Abstract
Background
No previous correlation between phenotype at diagnosis of Crohn's disease (CD) and mortality has been performed. We assessed the predictive value of phenotype at diagnosis on overall and disease related mortality in a European cohort of CD patients.
Methods
Overall and disease related mortality were recorded 10 years after diagnosis in a prospectively assembled, uniformly diagnosed European population based inception cohort of 380 CD patients diagnosed between 1991 and 1993. Standardised mortality ratios (SMRs) were calculated for geographic and phenotypic subgroups at diagnosis.
Results
Thirty seven deaths were observed in the entire cohort whereas 21.5 deaths were expected (SMR 1.85 (95% CI 1.30–2.55)). Mortality risk was significantly increased in both females (SMR 1.93 (95% CI 1.10–3.14)) and males (SMR 1.79 (95% CI 1.11–2.73)). Patients from northern European centres had a significant overall increased mortality risk (SMR 2.04 (95% CI 1.32–3.01)) whereas a tendency towards increased overall mortality risk was also observed in the south (SMR 1.55 (95% CI 0.80–2.70)). Mortality risk was increased in patients with colonic disease location and with inflammatory disease behaviour at diagnosis. Mortality risk was also increased in the age group above 40 years at diagnosis for both total and CD related causes. Excess mortality was mainly due to gastrointestinal causes that were related to CD.
Conclusions
This European multinational population based study revealed an increased overall mortality risk in CD patients 10 years after diagnosis, and age above 40 years at diagnosis was found to be the sole factor associated with increased mortality risk.
Keywords: mortality, Crohn's disease, population based, cohort
Crohn's disease (CD) is a chronic inflammatory condition of unknown origin that can be localised throughout the entire gastrointestinal tract. There has been debate as to whether this condition, that predominantly affects young adults, carries an increased mortality risk.1,2 Because of the heterogeneity of the disease, specification of high risk patient groups is desirable, based on demographic and initial disease behaviour characteristics.
It seems that overall mortality in CD has decreased over the last half century3 but based on recently published data there still remains excess mortality occurring late during the disease course.2 Mortality rates in CD may vary in various regions of the world because of different genetic, environmental, and health care related conditions.3 Mortality risk in CD is best evaluated in unselected patient samples acquired from randomly selected regions, prospectively incepted within a relatively short time frame, and using uniform diagnostic criteria.
The aim of this study was to evaluate whether the mortality risk in CD is different from the background population 10 years after diagnosis—using a population based prospectively and uniformly diagnosed European cohort—and to identify possible risk factors.
Methods
Patients and centres
Between October 1991 and September 1993, the European Collaborative study group of Inflammatory Bowel Disease (EC‐IBD) created a population based prospectively and uniformly diagnosed inception cohort of 2201 patients afflicted with inflammatory bowel disease (IBD) within 20 well described geographical areas in 12 European countries.4 In this cohort, 706 patients were diagnosed with CD, 1379 with ulcerative colitis, and 116 with indeterminate colitis,5 all according to the diagnostic criteria by Lennard‐Jones and Truelove and Witts.6
All centres that originally participated in the EC‐IBD cohort were approached to take part in the present follow up study. Thirteen of the original 20 centres distributed over nine countries participated, including 483 out of the original 706 CD patients (68.4%). Study areas and study populations of the participating centres have been described in detail previously.5 Patients were followed up from inception, between 1 October 1991 and 30 September 1993, until data inclusion of the present study between 1 August 2002 and 31 January 2004 or any date prior to this period, indicated as being the date of death or “lost to follow up” (LTFU). Seven of the original 20 centres refrained from participation because of technical and/or logistical reasons. This did not jeopardise the population based character of the study as all participating centres had individually met the criteria for population based patient inclusion when the cohort was initially formed in the period 1991–1993. Before the start of the current data collection, an arbitrary chosen minimum response rate per centre was set at 60%.
Ten year clinical follow up project
The EC‐IBD launched a large clinical follow up study project to investigate multiple facets of disease outcome in this cohort a decade after diagnosis. The project was planned since 1998, received a grant from the European Union, and started in 2001. Details of the methods used in this follow up study project were extensively described elsewhere.5,7 Data inclusion in this study project was supported by an electronic internet based facility.
Definitions
The vital status of each individual patient was assessed by review of the patients' hospital charts. If no satisfactory information could be obtained, patients' general practitioners or families were approached by telephone or regular mail or, in the participating Scandinavian countries, national death registries were searched. Dates of death were registered and causes of death were recorded according to the single level Clinical Classifications Software (CCS),8 a categorisation scheme based on the International Classification of Diseases (ICD‐10).9 In the CCS, ICD‐10 codes are collapsed into a smaller number of clinically meaningful categories, thus creating broad diagnosis groupings, more useful for descriptive statistics than individual ICD‐10 codes. The CCS aggregates illnesses and conditions into 259 mutually exclusive categories, most of which are clinically homogeneous. Expected mortality rates were calculated using aggregate codes in each main diagnosis category, as listed in both the ICD‐9 (Greece, Italy, Spain, and Portugal) and ICD‐10 (Denmark, Israel, Norway, and the Netherlands) of the World Health Organisation (WHO) mortality databases10 for the years 1995–1998.
In the original cohort, detailed information concerning disease location and presence or absence of fistula and strictures was recorded at diagnosis. In the present study, patients were retrospectively grouped regarding disease phenotype at diagnosis according to the Vienna classification11 using the detailed information as obtained at inception.
Statistical analysis
Standardised mortality ratios (SMRs) were calculated by dividing observed mortality rates by expected mortality rates using country, age, and sex specific mortality rates from the WHO mortality database (31 January 2004 update); 95% confidence intervals (95% CI) were calculated by means of Byar's approximation.12 The χ2 and t test were performed to identify possible differences in terms of sex, age, disease location, and behaviour at diagnosis between patients completely followed up and those LTFU. Cox regression analysis was applied to identify risk factors for mortality using the proportional hazards assumption. The proportional hazards assumption was tested using the scaled Schoenfeld residuals.13 The Stata statistical software package was used for the analysis.14 Observed survival curves were calculated using the Kaplan‐Meyer technique. Expected survival curves were calculated using the age specific mortality rates and age and sex specific distribution of person years of the cohort.
Results
Ten of the 13 participating centres complied with the minimum 60% response threshold, giving a total of 380 CD patients to follow of whom 348 (92%) were completely followed up until at least August 2002 or death. Thirty two patients were LTFU of whom 23 were followed during varying time intervals (median follow up time 15 months (range 1–84)) and nine patients appeared to have a date of last visit equal to the date of diagnosis. These nine patients were not incorporated into the analysis. Thus a total of 371 patients participated in the present study (183 males and 188 females with a median age of 31.0 years at diagnosis (range15–83)). Patients LTFU were not different from patients with a complete follow up in terms of sex, age, disease location, or behaviour at diagnosis.
