiac output and Child‐Pugh score.7 Moreover, systemic administration of a non‐selective nitric oxide synthase inhibitor increases systemic vascular resistance and blood pressure in patients with cirrhosis.8
Nitric oxide is generated from L‐arginine, a reaction catalysed by nitric oxide synthase (NOS). Three isoforms of NOS have been identified; endothelial, inducible, and neuronal.9 Endothelial nitric oxide synthase (eNOS) is expressed constitutively, and produces small amounts of nitric oxide for short periods of time in response to exogenous and endogenous stimuli. It is found in a number of cell types but principally endothelial cells.10 In contrast, inducible nitric oxide synthase (iNOS) is synthesised de novo in response to inflammation and is found in vascular smooth muscle cells, hepatocytes, macrophages, and many other cell types.9 Once expressed, it produces large amounts of nitric oxide for prolonged periods.9
In various pathological states, endotoxins, cytokines, and bacterial infections have promoted iNOS formation and are associated with overproduction of nitric oxide. This causes hyporesponsiveness to vasoconstrictors and a hyperdynamic circulation.1,2 Bacterial overgrowth and failure of the gut mucosal barrier commonly promotes bacterial translocation and endotoxaemia in patients with cirrhosis,11,12,13 and several reports have demonstrated an association between nitric oxide release and endotoxinaemia.5,14,15 Vallance and Moncada hypothesised that, through such mechanisms, iNOS formation may account for the hyperdynamic circulation of patients with advanced liver disease.16
Until recently, it has not been possible to distinguish clearly between endothelial and inducible NOS activity due to the lack of specific inhibitors. N‐(3‐(aminomethyl) benzyl) acetamidine (1400W) is a novel selective inhibitor of human iNOS that has recently become available for clinical use.17 It competes with L‐arginine to bind irreversibly with iNOS and is at least 5000‐fold more selective for iNOS than eNOS, making it one of the most selective iNOS inhibitor to date.17 Therefore, the aim of the present study was to assess the contribution of functional iNOS activity to peripheral vascular tone in patients with cirrhosis and ascites by determining the effect of direct local intra‐arterial 1400W on peripheral blood flow.
Methods
Study population
Eight patients with biopsy proven cirrhosis, ascites, and portal hypertension, and eight age and sex matched healthy subjects were recruited. All patients were ambulant, had endoscopically proven varices, and normal serum creatinine (<100 μmol/l). In order to avoid alcohol induced depression of vascular responses,18 patients were abstinent from alcohol for at least one month, confirmed by history and repeated blood ethanol testing. Patients with primary biliary cirrhosis were excluded due to the possible vasoactive effects of hyperbilirubinaemia. Patients with clinically overt bacterial infection, neutrophilia, or pyrexia (body temperature >37.5°C) were excluded from the study. To avoid the possibility of altering the endogenous vasopressor systems, all subjects were maintained on their normal sodium intake (∼150 mmol/day). None of the patients received vasoactive (including beta adrenoreceptor blockers) or non‐steroidal anti‐inflammatory drugs in the week before, and all patients abstained from food, tobacco, and caffeine containing drinks for at least four hours before each study. All female subjects were postmenopausal both for safety and to avoid the variability in vascular responses that may be associated with cyclical hormonal changes.19 The studies were undertaken in accordance with the Declaration of Helsinki of the World Medical Association, the approval of the local research ethics committee, and the written informed consent of each subject.
Drugs and intra‐arterial administration
The brachial artery of the non‐dominant arm was cannulated with a 27 standard wire gauge steel needle (Cooper's Needle Works Ltd, Birmingham, UK) under local anaesthesia. The cannula was attached to a 16 gauge epidural catheter (Portex Ltd, Hythe, Kent, UK) and patency was maintained by infusion of saline (0.9%: Baxter Health Care Ltd, Thetford, UK) via an IVAC P1000 syringe pump (IVAC Ltd, Basingstoke, UK). The rate of intra‐arterial infusions was maintained constant throughout all studies at 1 ml/min. Pharmaceutical grade 1400W (Merck Biosciences AG, Läufelfingen, Switzerland), L‐NMMA (Merck Biosciences AG), and norepinephrine (Levophed, Sanofi‐Winthrop Ltd, Guildford, UK) were dissolved in saline on the day of the study.
