Abstract
Background
The 13C‐urea breath test (UBT) for detecting Helicobacter pylori infection is a non‐invasive method based on the organism's urease activity. Since its first description, the method has been extensively modified. However, only the dose of 13C‐urea and the measurement equipment are directly related to the cost of the test.
Aims
(1) To assess the diagnostic accuracy before eradication therapy of three UBTs using 25, 15, and 10 mg of 13C‐urea, respectively; and (2) to determine diagnostic performance in the post‐eradication setting showing the highest values for sensitivity and specificity with the lowest dose of 13C‐urea.
Methods
Three hundred consecutive patients were randomised to be tested with one of the three UBTs. All patients underwent upper endoscopy with biopsies. A total of 222 more patients were enrolled to evaluate the second aim. Infected patients were offered treatment and asked to return 4–6 weeks after the end of therapy to perform endoscopic follow up and to carry out 13C‐UBT.
Results
In the pretreatment setting, 13C‐UBT 25 mg had a sensitivity of 100% (95% confidence interval (CI) 91.8–100) and a specificity of 100% (95% CI 93.7–100); 13C‐UBT 15 mg had a sensitivity of 96.1% (95% CI 86.8–98.9) and a specificity of 100% (95% CI 92.6–100); and 13C‐UBT 10 mg had a sensitivity of 89.1% (95% CI 77–95.3) and a specificity of 100% (95% CI 93.3–100). As the test with the best performance and the lowest dose of 13C‐urea was 13C‐UBT 15 mg, it was evaluated after treatment, reporting a sensitivity of 100% (95% CI 79.6–100) and a specificity of 98.9% (95% CI 94.3–99.8).
Discussion
UBTs using 25 and 15 mg of 13C‐urea were both accurate in the diagnosis of H pylori infection in untreated patients. 13C‐UBT 15 mg was also accurate for follow up of patients after treatment.
Keywords: 13C‐urea breath test, Helicobacter pylori, dyspepsia, diagnosis
The urea breath test (UBT) for detecting Helicobacter pylori infection is a non‐invasive method based on the organism's urease activity. Urea can be labelled with two different carbon isotopes: 14C and 13C. The main difference between them is that the former is radioactive whereas the latter is stable. As there are several concerns with the use of a radioactive isotope,1 labelling urea with 13C has become popular,2 and numerous studies have shown that it is highly accurate both in the initial diagnosis of infection and in confirming eradication after therapy.3 However, the cost of producing 13C urea is much higher than that of 14C and the equipment used to measure 13C enrichment of the breath is also expensive.4
Since its first description,5 the 13C‐UBT has been extensively modified, including variations in the cut off values, test meal, dose of labelled urea, and sampling time.6 These modifications have been designed to improve accuracy, reduce the amount of the substrate used (13C‐urea), and the duration of the test. Reduction of fixed (amount of 13C used, cost of measurement equipments) and indirect (for example, duration of the test) costs can both produce a reduction in the overall cost of the UBT.
Recent studies have suggested that the dose of 13C‐urea can be reduced without affecting the performance of the device, both before and after treatment.7,8,9,10 We sought to determine the efficacy of breath tests performed with three doses of 13C urea (25, 15, and 10 mg) that are substantially lower than conventional doses of 13C urea (75–100 mg) used in breath testing.
Hence the aims of this study were: (1) to assess the diagnostic accuracy before eradication therapy of three UBTs, using 25, 15 and 10 mg of 13C‐urea, respectively; and (2) to determine diagnostic performance in the post‐eradication setting showing the highest values for sensitivity and specificity with the lowest dose of 13C‐urea.
Materials and methods
Patients, endoscopy, and histology
In the initial study, 300 consecutive dyspeptic patients referred to our unit for upper endoscopy were studied in a prospective, open, randomised, controlled trial. Dyspepsia was defined as pain or discomfort in the upper abdomen (Rome II classification).11 Patients were excluded from the study if they had been previously treated for H pylori infection or were taking proton pump inhibitors, H2 receptor antagonists, antibiotics, or bismuth compounds in the four weeks preceding the initial visit. Height and weight were noted in order to calculate body mass index (BMI, calculated as weight in kilograms divided by the square of height in metres). Patients who agreed to participate in the study were randomly assigned in 1:1:1 proportions using a computer generated randomisation chart to be tested with one of the following UBTs: (a) UBT using 25 mg of 13C‐urea (25 mg‐13C‐UBT), (b) UBT using 15 mg of 13C‐urea (15 mg‐13C‐UBT), or (c) UBT using 10 mg of 13C‐urea (10 mg‐13C‐UBT). Allocation was concealed from the investigators and patients by using a set of sealed opaque envelopes, numbered sequentially. All patients underwent upper endoscopy, and five biopsy samples were obtained during the procedure. Two biopsies were taken from the antrum and two from the corpus for histology. Biopsies were stained with haematoxylin‐eosin and Giemsa stains, and gastritis was scored using the updated Sydney system.12 The pathologist who performed the histological examination (CR) was blinded to the results of all of the other tests. One biopsy specimen was obtained from the antrum for the rapid urease test (CP‐test; Yamamouchi Pharma SpA, Corrugate, Milan, Italy). Patients were classified infected with H pylori only if both the rapid urease test and histology were positive (gold standard before treatment). These criteria have been recommended by an expert panel for use in clinical trials of H pylori.13 All patients found to be infected were offered one week triple therapy according to current European guidelines.14
To achieve our second aim, we performed a post‐treatment evaluation with the UBT that had the best results with the lowest dose of 13C in the pretreatment evaluation. In order to increase the data available, we decided to enrol at least 200 more consecutive dyspeptic patients to be tested with this UBT. For these new patients, exclusion criteria, endoscopic procedures, and criteria for infection with H pylori (gold standard before treatment) were the same as those used in the first part of the study.