Overall mortality
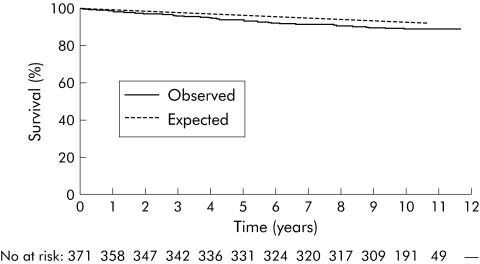
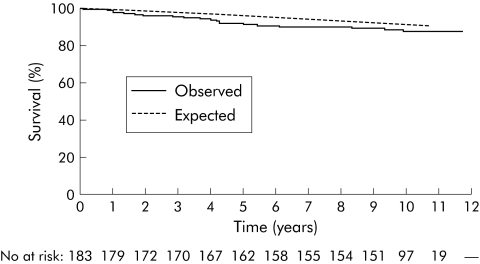
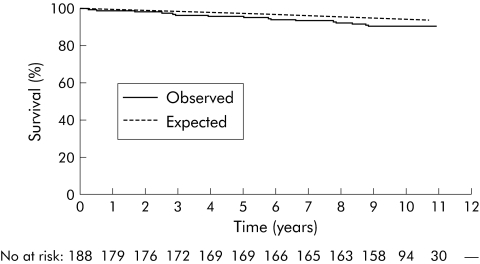
Table 1 shows male, female, and total mortality rates according to age, disease phenotype at diagnosis, and residence. At the end of follow up, 311 patients were known to be alive (median follow up time 123 months (range 107–141)).Thirty seven patients had died (median time from diagnosis until death 51 months (range 3–120)) whereas 21.5 deaths were expected (SMR 1.85 (95% CI 1.30–2.55)). Mortality risk was significantly increased in both females (SMR 1.93 (95% CI 1.10–3.14)) and males (SMR 1.79 (95% CI 1.11–2.73)). Patients from northern European centres had a significantly increased mortality risk (SMR 2.04 (95% CI 1.32–3.01)) whereas a tendency towards increased overall mortality risk was observed in the south (SMR 1.55 (95% CI 0.80–2.70)). Figures 1–3 show the total, male, and female survival curves, as observed in the cohort, compared with the expected survival based on aggregate mortality statistics of all participating countries.
Table 1 Male, female, and cohort specific mortality according to age (sex and country adjusted), disease phenotype at diagnosis (age, sex, and country adjusted), and residence (age and sex adjusted).
| Male | Female | Entire cohort | Groupings entire cohort | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Dead | Exp | SMR (95%CI) | No | Dead | Exp | SMR (95%CI) | No | Dead | Exp | SMR (95%CI) | No | Dead | Exp | SMR (95%CI) | |
| Total | 183 | 21 | 11.8 | 1.79 (1.11–2.73) | 188 | 16 | 8.28 | 1.93 (1.10–3.14) | 371 | 37 | 21.5 | 1.85 (1.30–2.55) | ||||
| Age at diagnosis | ||||||||||||||||
| <40 y | ||||||||||||||||
| 10–19 | 25 | 1 | 0.22 | 4.51 (0.06–25.1) | 26 | 0 | 0.07 | 0 (–) | 51 | 1 | 0.29 | 3.39 (0.04–18.9) | 241 | 3 | 2.32 | 1.29 (0.26–3.78) |
| 20–29 | 58 | 1 | 0.65 | 1.53 (0.02–8.51) | 67 | 1 | 0.34 | 2.95 (0.04–16.4) | 125 | 2 | 0.99 | 2.01 (0.23–7.27) | ||||
| 30–39 | 28 | 0 | 0.50 | 0 (–) | 38 | 0 | 0.41 | 0 (–) | 66 | 0 | 0.91 | 0 (–) | ||||
| ⩾40 y | ||||||||||||||||
| 40–49 | 28 | 1 | 1.28 | 0.78 (0.01–4.34) | 21 | 2 | 0.53 | 3.80 (0.43–13.7) | 49 | 3 | 1.81 | 1.66 (0.33–4.85) | 117 | 33 | 16.6 | 1.99 (1.37–2.80) |
| 50–59 | 18 | 2 | 1.94 | 1.03 (0.12–3.71) | 15 | 1 | 0.90 | 1.11 (0.01–6.15) | 33 | 3 | 2.85 | 1.05 (0.09–39.8) | ||||
| 60–69 | 14 | 6 | 3.65 | 1.64 (0.60–3.58) | 8 | 3 | 1.10 | 2.72 (0.55–7.94) | 22 | 9 | 4.75 | 1.89 (0.86–3.59) | ||||
| 70–79 | 11 | 9 | 3.48 | 2.58 (1.18–4.91) | 11 | 7 | 3.36 | 2.08 (0.84–4.30) | 22 | 16 | 6.84 | 2.34 (1.34–3.80) | ||||
| 80–89 | 1 | 1 | 0.01 | 82.8 (1.08–461) | 2 | 2 | 1.56 | 1.28 (0.14–4.62) | 3 | 3 | 1.57 | 1.91 (0.38–5.57) | ||||
| Disease location at diagnosis | ||||||||||||||||
| Ileum | 26 | 6 | 3.68 | 1.63 (0.60–3.55) | 27 | 4 | 2.15 | 1.86 (0.50–4.77) | 53 | 3 | 2.41 | 1.24 (0.25–3.63) | ||||
| Colon | 73 | 13 | 5.83 | 2.23 (1.19–3.81) | 78 | 8 | 4.41 | 1.81 (0.78–3.57) | 151 | 21 | 10.2 | 2.05 (1.27–3.13) | ||||
| Ileocolon | 69 | 1 | 1.67 | 0.60 (0.01–3.33) | 65 | 2 | 0.74 | 2.69 (0.30–9.73) | 134 | 10 | 5.83 | 1.72 (0.82–3.16) | ||||
| Upper GI | 9 | 1 | 0.29 | 3.44 (0.04–19.1) | 11 | 1 | 0.13 | 7.75 (0.10–43.1) | 20 | 2 | 0.42 | 4.76 (0.54–17.2) | ||||
| Disease behaviour at diagnosis | ||||||||||||||||
| Inflammatory | 132 | 18 | 8.47 | 2.12 (1.26–3.36) | 132 | 10 | 4.24 | 2.36 (1.13–4.34) | 264 | 28 | 12.7 | 2.20 (1.46–3.18) | ||||
| Stricturing | 29 | 3 | 1.85 | 1.62 (0.33–4.74) | 28 | 3 | 2.52 | 1.19 (0.24–3.48) | 57 | 6 | 4.37 | 1.37 (0.50–2.99) | ||||
| Penetrating | 14 | 0 | 1.12 | 0 (–) | 16 | 2 | 0.64 | 3.10 (0.35–11.2) | 30 | 2 | 1.77 | 1.13 (0.13–4.08) | ||||
| Penetrating and stricturing | 2 | 0 | 0.02 | 0 (–) | 5 | 0 | 0.03 | 0 (–) | 7 | 0 | 0.05 | 0 (–) | ||||
| Country | ||||||||||||||||
| South | ||||||||||||||||
| Greece | 16 | 4 | 1.51 | 2.65 (0.71–6.78) | 5 | 0 | 0.02 | 0 (–) | 21 | 4 | 1.53 | 2.61 (0.70–6.67) | 130 | 12 | 7.76 | 1.55 (0.80–2.70) |
| Italy | 20 | 2 | 2.05 | 0.98 (0.11–3.53) | 22 | 2 | 1.43 | 1.39 (0.16–5.04) | 42 | 4 | 3.48 | 1.15 (0.31–2.94) | ||||
| Israel | 5 | 0 | 0.16 | 0 (–) | 16 | 1 | 0.33 | 3.02 (0.04–16.8) | 21 | 1 | 0.49 | 2.03 (0.03–11.3) | ||||
| Portugal | 5 | 0 | 0.40 | 0 (–) | 8 | 0 | 0.09 | 0 (–) | 13 | 0 | 0.49 | 0 (–) | ||||
| Spain | 25 | 3 | 1.72 | 1.74 (0.35–5.08) | 8 | 0 | 0.04 | 0 (–) | 33 | 3 | 1.77 | 1.70 (0.34–4.97) | ||||
| North | ||||||||||||||||
| Denmark | 20 | 5 | 2.01 | 2.49 (0.80–5.81) | 38 | 5 | 2.35 | 2.12 (0.68–4.96) | 58 | 10 | 4.36 | 2.29 (1.10–4.22) | 241 | 25 | 12.3 | 2.04 (1.32–3.01) |