Haemodynamic measurements
Blood flow was measured in both forearms by venous occlusion plethysmography, as described previously.20 Blood pressure and heart rate were monitored in the non‐infused arm at intervals throughout each study with a semi automated non‐invasive oscillometric sphygmomanometer.
Study design
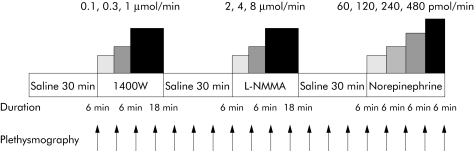
Subjects attended on a single occasion, at 9am, and were recumbent throughout the study. Strain gauges and cuffs were applied, and the brachial artery of the non‐dominant arm was cannulated. Forearm blood flow was measured every 6–10 minutes. Following a 30 minute equilibration period, during which saline was infused, subjects received intra‐arterial infusions of 1400W (iNOS inhibitor) at 0.1, 0.3, and 1 μmol/min for 6–18 minutes at each dose, L‐NMMA (non‐selective NOS inhibitor) at 2, 4, and 8 μmol/min for 6–18 minutes at each dose,21 and norepinephrine (control vasoconstrictor) at 60, 120, 240, and 480 pmol/min for six minutes at each dose,22,23 with 30 minute saline washout periods between each drug infusion (fig 1).
Figure 1 Study protocol. L‐NMMA, NG‐monomethyl‐L‐arginine.
Data analysis and statistics
Plethysmographic data were extracted from chart data files, and the last five linear recordings in each measurement period were averaged. Forearm blood flows were calculated from the plethysmographic data, as described previously.20
Data were examined, where appropriate, by ANOVA with repeated measures and two tailed Student's t test using Microsoft Excel 2002. Data are expressed as mean (SEM). Statistical significance was taken at the 5% level.
Results
Patients with cirrhosis were well matched to control subjects (table 1) although patients had a higher heart rate, in keeping with their hyperdynamic circulation. Throughout each study, there were no significant changes in mean arterial pressure, heart rate, or forearm blood flow in the non‐infused arm. There was no significant difference in baseline forearm blood flow in the infused arm between patients with cirrhosis and control subjects (3.9 (0.7) and 3.9 (0.7) ml/100 ml/min, respectively).
Table 1 Patient and subject characteristics.
| Variable | Patient with cirrhosis (n = 8) | Healthy controls (n = 8) |
|---|---|---|
| Age (y) | 58 (2) | 59 (3) |
| Sex (M:F) | 7:1 | 7:1 |
| Aetiology of liver disease: | ||
| Alcoholic | 8 | – |
| Child Pugh Score | 9 (1) | – |
| Child Grade | ||
| A | 1 | |
| B | 3 | – |
| C | 4 | – |
| Serum albumin (g/l) | 32 (3) | – |
| Serum bilirubin (mmol/l) | 46 (9) | – |
| Serum creatinine (μmol/l) | 82 (6) | |
| Prothrombin time (s) | 14 (1) | – |
| Oesophageal varices | 8 | – |
| Ascites | 8 | – |
| Baseline blood pressure | ||
| Systolic (mm Hg) | 138 (5) | 143 (6) |
| Diastolic (mm Hg) | 76 (4) | 81 (3) |
| Baseline heart rate (/min) | 89 (4) | 59 (3)* |
| Resting forearm blood flow (ml/100 ml tissue/min) | 2.4 (0.3) | 2.1 (0.1) |
Results are expressed as mean (SEM).