Patients who agreed to undergo treatment were asked to return 4–6 weeks after the end of therapy for endoscopic follow up with the same schedule as for pretreatment. However, according to the guidelines,14 patients were considered eradicated only if both histology and the rapid urease test were negative (gold standard after treatment).
Low dose 13C‐UBT
Breath tests were carried out after an overnight fast. Citric acid (1 g) was used as the test meal and 25, 15, or 10 mg of 13C‐urea, as a water solution, was given to patients after collection of a baseline sample, obtained by blowing through a disposable plastic straw into a 20 ml container (AB Analitica, Padua, Italy). A further breath sample was collected 30 minutes later.
All breath samples were analysed by a gas isotope ratio mass spectrometer (Finnigan, Bremen, Germany), and differences over baseline (DOB) were acquired. Best cut offs were calculated using receiver operating characteristic (ROC) analysis. The doctor who performed the UBTs (AT) was blinded to the results of all of the other tests.
Statistics
This was a prospective comparison study designed to fulfil STARD (standards for reporting of diagnostic accuracy) recommendations.15 Proportions, their differences, and 95% confidence intervals (95% CI) were calculated using the method recommended by Newcombe and Altman.16 Sensitivity, specificity, likelihood ratios for a positive (LR+ve) or negative (LR−ve) test, and their 95% CI, were calculated against the defined gold standards, using methods recommended by Altman.17 As they are dependent on the prevalence of infection, positive and negative predictive values were not calculated because they are not indicative of values that might be observed in other clinical settings. LRs are presented instead. Fisher's exact test, χ2 test, χ2 test for trend, Mann‐Whitney test, and Kruskal‐Wallis test with post test were used as appropriate. ROC analysis was performed using non‐parametric methods. A two sided p<0.05 was considered to indicate statistical significance.
Spectrum effect18 for the performance of the breath tests was assessed with ROC analysis for the following factors: age (dichotomised as ⩽50 or >50 years), sex, peptic ulcer disease (dichotomised as present or absent), and atrophic gastritis of corpus (dichotomised as present or absent).
These criteria were determined before commencement of the study. Statistical analysis was performed with Intercooled STATA 8.2 (Stata Corporation, College Station, Texas, USA) and with GraphPad InStat 3.06 (GraphPad Software, San Diego, California, USA).
Ethics committee
All patients gave written informed consent. The protocol was approved by the ethics committee of S Orsola Hospital.
Results
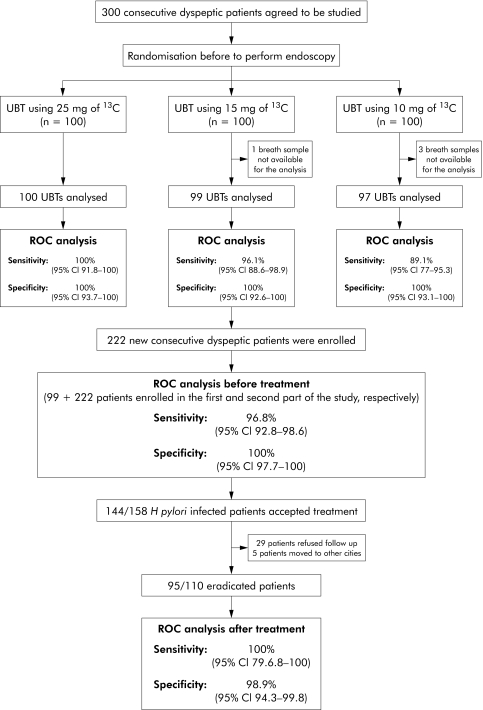
The overall design of the study is shown in fig 1. Demographic characteristics of the 300 patients enrolled for the first part of the study are shown in table 1.
Figure 1 Consort diagram of patient flow in the study. UBT, urea breath test; ROC, receiver operating characteristic; 95% CI, 95% confidence interval.