| Norway | 56 | 5 | 2.85 | 1.75 (0.56–4.09) | 50 | 6 | 3.57 | 1.68 (0.61–3.65) | 106 | 11 | 6.43 | 1.71 (0.85–3.06) | ||||
| the Netherlands | 36 | 2 | 1.05 | 1.91 (0.21–6.91) | 41 | 2 | 0.43 | 4.64 (0.52–16.7) | 77 | 4 | 1.48 | 2.71 (0.73–6.94) | ||||
Thirteen patients with no known phenotype at diagnosis, of whom one female died due to pulmonary cause, were excluded from analysis in the age (<40 years and ⩾40 years), disease location, and behaviour at diagnosis categories.
No, number of patients; Exp, expected number of deaths; upper GI, upper gastrointestinal; SMR, standardised mortality ratio; 95% CI, 95% confidence interval.
Figure 1 Total observed survival of the entire cohort versus expected survival based on aggregate mortality statistics of all participating countries. Overall expected versus observed survival rates were 99.5% versus 98.9%, 97.0% versus 94.1%, and 93.0% versus 89.6%, one, five, and 10 years after diagnosis, respectively. No, number of patients.
Figure 2 Male observed survival of the entire cohort versus expected survival based on aggregate mortality statistics of all participating countries. Male expected versus observed survival rates were 99.6% versus 98.9%, 96.2% versus 91.8%, and 91.2% versus 87.5%, one, five, and 10 years after diagnosis, respectively. No, number of patients.
Figure 3 Female observed survival of the entire cohort versus expected survival based on aggregate mortality statistics of all participating countries. Female expected versus observed survival rates were 99.7% versus 98.9%, 97.8% versus 96.1%, and 94.6% versus 90.6%, one, five, and 10 years after diagnosis, respectively. No, number of patients.
Phenotype at diagnosis associated mortality
Increased mortality risks were observed for both males (19 deaths observed versus 10 expected, SMR 1.90 (95% CI 1.14–2.97)) and females (14 deaths observed versus 6.58 expected, SMR 2.13 (95% CI 1.16–3.57)) older than 40 years at diagnosis. Table 1 shows that males with isolated colonic localisation at diagnosis (SMR 2.23 (95% CI 1.19–3.36)) and both males (SMR 2.12 (95% CI 1.26–3.36)) and females (SMR 2.36 (95% CI 1.13–4.34)) with inflammatory disease behaviour at diagnosis had increased mortality risks.
Cause specific mortality
Table 2 shows cause specific mortality rates for the entire cohort. The cause of death of one patient (2.7%) remained unknown. Increased mortality risks were observed in all cause specific groups but reached statistical significance only for gastrointestinal causes (SMR 9.77 (95% CI 4.20–19.2)). Male patients, patients older than 40 years at diagnosis, and patients with inflammatory disease behaviour at diagnosis were observed to have excess risks for cancer, gastrointestinal, and all causes. Female patients and patients with isolated colonic disease at diagnosis had increased mortality risks for both gastrointestinal and all causes. Increased mortality risks by gastrointestinal causes only occurred in patients younger than 40 years at diagnosis (SMR 22.6 (95% CI 2.54–81.8)), with upper gastrointestinal disease location at diagnosis (SMR (95% CI 117 13.1–422)), and those with penetrating disease behaviour at diagnosis (SMR 9.77 (95% CI 4.20–19.2)). Cause specific mortality rates for male patients only, portrayed an almost identical cause specific mortality pattern as for the entire cohort. The observed increased mortality risk by gastrointestinal causes was due to the death of two young male patients. A substantial part of the observed increased mortality risk in the male population with isolated colonic disease localisation at diagnosis was due to pulmonary causes (SMR 6.14 (95% CI 1.23–18.0)). Female patients older than 40 years at diagnosis had excess mortality risks for both gastrointestinal and all causes. The majority of deaths in female patients with inflammatory disease behaviour at diagnosis were due to cardiovascular causes (SMR 3.25 (95% CI 1.05–7.59)). When female patients with isolated colonic disease localisation at diagnosis were considered as a group, a tendency to increased mortality was observed (eight deaths observed versus 4.24 expected, SMR 1.81 (95% CI 0.78–3.57)).
Table 2 Overall cause specific mortality for all patients (age, sex, and country adjusted), according to sex (age and country adjusted), age (sex and country adjusted), disease location and behaviour at diagnosis (age, sex, and country adjusted), and residence (age and sex adjusted).