*p<0.05 versus controls.
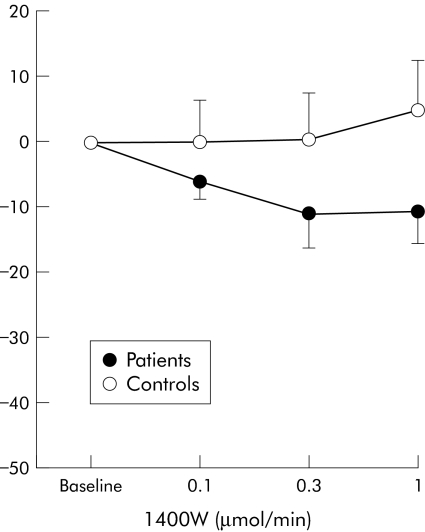
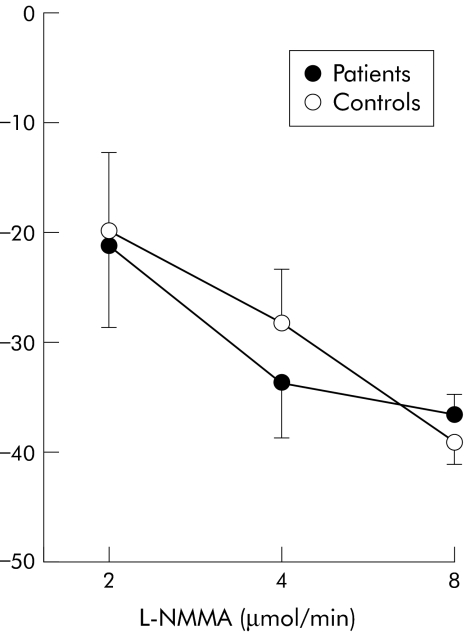
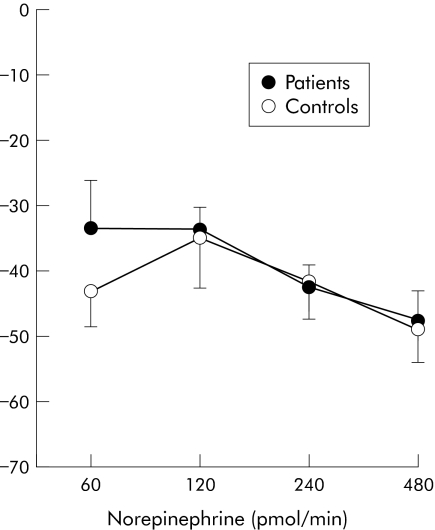
In patients with cirrhosis, intrabrachial infusion of 1400W, L‐NMMA, and norepinephrine caused dose dependent reductions in infused forearm blood flow: peak reductions of 11 (5)%, 37 (4)%, and 48 (5)%, respectively (p<0.05 for all). In contrast, 1400W had no effect on blood flow (+4 (8)%; NS) in healthy controls despite similar reductions in blood flow with L‐NMMA and norepinephrine (39 (5)% and 49 (5)% respectively; p<0.05 for both) (figs 2–4; table 2).
Figure 2 Percentage change in forearm blood flow after infusion of the inducible nitric oxide synthase inhibitor 1400W in patients and healthy controls.
Figure 3 Percentage change in forearm blood flow after infusion of the nitric oxide synthase inhibitor NG‐monomethyl‐L‐arginine (L‐NMMA) in patients and healthy controls.
Figure 4 Percentage change in forearm blood flow after norepinephrine infusion in patients and healthy controls.
Table 2 Forearm blood flow responses in patients and healthy controls.
| Patients with cirrhosis | Healthy controls | |
|---|---|---|
| 1400W (μmol/min) | ||
| 0.1 | −7 (4) | 0 (7) |
| 0.3 | −11 (5)* | 0 (7) |
| 1 | −11 (5)* | 4 (8) |
| L‐NMMA (μmol/min) | ||
| 2 | −21 (6) | −20 (7) |
| 4 | −34 (4) | −29 (5) |
| 8 | −35 (5) | −39 (4) |
| Norepinephrine (pmol/min) | ||
| 60 | −34 (7) | −43 (5) |
| 120 | −34 (4) | −35 (7) |
| 240 | −43 (3) | −42 (5) |
| 480 | −48 (5) | −49 (5) |
Results are mean (SEM).
*p<0.05 versus controls.
Discussion
We have, for the first time, demonstrated that inhibition of inducible NOS causes vasoconstriction in the forearm circulation of patients with cirrhosis due to alcoholic liver disease. This finding suggests that inducible NOS may be partly responsible for the overproduction of nitric oxide associated with the hyperdynamic circulation of cirrhosis.
In 1991, Vallance and Moncada16 suggested that the characteristic hyperdynamic circulation of liver cirrhosis resulted from increased production of nitric oxide secondary to endotoxaemia induced expression of iNOS. Both animal24,25,26,27 and clinical28 studies have variably provided support for this hypothesis, with significant tissue expression of iNOS in cirrhosis. However, current opinion suggests that eNOS rather than iNOS is the isoform responsible for the circulatory dysfunction associated with cirrhosis.26,27 The majority of the data supporting a role for eNOS comes from rodent models without advanced liver disease. In a rodent model of cirrhosis with advanced liver disease, clear induction of iNOS was demonstrated in the splanchnic vasculature.29 This suggests that differing isoforms of NOS may play varying roles through the course of cirrhosis, with eNOS the dominant isoform in early cirrhosis and iNOS the dominant isoform in advanced cirrhosis. For the first time, our data suggest that iNOS appears to be functionally active and may contribute to the regulation of vascular tone in patients with cirrhosis and ascites.
During our study and in previous work,21,23,30 infusion of the non‐selective NOS inhibitor, L‐NMMA, produced a similar vasoconstriction in healthy controls and patients with cirrhosis. This suggests that if overall NOS activity is similar, then the contribution from eNOS must be downregulated. Indeed, decreased eNOS expression and increased iNOS expression occurs in other inflammatory conditions31,32,33 and is consistent with our findings in patients with cirrhosis.
The forearm circulation is generally representative of other systemic vascular resistance beds.34 In the present clinical study, we have not established the role of iNOS in the splanchnic or portal circulations of patients with cirrhosis. However, if the forearm circulation is a valid surrogate, then selective iNOS inhibition would be expected to improve the portal hypertension and systemic hypotension associated with cirrhosis. We therefore believe that systemic studies are needed to assess the role of iNOS inhibition in patients with cirrhosis and portal hypertension.
Study limitations
The present study is the first to use the iNOS inhibitor 1400W in humans in vivo. 1400W is a monoamidine monoamine analogue that competes with L‐arginine to bind tightly and irreversibly to iNOS. It is more than 5000‐fold more potent against purified human iNOS than eNOS and is one of the most selective inhibitors of iNOS reported to date.17 The selectivity of 1400W, both in vitro and in vivo, makes it an attractive tool for assessing the contribution of iNOS. However, an important concern is whether the dose of 1400W chosen is appropriate to achieve complete inhibition of iNOS activity in vivo. 1400W inhibits rodent vascular iNOS with an EC50 value of 0.8 μM, an eightfold higher potency than L‐NMMA which inhibits iNOS with an EC50 value of 6 μM.35 During infusion of 1400W at 1 μmol/min, with calculated blood flow rates of approximately 20–25 ml/min, we would predict effective end organ concentrations to be between 40 and 50 μM—that is, more than 50‐fold higher than the EC50. We are therefore confident that we have achieved complete and selective iNOS inhibition in our study. During the study, forearm blood flow did not return to baseline after infusion of 1400W in patients with cirrhosis. This is not unexpected as 1400W is an irreversible inhibitor of iNOS17 and why we designed the study with 1400W as the first infusion.