| No | Cancer | Cardiovascular causes | Pulmonary causes | Gastrointestinal causes | All causes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCS ICD‐10: 011–044 | CCS ICD‐10: 099–118 | CCS ICD‐10: 122–134 | CCS ICD‐10: 138–155 | CCS ICD‐10: 1–259 | |||||||||||||
| Dead | Exp | SMR (95%CI) | Dead | Exp | SMR (95%CI) | Dead | Exp | SMR (95%CI) | Dead | Exp | SMR (95%CI) | Dead | Exp | SMR (95%CI) | |||
| All patients | 371 | 11 | 5.60 | 1.96 (0.98–3.51) | 11 | 7.39 | 1.49 (0.74–2.66) | 4 | 1.50 | 2.66 (0.72–6.80) | 8 | 0.82 | 9.77 (4.20–19.2) | 37 | 21.5 | 1.85 (1.30–2.55) | |
| Sex | |||||||||||||||||
| Male | 183 | 8 | 3.43 | 2.33 (1.00–4.59) | 4 | 4.17 | 0.96 (0.26–2.46) | 3 | 0.83 | 3.61 (0.73–10.6) | 5 | 0.50 | 10.0 (3.23–23.4) | 21 | 11.6 | 1.79 (1.11–2.73) | |
| Female | 188 | 3 | 2.17 | 1.38 (0.28–4.04) | 7 | 3.22 | 2.17 (0.87–4.48) | 1 | 0.67 | 1.48 (0.02–8.25) | 3 | 0.32 | 9.37 (1.88–27.4) | 16 | 8.28 | 1.93 (1.10–3.14) | |
| Age at diagnosis | |||||||||||||||||
| <40 y | 241 | 0 | 0.54 | 0 (–) | 1 | 0.31 | 3.18 (0.04–17.7) | 0 | 0.05 | 0 (–) | 2 | 0.09 | 22.6 (2.54–81.8) | 3 | 2.32 | 1.29 (0.26–3.78) | |
| ⩾40 y | 117 | 11 | 4.83 | 2.28 (1.14–4.08) | 10 | 6.60 | 1.51 (0.73–2.79) | 3 | 1.35 | 2.22 (0.45–6.48) | 6 | 0.68 | 8.78 (3.21–19.1) | 33 | 16.6 | 1.99 (1.37–2.80) | |
| Disease location at diagnosis | |||||||||||||||||
| Ileal | 53 | 2 | 0.77 | 2.59 (0.29–9.34) | 1 | 0.84 | 1.20 (0.02–6.66) | 0 | 0.13 | 0 (–) | 0 | 0.10 | 0 (–) | 3 | 2.41 | 1.24 (0.25–3.63) | |
| Colonic | 151 | 5 | 2.76 | 1.81 (0.58–4.23) | 8 | 4.01 | 2.00 (0.86–3.93) | 3 | 0.86 | 3.49 (0.70–10.2) | 4 | 0.40 | 9.91 (2.67–25.4) | 21 | 10.2 | 2.05 (1.27–3.13) | |
| Ileocolonic | 134 | 4 | 1.71 | 2.35 (0.63–6.00) | 2 | 1.96 | 1.02 (0.11–3.69) | 0 | 0.40 | 0 (–) | 2 | 0.26 | 7.84 (0.88–28.3) | 10 | 5.83 | 1.72 (0.82–3.16) | |
| Upper GI | 20 | 0 | 0.13 | 0.00 (0.00–27.6) | 0 | 0.11 | 0 (–) | 0 | 0.02 | 0 (–) | 2 | 0.02 | 117 (13.1–422) | 2 | 0.42 | 4.76 (0.54–17.2) | |
| Disease behaviour at diagnosis | |||||||||||||||||
| Inflammatory | 264 | 10 | 3.68 | 2.71 (1.30–4.99) | 8 | 4.64 | 1.73 (0.74–3.40) | 2 | 0.91 | 2.21 (0.25–7.97) | 6 | 0.50 | 12.0 (4.39–26.2) | 28 | 12.7 | 2.20 (1.46–3.18) | |
| Stricturing | 57 | 1 | 1.11 | 0.90 (0.01–5.00) | 3 | 1.73 | 1.74 (0.35–5.08) | 1 | 0.34 | 2.91 (0.04–16.2) | 0 | 0.18 | 0 (–) | 6 | 4.37 | 1.37 (0.50–2.99) | |
| Penetrating | 30 | 0 | 0.56 | 0 (–) | 0 | 0.55 | 0 (–) | 0 | 0.15 | 0 (–) | 2 | 0.09 | 22.5 (2.52–81.1) | 2 | 1.77 | 1.13 (0.13–4.08) | |
| Penetrating and stricturing | 7 | 0 | 0.01 | 0 (–) | 0 | 0.01 | 0 (–) | 0 | 0.00 | 0 (–) | 0 | 0.00 | 0 (–) | 0 | 0.05 | 0 (–) | |
| Greece | |||||||||||||||||
| Heraklion | 15 | 21 | 3 | 0.40 | 7.52 (1.51–22.0) | 0 | 0.70 | 0 (–) | 1 | 0.09 | 11.3 (0.15–62.9) | 0 | 0.04 | 0 (–) | 4 | 1.53 | 2.61 (0.70–6.67) |
| Ioannina | 6 | ||||||||||||||||
| Italy | |||||||||||||||||
| Cremona | 10 | 42 | 0 | 1.02 | 0 (–) | 2 | 1.38 | 1.45 (0.16–5.25) | 0 | 0.19 | 0 (–) | 1 | 0.18 | 5.62 (0.07–31.3)) | 4 | 3.48 | 1.15 (0.31–2.94) |
| Reggio Emilia | 32 | ||||||||||||||||
| Israel (Beer Sheeva) | 21 | 0 | 0.17 | 0 (–) | 0 | 0.12 | 0 (–) | 0 | 0.02 | 0 (–) | 1 | 0.01 | 70.1 (0.92–390) | 1 | 0.49 | 2.03 (0.03–11.3) | |
| Portugal (Almada) | 13 | 0 | 0.14 | 0 (–) | 0 | 0.12 | 0 (–) | 0 | 0.03 | 0 (–) | 0 | 0.04 | 0 (–) | 0 | 0.49 | 0 (–) | |
| Spain (Vigo) | 33 | 1 | 0.49 | 2.05 (0.03–11.4) | 0 | 0.52 | 0 (–) | 0 | 0.19 | 0 (–) | 2 | 0.09 | 21.7 (2.44–78.5) | 3 | 1.77 | 1.70 (0.34–4.97) | |
| Denmark (Copenhagen) | 58 | 5 | 1.29 | 3.88 (1.25–9.05) | 3 | 1.52 | 1.98 (0.40–5.78) | 1 | 0.39 | 2.56 (0.03–14.3) | 1 | 0.20 | 4.89 (0.06–27.2) | 10 | 4.36 | 2.29 (1.10–4.22) | |
| Norway (Oslo) | 106 | 1 | 1.53 | 0.65 (0.01–3.63) | 5 | 2.64 | 1.89 (0.61–4.42) | 2 | 0.55 | 2.63 (0.41–13.1) | 1 | 0.20 | 5.09 (0.07–28.3) | 11 | 6.43 | 1.71 (0.85–3.06) | |
| the Netherlands (South Limburg) | 77 | 1 | 0.56 | 1.79 (0.02–9.97) | 1 | 0.39 | 2.56 (0.03–14.3) | 0 | 0.05 | 0 (–) | 2 | 0.05 | 37.8 (4.24–136) | 4 | 1.48 | 2.71 (0.73–6.94) | |
Thirteen patients with no known phenotype at diagnosis, of whom one female died due to pulmonary cause, were excluded from analysis in the age (<40 years and ⩾40 years), disease location, and disease behaviour at diagnosis categories. Three patients died due to other causes than listed (two infectious and one unknown).
No, number of patients; Exp, expected number of deaths; upper GI: upper gastrointestinal; SMR, standardised mortality ratio; 95% CI, 95% confidence interval; CCS, clinical classifications software; ICD, International Classification of Diseases.
CD related mortality
Fourteen (seven males and seven females) of the 37 observed deaths (37.8%) had a certain (6) or possible (8) CD related cause of death (table 3). Median age at diagnosis of patients with a CD related cause of death was 64 years (range 18–83). Median survival after diagnosis of this group was 39 months (range 1–112). Median age at diagnosis of patients with a cause of death not related to CD (table 4) was 71 years (range 41–81) and median survival after diagnosis of these patients was 51 months (range 6–119). All eight observed deaths due to gastrointestinal causes had a certain (4) or possible (4) causal relationship with CD. Four patients in this category died because of abdominal sepsis, one due to intestinal ischaemia, one because of intestinal haemorrhage, and one because of surgery related complications. One patient died because of gall stone mediated biliary sepsis that was interpreted as possibly CD related because of the known association of CD and biliary stones. The three deaths in patients younger than 40 years at diagnosis were certainly (2) or possibly (1) CD related. Twelve of the observed 28 deaths in the patient group with inflammatory disease behaviour at diagnosis were certainly (5) or possibly (7) CD related. Six of the observed 21 deaths in patients with isolated colonic disease location had a certain (4) or possible (2) CD related cause of death. One female patient with an unknown disease phenotype at diagnosis died because of aspiration pneumonia as a result of CD mediated bowel obstruction and ileus. Three males with isolated colonic disease location at diagnosis died due to pulmonary causes not related to CD. Two of the five female patients with inflammatory disease behaviour at diagnosis died due to cardiovascular causes that occurred in association with CD activity. None of the observed cancer associated deaths had a causal relationship with CD.
Table 3 Causes of death possibly or certainly related to Crohn's disease (CD).
| Patient No and causality of death and CD | Centre | Sex | Disease location at diagnosis | Disease behaviour at diagnosis | Age at diagnosis (y) | Survival after diagnosis (months) | Cause of death | CCS ICD‐10 code |
|---|---|---|---|---|---|---|---|---|
| Infections | ||||||||
| 1 Certain | Oslo | F | Ileocolonic | Stricturing | 65 | 34 | Sepsis, steroid use for CD activity | 2 |
| 2 Possible | Reggio Emilia | M | Ileocolonic | Inflammatory | 67 | 112 | Sepsis, steroid use for CD activity | 2 |
| Cardiovascular causes | ||||||||
| 3 Possible | South Limburg | F | Ileocolonic | Inflammatory | 26 | 94 | Acute myocardial infarction during CD activity (autopsy confirmed). Steroid use | 100 |
| 4 Possible | Reggio Emilia | F | Colonic | Inflammatory | 80 | 69 | Cardiac failure during CD activity. Steroid use | 108 |
| 5 Certain | Copenhagen | M | Colonic | Inflammatory | 78 | 34 | Congestive heart failure after elective subtotal colectomy. Steroid use | 108 |
| Pulmonary causes | ||||||||
| 6 Possible | Copenhagen | F | Unknown | Unknown | 71 | 8 | Aspiration pneumonia due to CD mediated bowel obstruction. Steroid use. | 122 |
| Gastrointestinal causes | ||||||||
| 7 Certain | Reggio Emilia | M | Colonic | Inflammatory | 63 | 44 | Toxic megacolon. Steroid and cyclosporine use | 144 |
| 8 Possible | Beer Sheeva | F | Upper GI | Inflammatory | 60 | 60 | Jejunal infarction. Azathioprine and steroid use. | 144 |
| 9 Possible | Copenhagen | F | Colonic | Penetrating | 71 | 4 | Sepsis after elective subtotal colectomy. Topical steroid use | 148 |
| 10 Certain | South Limburg | M | Ileocolonic | Inflammatory | 20 | 23 | Sepsis after acute ileocaecal resection | 148 |
| 11 Certain | Vigo | M | Colonic | Inflammatory | 18 | 50 | Acute subtotal colectomy for perforation followed by sepsis. Azathioprine and steroid use | 148 |
| 12 Certain | Oslo | M | Colonic | Inflammatory | 83 | 1 | Acute subtotal colectomy for perforation followed by sepsis. Steroid use. | 148 |
| 13 Possible | South Limburg | F | Ileocolonic | Inflammatory | 46 | 94 | Gall bladder stones, biliary sepsis | 149 |
| 14 Possible | Vigo | M | Upper GI | Inflammatory | 60 | 16 | Gastrointestinal haemorrhage | 153 |
CCS, clinical classifications software; ICD, International Classification of Diseases.
Table 4 Causes of death not related to Crohn's disease (CD).
| Patient No | Centre | Sex | Disease location at diagnosis | Disease behaviour at diagnosis | Age at diagnosis (y) | Survival after diagnosis (months) | Cause of death | CCS ICD‐10 code | Circumstances at death |
|---|---|---|---|---|---|---|---|---|---|
| Cancer | |||||||||
| 15 | Heraklion | M | Colonic | Inflammatory | 76 | 12 | Bronchus carcinoma | 19 | No immunosuppressives |
| 16 | Vigo | M | Ileocolonic | Inflammatory | 70 | 65 | Bronchus carcinoma | 19 | No immunosuppressives |
| 17 | South Limburg | M | Ileal | Stricturing | 60 | 100 | Bronchus carcinoma | 19 | No immunosuppressives |
| 18 | Oslo | F | Ileocolonic | Inflammatory | 53 | 35 | Breast cancer | 24 | No immunosuppressives |
| 19 | Heraklion | M | Colonic | Inflammatory | 65 | 38 | Urinary bladder cancer | 32 | No immunosuppressives |
| 20 | Copenhagen | M | Colonic | Inflammatory | 56 | 21 | Leukaemia | 39 | HIV, heart failure |
| 21 | Copenhagen | M | Ileocolonic | Inflammatory | 46 | 119 | Leukaemia | 39 | No immunosuppressives |
| 22 | Copenhagen | F | Colonic | Inflammatory | 71 | 79 | Multiple myeloma | 40 | No immunosuppressives |
| 23 | Copenhagen | M | Colonic | Inflammatory | 71 | 48 | Pancreatic cancer | 41 | No immunosuppressives |
| 24 | Ioannina | M | Ileocolonic | Inflammatory | 77 | 51 | Unknown primary cancer | 41 | Azathioprine during 3 y previously |
| 25 | Copenhagen | F | Ileal | Inflammatory | 67 | 47 | Unspecified cancer of peritoneum, probably metastasis of gynaecological or lung cancer | 43 | No immunosuppresives |
| Cardiovascular causes | |||||||||
| 26 | Oslo | M | Colonic | Inflammatory | 73 | 10 | Acute myocardial infarction | 100 | No CD activity |
| 27 | Oslo | M | Colonic | Inflammatory | 75 | 73 | Acute myocardial infarction | 100 | No CD activity |
| 28 | Oslo | F | Colonic | Stricturing | 81 | 105 | Coronary atherosclerosis | 101 | No CD activity |
| 29 | Oslo | F | Colonic | Inflammatory | 78 | 30 | Acute cerebrovascular disease | 109 | No CD activity |
| 30 | Copenhagen | M | Ileocolonic | Inflammatory | 73 | 65 | Acute cerebrovascular disease | 109 | No CD activity |
| 31 | Copenhagen | F | Colonic | Inflammatory | 41 | 93 | Acute cerebrovascular disease | 109 | No CD activity |
| 32 | Cremona | F | Ileal | Stricturing | 70 | 100 | Acute cerebrovascular disease | 109 | No CD activity |
| 33 | Oslo | F | 79 | 70 | Pulmonary embolism | 118 | No CD activity or medication use during years previously | ||
| Pulmonary causes | |||||||||
| 34 | Oslo | M | Colonic | Inflammatory | 67 | 12 | Pneumonia | 122 | No immunosuppressives |
| 35 | Oslo | M | Colonic | Stenotic | 77 | 51 | Pneumonia | 122 | No immunosuppressives |
| 36 | Heraklion | M | Colonic | Inflammatory | 64 | 60 | COPD | 127 | No CD activity |
| Unknown | |||||||||
| 37 | Oslo | F | Colonic | Inflammatory | 74 | 6 | Unknown | – | No CD activity |
CCS, clinical classifications software; ICD, International Classification of Diseases.