Another potential limitation is that all patients had alcohol induced liver disease and our findings may not be applicable to other forms of liver cirrhosis. Excess alcohol intake is known to alter the response to exogenous vasopressor agents, such as norepinephrine.18 However, all our subjects were abstinent from alcohol for a minimum of one month, as determined by clinical history and random ethanol testing. Moreover, consistent with previous work,22,23 we have demonstrated a normal response to norepinephrine infusion, suggesting no significant derangements in vascular smooth muscle function.
In conclusion, we have, for the first time, demonstrated that 1400W causes peripheral vasoconstriction in patients with cirrhosis but not in healthy matched controls. This suggests that iNOS contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites, and may contribute towards the hyperdynamic circulation associated with this condition. Further studies are required to look at the role of iNOS in differing stages of cirrhosis and in patients with advanced cirrhosis undergoing bacterial decontamination.
Abbreviations
NOS - nitric oxide synthase
eNOS - endothelial nitric oxide synthase
iNOS - inducible nitric oxide synthase
L‐NMMA - NG‐monomethyl‐L‐arginine
Footnotes
Conflict of interest: None declared.
References
- 1.Groszmann R J. Hyperdynamic state in chronic liver diseases. J Hepatol 199317(suppl 2)38–40. [DOI] [PubMed] [Google Scholar]
- 2.Groszmann R J. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology 1994201359–1363. [PubMed] [Google Scholar]
- 3.Lopez‐Talavera J C, Groszmann R J. Hyperdynamic circulatory syndrome of chronic liver disease: physiopathology of a multiorganic entity. Med Clin (Barc) 199510513–15. [PubMed] [Google Scholar]
- 4.Wiest R, Groszmann R J. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology 200235478–491. [DOI] [PubMed] [Google Scholar]
- 5.Guarner C, Soriano G, Tomas A.et al Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology 1993181139–1143. [PubMed] [Google Scholar]
- 6.Battista S, Bar F, Mengozzi G.et al Hyperdynamic circulation in patients with cirrhosis: direct measurement of nitric oxide levels in hepatic and portal veins. J Hepatol 19972675–80. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto A, Ogura K, Hirata Y.et al Increased nitric oxide in the exhaled air of patients with decompensated liver cirrhosis. Ann Intern Med 1995123110–113. [DOI] [PubMed] [Google Scholar]
- 8.Forrest E H, Jones A L, Dillon J F.et al The effect of nitric oxide synthase inhibition on portal pressure and azygos blood flow in patients with cirrhosis. J Hepatol 199523254–258. [DOI] [PubMed] [Google Scholar]
- 9.Sessa W C. The nitric oxide synthase family of proteins. J Vasc Res 199431131–143. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Ishida T, Traub O.et al Mechanotransduction in endothelial cells: temporal signalling in response to shear stress. J Vasc Res 199734212–219. [DOI] [PubMed] [Google Scholar]
- 11.Bauer T M, Schwacha H, Steinbruckner B.et al Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol 2002972364–2370. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Chen M, Sun G.et al Small bowel bacterial overgrowth and endotoxemia in cirrhosis. Zhonghua Nei Ke Za Zhi 200241459–461. [PubMed] [Google Scholar]
- 13.Zhang S, Wang W, Ren W.et al Effects of lactulose on intestinal endotoxin and bacterial translocation in cirrhotic rats. Chin Med J (Engl) 2003116767–771. [PubMed] [Google Scholar]
- 14.Albillos A, de la Hera A, Gonzalez M.et al Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 200337208–217. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Liang K, Yin C. Nitric oxide levels in cirrhotic patients. Zhonghua Nei Ke Za Zhi 19973625–27. [PubMed] [Google Scholar]
- 16.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet 1991337776–778. [DOI] [PubMed] [Google Scholar]