Cox regression analysis
Age and sex as well as disease location and behaviour were the factors analysed in both the uni‐ and multivariate analysis. Univariate analysis showed hazard ratios of 1.10 and 1.07 per year with increasing age at diagnosis for all causes of mortality (95% CI 1.08–1.12) and CD related mortality (95% CI 1.04–1.10), respectively. No other factor reached statistical significance in the univariate model. In the multivariate model, increasing age remained the only independent risk factor for both total and CD related mortality causes.
Discussion
Increased overall mortality was observed 10 years after diagnosis in this European population based cohort of CD patients. Mortality risk appeared to be increased in the age group above 40 years at diagnosis. Excess mortality was mainly due to gastrointestinal causes that were either certainly or possibly related to CD.
Previous studies reporting on CD associated mortality differed in terms of patient selection, patient inception, follow up rates, and follow up duration. Referral centre based studies have mostly reported on mortality values in CD that were increased.15,16,17,18,19,20,21,22,23,24,25 These studies were based on selected patient samples and must be interpreted with caution.26 Most population based studies with good methodology, reporting on mortality in CD, had patients enrolled over decades.2,27,28,29,30 Changes in treatment practice may have influenced mortality rates observed during the course of time. Follow up duration can influence outcome considerably: excess mortality in a Danish study was not observed after 10 years31 but appeared after a median of 17 years of follow up in the same cohort of CD patients.2
The methodological strengths of the current study are threefold. Firstly, this study was based on a population based, prospectively, and uniformly diagnosed inception cohort of CD patients originating from 10 well described geographical areas in seven European countries and Israel. This multinational population based cohort is unique on the European continent and possibly also in the world. Secondly, patient inception at diagnosis took place during a short period of two years. All patients were followed within the same time period and had, except for those who died and were found lost to follow up, approximately identical observation times. Thirdly, no previous cohort study has been able to correlate disease phenotype at diagnosis according to the Vienna classification with mortality. Our applied prognostic model could also be tested in other contemporary and future cohorts.
There were some limitations to the study. Firstly, even though the total number of patients may seem sufficient and the 92% follow up rate must be regarded as excellent, some participating centres and clinical subgroups had low sample sizes. This has biased the actual mortality risk in certain described geographical and phenotypic patient subsets. Multivariate analysis carried out in this study did not produce a robust model as no deaths occurred in some patient subsets. Confounding may have occurred in the stratified analyses of disease phenotype at diagnosis, as these analyses were only adjusted for age, sex, and residence. In these analyses, colonic location and inflammatory disease behaviour were associated with increased mortality. It is possible that these results were not independent. Secondly, 50% of the original EC‐IBD centres participated in this study, leaving 52% of the original CD patients. It is possible that centres with more of an interest in IBD and therefore with more expertise in treatment, participated, which may have been a source of bias. Thirdly, retrospective data collection concerning mortality and causes of death was used. However, for all except one patient, the exact cause of death and its relationship to CD was established. In 23 included cases the vital status remained unclear. These patients were not different in terms of baseline characteristics from those with complete follow up, and any deaths that could have occurred in this subset would probably have further upgraded the increased mortality risk observed in this cohort. Fourthly, CD patient classification by pattern of disease behaviour at diagnosis was previously shown to yield only fair interobserver agreement.32 In the present study, uniform definitions were used to retrospectively classify patients at diagnosis regarding disease location and disease behaviour. These findings were clearly reported in patient files. Accordingly, the risk of interpretative classification was minimised.
An increased mortality risk in CD patients was described until the mid 1970s,3,33,34 with a subsequent decrease thereafter.3 Seven population based studies have reported on mortality risk in CD. Most of these showed an increased SMR, but decreasing to unity during the last 40 years.1 Although comparison was hampered by differences in follow up duration and geographical backgrounds, four of these studies showed increased mortality risks during the first 2–9 years after diagnosis,23,27,29 a subsequent plateau phase, and thereafter an increasing risk from 14.5 to 25 years after diagnosis.2,23 In most of these studies, early deaths occurred due to non‐surgical and/or surgical complications of the disease whereas the deaths due to gastrointestinal cancer occurred late during the disease course. Findings in this study confirmed both the increased mortality risk during the first years after diagnosis and its direct relation to CD. Further follow up of this cohort in the future may reveal the impact of possible late sequelae on CD related mortality, such as intestinal cancer. Interestingly, the previously assumed geographical differences may be confirmed by findings in this study, as the northern European patient group demonstrated a slightly higher mortality risk than the southern European cohort. Apart from the relatively small sample size in the south, true differences in terms of more aggressive phenotype in the north could be responsible for this gradient. Interestingly in this context is that the use of corticosteroids did not differ between the northern and southern European centres but the use of azathioprine did. Of 237 patients from northern Europe, 74 (31%) had used azathioprine whereas in the south 21 of 121 patients (17%) had used the drug (p = 0.005). Median duration of azathioprine use was 24 months (range 1–114) in the north versus 36 months (range 1–87) in the south (p = 0.009). This discrepancy was caused by a relatively high number of patients from northern European centres in whom azathioprine had to be discontinued early after treatment initiation because of side affects (20/74 patients from the north used azathioprine for less than six months versus 2/21 patients from the south). The use of immunosuppressive drugs could be interpreted as a surrogate marker of disease severity and hence, given the percentages of patients in whom azathioprine was considered to be indicated, disease phenotype might be estimated as more severe in the north of Europe compared with the south.
Population based findings concerning the correlation of phenotypic characteristics at diagnosis and subsequent mortality risk in CD are scarce. In one Danish and two large Swedish cohort studies, no differences in mortality risk between the different disease location groups at diagnosis were observed.2,28,29 A population based study from the UK reported increased mortality in patients with colonic disease location. In that study, however, it was not explicitly indicated whether disease location was recorded at diagnosis or during the course of the disease.35 Few population based studies analysed age at diagnosis as a possible risk factor for mortality and the results were conflicting. Mayberry et al observed increased mortality rates in patients between the ages of 10–19 and 50–59 years at diagnosis,23 whereas Jess et al observed an increased mortality risk in female patients between 20–29 and 40–49 years at diagnosis,2 and Loftus in patients with “older” age at diagnosis.30 Previous population based reports on disease behavioural characteristics at diagnosis as risk factors are lacking.
In the multivariate analysis, age above 40 years at diagnosis was the only independent risk factor. It should be noted however that the multivariate analysis was hampered by the number of patients and that deaths were few or even absent in some phenotypic subgroups. SMRs were increased in phenotypic subgroups such as upper gastrointestinal disease location and stricturing disease but differences with the background population did not reach significance, presumably because of the small patient samples. Although comorbidity was not recorded for the purpose of this study, it may have played an important role in explaining the observed increased mortality risk in the older age group at diagnosis in this cohort. The additional effect of disease and therapy related complications may have increased the chance of death in older patients with comorbidity. Eleven of the 14 patients who had a CD related cause of death were on corticosteroid therapy at the moment of death, of whom six had a septic cause of death. Two of the patients on steroid therapy also were using azathioprine, of whom one had a septic cause of death. One patient was treated with ciclosporin in addition to corticosteroids and died because of a toxic megacolon. The use of immunosuppressive medication is a reflection of disease severity and, in addition, may have exerted a contributory effect to death in the critically ill. The availability of anti‐tumour necrosis factor α (TNF‐α) for severe endoluminal and perianal CD dates from 1999. The effect of treatment with anti‐TNF‐α and other biologicals on mortality in CD is important but could not be addressed in the context of this study. Anti‐TNF‐α was available only during the last three years of the observation period of the present study. Only 16 patients were treated with anti‐TNF‐α, of whom 14 received it for less than 10% of the entire observation period. A planned new inception cohort will address the effect of treatment with biologicals on mortality in CD, especially in comparison with the mainly “pre‐biological” findings of the present study. The effect of smoking could not be tested reliably because patients who died during follow up were not available for the questionnaire in this present study. Data concerning smoking behaviour, as obtained at patient inception during 1991–1993, were used as a surrogate marker but appeared rather incomplete.
A consistent finding in previously reported population based studies was an increased mortality risk from gastrointestinal causes either with27 or without2,28,29 CD related causes incorporated. This was confirmed in the present study. Increased mortality due to gastrointestinal causes was observed in both males and females, and in age and various disease location and behaviour groups. It may explain an important part of the excess mortality in this cohort, as all eight observed deaths due to gastrointestinal causes had a causal relationship with CD.
In conclusion, this European and Israeli multinational population based study revealed an increased overall mortality risk in CD patients 10 years after diagnosis, and age greater than 40 years at diagnosis was the sole factor associated with increased mortality risk.
Acknowledgements.
| Centre | Investigator | Contribution |
|---|---|---|
| Maastricht (project coordinotor), Department of Gastroenterology and Hepatology, University Hospital Maastricht, Maastricht, the Netherlands | Mia Cilissen | Collected data |
| Marielle Romberg | Collected data | |
| Almada, Departments of Gastroenterology and Pathology, Almada Regional Health Departments, Portugal | João Freitas | Collected data |
| Paula Borrhalho Nunes | Collected data | |
| Copenhagen, Department of Medical Gastroenterology, Herlev Hospital, University of Copenhagen, Herlev, Denmark | Vibeke Binder | Scientific advisor |
| Ebbe Langholz | Scientific advisor | |
| Lene Riis | Collected data | |
| Cremona, Servizio di Gastroenterologia, Ospedale di Cremona, Cremona, Italy | Patrizia Politi | Collected data |
| Dublin, Adelaide and Meath Hospital, Department of Gastroenterology, Trinity College, Tallaght, Dublin, Ireland | Asghar Qasim | Collected data |
| Angie Harrington | Collected data | |
| Firenze, UO di Gastroenterologia, Policlinico di Careggi, Firenze, Italy | Guiseppe d' Albasio | Scientific advisor |
| Andrea Messori | Scientific advisor | |
| Global Vitis, Wattstraat 52, Sassenheim, the Netherlands | Frederik Wessels | Developed data acquisition tools |
| Ed van Hees | ||
| Robert van Hees | ||
| Heerlen, Department of Pathology, Atrium Medisch Centrum Heerlen, the Netherlands | Marius Nap | Scientific advisor |
| Heraklion, Departments of Gastroenterology, University General Hospital, Heraklion, Crete, Greece | Athanasios G. Pallis | Collected data |
| Ioannis G. Vlachonikolis | ||
| Ioannis Koutroubakis | ||
| Maria Tzardi | ||
| Ioannina, Division of Internal Medicine, University of Ioannina, Ioannina, Greece | Kostas Katsanos | Collected data |
| Michel Economou | Collected data | |
| Leuven, Department of Gastroenterology, UZ Gasthuisberg, Universiteit Leuven, Leuven, Belgium | Paul Rutgeerts | Scientific advisor |
| Sofie Joossens | Collected data | |
| Greet Claessens | Collected data | |
| Maastricht MEMIC, University of Maastricht, Maastricht, the Netherlands | Gilbert van Zeijl | Data manager |
| Milan, Universita degli Studi di Milano, Cattedra di Gastroenterologia, Milano, Italy | Tullio Ranzi | Scientific advisor |
| Claudio Cortelezzi | Collected data | |
| Oslo, Medical Department A, Rikshospitalet, University of Oslo, Oslo, Norway | Idar Lygren | Collected data |
| Tomm Bernklev | Collected data | |
| Camilla Solberg | Collected data | |
| Ole Høie | Collected data | |
| Department of Internal Medicine and Gastroenterology, Arcispedale S Maria Nuova, Reggio Emilia, Italy | Maria Grazia Mortilla | Collected data |
| Marina Beltrami | Collected data | |
| Torino, Divisione di Gastroenterologica, Largo Turati 62, Torin, Italy | Angelo Pera | Scientific advisor |
| Vigo, Hospital Xeral de Vigo, Vigo, Spain | Juan Clofent | Collected data |
| Mercedes Butron | Collected data |
Abbreviations
CD - Crohn's disease
CCS - clinical classifications software
EC‐IBD - European Collaborative study group of Inflammatory Bowel Disease
IBD - inflammatory bowel disease
ICD - International Classification of Diseases
LTFU - lost to follow up
SMR - standardised mortality ratio
TNF‐α - tumour necrosis factor α
WHO - World Health Organisation
Footnotes
This study was granted by the European Commission as a fifth framework shared cost action (QLG4‐CT‐2000‐01414)
Conflict of interest: None declared.