- 17.Garvey E P, Oplinger J A, Furfine E S.et al 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric‐oxide synthase in vitro and in vivo. J Biol Chem 19972724959–4963. [DOI] [PubMed] [Google Scholar]
- 18.Howes L G, Reid J L. Decreased vascular responsiveness to noradrenaline following regular ethanol consumption. Br J Clin Pharmacol 198520669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto M, Akishita M, Eto M.et al Modulation of endothelium‐dependent flow‐mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 1995923431–3435. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin N, Calver A, Collier J.et al Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension 199525918–923. [DOI] [PubMed] [Google Scholar]
- 21.Calver A, Harris A, Maxwell J D.et al Effect of local inhibition of nitric oxide synthesis on forearm blood flow and dorsal hand vein size in patients with alcoholic cirrhosis. Clin Sci 199486203–208. [DOI] [PubMed] [Google Scholar]
- 22.Helmy A, Jalan R, Newby D E.et al Role of angiotensin II in regulation of basal and sympathetically stimulated vascular tone in early and advanced cirrhosis. Gastroenterology 2000118565–572. [DOI] [PubMed] [Google Scholar]
- 23.Newby D E, Jalan R, Masumori S.et al Peripheral vascular tone in patients with cirrhosis: role of the renin‐angiotensin and sympathetic nervous systems. Cardiovasc Res 199838221–228. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Song D, Lee S S. Increased nitric oxide synthase expression in aorta of cirrhotic rats. Life Sci 1999641753–1759. [DOI] [PubMed] [Google Scholar]
- 25.Mizumoto M, Arii S, Furutani M.et al NO as an indicator of portal hemodynamics and the role of iNOS in increased NO production in CCl4‐induced liver cirrhosis. J Surg Res 199770124–133. [DOI] [PubMed] [Google Scholar]
- 26.Stumm M M, D'Orazio D, Sumanovski L T.et al Endothelial, but not the inducible, nitric oxide synthase is detectable in normal and portal hypertensive rats. Liver 200222441–450. [DOI] [PubMed] [Google Scholar]
- 27.Wiest R, Das S, Cadelina G.et al Bacterial translocation in cirrhotic rats stimulates eNOS‐derived NO production and impairs mesenteric vascular contractility. J Clin Invest 19991041223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNaughton L, Puttagunta L, Martinez‐Cuesta M A.et al Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A 20029917161–17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angelini P, Fernandez‐Vara G, Fasolato S, et al. The role of nitric oxide in the pathogenesis of systemic and spanchnic vasodilatation in cirrhotic rats before and after the onset of ascites. Liver Int 200525429–437. [DOI] [PubMed] [Google Scholar]
- 30.Helmy A, Newby D E, Jalan R.et al Nitric oxide mediates the reduced vasoconstrictor response to angiotensin II in patients with preascitic cirrhosis. J Hepatol 20033844–50. [DOI] [PubMed] [Google Scholar]
- 31.Leifeld L, Fielenbach M, Dumoulin F L.et al Inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) expression in fulminant hepatic failure. J Hepatol 200237613–619. [DOI] [PubMed] [Google Scholar]
- 32.Cardaropoli S, Silvagno F, Morra E.et al Infectious and inflammatory stimuli decrease endothelial nitric oxide synthase activity in vitro. J Hypertens 2003212103–2110. [DOI] [PubMed] [Google Scholar]
- 33.Venugopal S K, Devaraj S, Yuhanna I.et al Demonstration that C‐reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 20021061439–1441. [DOI] [PubMed] [Google Scholar]
- 34.Webb D J. The pharmacology of human blood vessels in vivo. J Vasc Res 1995322–15. [DOI] [PubMed] [Google Scholar]
- 35.Boer R, Ulrich W R, Klein T.et al The inhibitory potency and selectivity of arginine substrate site nitric‐oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol 2000581026–1034. [PubMed] [Google Scholar]