References
- 1.Wolters F L, Russel M G, Stockbrugger R W. Systematic review: has disease outcome in Crohn's disease changed during the last four decades? Aliment Pharmacol Ther 200420483–496. [DOI] [PubMed] [Google Scholar]
- 2.Jess T, Winther K V, Munkholm P.et al Mortality and causes of death in Crohn's disease: follow‐up of a population‐based cohort in Copenhagen County, Denmark. Gastroenterology 20021221808–1814. [DOI] [PubMed] [Google Scholar]
- 3.Sonnenberg A. Mortality from Crohn's disease and ulcerative colitis in England‐Wales and the U.S. from 1950 to 1983. Dis Colon Rectum 198629624–629. [DOI] [PubMed] [Google Scholar]
- 4.Stockbrugger R, Russel M G, van Blankenstein M.et al EC‐IBD: a European effort in inflammatory bowel disease. Eur J Intern Med 200011187–190. [DOI] [PubMed] [Google Scholar]
- 5.Shivananda S, Lennard Jones J, Logan R.et al Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC‐IBD). Gut 199639690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lennard Jones J E. Classification of inflammatory bowel disease. Scand J Gastroenterol 1989170(suppl)2–6. [DOI] [PubMed] [Google Scholar]
- 7.Wolters F L, Zeijl G V, Wessels F.et al Internet‐based data inclusion in a European epidemiological follow‐up study of IBD patients: a description of methods used. World J Gastroenterol 2005117152–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical Classifications Software for ICD‐10 Data. Software and User's Guide, January 2003. Rockville, MD, Agency for Healthcare Research and Quality ( http://www.ahrq.gov/data/hcup/icd10usrgd.htm )
- 9.WHO The tenth revision of the international statistical classification of diseases and related health problems (ICD‐10). Geneva: WHO Press, 1992 (ISBN 92 4 154419)
- 10. Mortality Database, World Health Organization, July 2000. http://www3.who.int/whosis/mort/table1.cfm?path = mort,mort_table1&language = english (accessed 16 January 2006)
- 11.Gasche C, Scholmerich J, Brynskov J.et al A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 200068–15. [DOI] [PubMed] [Google Scholar]
- 12.Breslow M D. Comparisons among exposure groups. In: Heseltine E, ed. Statistical methods in cancer research, the design and analysis of cohort studies. Lyon: Oxford University Press, 198648–79.
- 13.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 198269239–241. [Google Scholar]
- 14.Cleves M A G W W, Gutierrez R G. Cox regression models. In: An introduction to survival analysis using Stata. Texas: Stata Press, 2002119–195.
- 15.Prior P, Gyde S, Cooke W T.et al Mortality in Crohn's disease. Gastroenterology 198180307–312. [PubMed] [Google Scholar]
- 16.Weterman I T, Biemond I, Pena A S. Mortality and causes of death in Crohn's disease. Review of 50 years' experience in Leiden University Hospital. Gut 1990311387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banks B M, Zetzel L, Richter H S. Morbidity and mortality in regional enteritis. Report of 168 cases. Am J Dig Dis 196914369–379. [DOI] [PubMed] [Google Scholar]
- 18.Burton I, Dombal F T, Goligher J C. The prognosis of Crohn's disease. Br J Surg 196956692. [PubMed] [Google Scholar]
- 19.Brill C B, Klein S F, Kark A E. Regional enteritis and entero‐colitis: a study of 74 patients over 15 years. Ann Surg 1969170766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer R G, Michener W M. Prognosis of Crohn's disease with onset in childhood or adolescence. Dig Dis Sci 197924752–757. [DOI] [PubMed] [Google Scholar]
- 21.Storgaard L, Bischoff N, Henriksen F W.et al Survival rate in Crohn's disease and ulcerative colitis. Scand J Gastroenterol 197914225–230. [DOI] [PubMed] [Google Scholar]
- 22.Cooke W T, Mallas E, Prior P.et al Crohn's disease: course, treatment and long term prognosis. Q J Med 198049363–384. [PubMed] [Google Scholar]
- 23.Mayberry J F, Newcombe R G, Rhodes J. Mortality in Crohn's disease. Q J Med 19804963–68. [PubMed] [Google Scholar]
- 24.Lind E, Fausa O, Gjone E.et al Crohn's disease. Treatment and outcome. Scand J Gastroenterol 1985201014–1018. [DOI] [PubMed] [Google Scholar]
- 25.Andrews H A, Lewis P, Allan R N. Mortality in Crohn's disease—a clinical analysis. Q J Med 198971399–405. [PubMed] [Google Scholar]
- 26.Truelove S C, Pena A S. Course and prognosis of Crohn's disease. Gut 197617192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palli D, Trallori G, Saieva C.et al General and cancer specific mortality of a population based cohort of patients with inflammatory bowel disease: the Florence Study. Gut 199842175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persson P G, Bernell O, Leijonmarck C E.et al Survival and cause‐specific mortality in inflammatory bowel disease: a population‐based cohort study. Gastroenterology 19961101339–1345. [DOI] [PubMed] [Google Scholar]
- 29.Ekbom A, Helmick C G, Zack M.et al Survival and causes of death in patients with inflammatory bowel disease: a population‐based study. Gastroenterology 1992103954–960. [DOI] [PubMed] [Google Scholar]
- 30.Loftus E V, Jr, Silverstein M D, Sandborn W J.et al Crohn's disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology 19981141161–1168. [DOI] [PubMed] [Google Scholar]
- 31.Munkholm P, Langholz E, Davidsen M.et al Intestinal cancer risk and mortality in patients with Crohn's disease. Gastroenterology 19931051716–1723. [DOI] [PubMed] [Google Scholar]
- 32.Steinhart A H, Girgrah N, McLeod R S. Reliability of a Crohn's disease clinical classification scheme based on disease behavior. Inflamm Bowel Dis 19984228–234. [DOI] [PubMed] [Google Scholar]
- 33.Newcombe R G, Mayberry J F, Rhodes J. An international study of mortality from inflammatory bowel disease. Digestion 19822473–78. [DOI] [PubMed] [Google Scholar]
- 34.Sonnenberg A. Geographic variation in the incidence of and mortality from inflammatory bowel disease. Dis Colon Rectum 198629854–861. [DOI] [PubMed] [Google Scholar]
- 35.Probert C S, Jayanthi V, Wicks A C.et al Mortality from Crohn's disease in Leicestershire, 1972–1989: an epidemiological community based study. Gut 1992331226–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]