Table 1 Demographic characteristics of the patients enrolled in the first part of the study.
| Characteristic | Total (n = 300) | 25 mg‐13C‐UBT (n = 100) | 15 mg‐13C‐UBT (n = 100) | 10 mg‐13C‐UBT (n = 100) | p Value |
|---|---|---|---|---|---|
| Sex (M/F) | 119/181 | 43/57 | 41/59 | 35/65 | 0.48* |
| Age (y) (median (IQR)) | 51 (38–63) | 53 (40.75–64) | 50 (35–63) | 49 (39–64.75) | 0.29† |
| BMI (kg/m2) (median (IQR)) | 24.60 (22.04–26.8) | 24.34 (21.99–26.47) | 24.69 (21.99–26.84) | 24.14 (21.36–27.05) | 0.56† |
| Patients with PUD (%; 95% CI) | 7 (2.3; 1.1–4.7) | 4 (4.0; 1.6–9.8) | 1 (1.0; 0.2–5.4) | 2 (2.0; 0.6– 7.0) | 0.35* |
| H pylori infected patients (%; 95% CI) | 142 (47.3; 41.8–53) | 48 (48; 38.5–57.7) | 51 (51; 41.3–60.6) | 43 (43; 33.7–52.8) | 0.51† |
IQR, interquartile range; 95% CI, 95% confidence interval; BMI, body mass index; PUD, peptic ulcer disease; UBT, urea breath test.
*χ2 test for difference among the three randomised groups; †Kruskal‐Wallis test for differences among the three randomised groups.
There was no significant difference among the three groups regarding sex, age, BMI, prevalence of peptic ulcer disease, or H pylori infection. The breath samples of three (one male H pylori positive; one male and one female H pylori negative) and one (female H pylori negative) patients, tested with 10 and 15 mg of 13C‐urea, respectively, were not available for the investigation, and therefore not included in the analysis.
Performance of the low dose UBTs before treatment
Sensitivities, specificities, and LRs for a positive and negative test according to ROC analysis are reported in table 2.
Table 2 Diagnostic accuracy of the three low dose urea breath tests (UBTs) according to receiver operating characteristic analysis.
| DOB best cut offs | TP | FN | TN | FP | Sensitivity (95% CI) | Specificity (95% CI) | LR+ve (95% CI) | LR−ve (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| 25 mg‐13C‐UBT | 4.40–6.26 | 43 | 0 | 57 | 0 | 100% (91.8–100) | 100% (93.7–100) | – | – |
| 15 mg‐13C‐UBT | 4.3–6.19 | 49 | 2 | 48 | 0 | 96.1% (86.8–98.9) | 100% (92.6–100) | ∞ (12.96–∞) | 0.039 (∞–∞) |
| 10 mg‐13C‐UBT | 1.67–2.62 | 41 | 5 | 52 | 0 | 89.1% (77–95.3) | 100% (93.1–100) | ∞ (12.94–∞) | 0.109 (∞–∞) |
DOB, difference over baseline; TP, true positive; FN, false negative; TN, true negative; FP, false positive; LR+ve, likelihood ratio for a positive test; LR−ve, likelihood ratio for a negative test; 95% CI, 95% confidence interval.
ROC analysis established that for the 25 mg‐13C‐UBT, a value of 4.40–6.26 DOB as the cut off would result in sensitivity and specificity values of 100%; for the 15 mg‐13C‐UBT, a value of 4.30–6.19 DOB as the cut off would result in a sensitivity of 96.1% and a specificity of 100%; and for the 10 mg‐13C‐UBT, a value of 1.67–2.62 DOB as the cut off would produce a sensitivity of 89% and a specificity of 100%. As shown in table 2, the number of false negative results increased significantly with reduction of 13C‐urea used (χ2 for trend, p = 0.01)
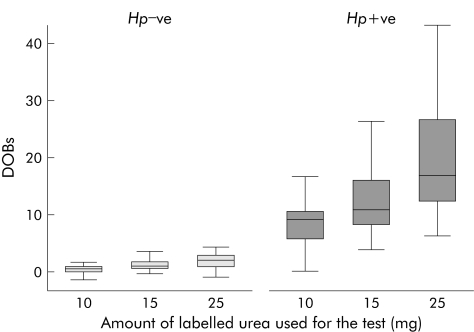
In H pylori infected patients (defined by the gold standard before treatment), the median values of DOB increased significantly with the dose of labelled urea used (fig 2). Median value for DOB of the 10 mg‐13C‐UBT was significantly lower compared with that of the 15 mg‐13C‐UBT (p<0.01) and the 25 mg‐13C‐UBT (p<0.001). Median value for DOB of the 15 mg‐13C‐UBT was also significantly lower than that for the 25 mg‐13C‐UBT (p<0.01).
Figure 2 Box and whisker plot of difference over baseline (DOB) values among the three different urea breath test groups (10, 15, and 25 mg) according to Helicobacter pylori status (Hp−ve, H pylori negative; Hp+ve, H pylori positive).
In patients not infected with H pylori there was no significant difference between median values for DOB of the 15 mg‐13C‐UBT and 25 mg‐13C‐UBT (p>0.05). However, median value for DOB of the 10 mg‐13C‐UBT was significantly lower than those of the 15 mg‐13C‐UBT (p<0.001) and 25 mg‐13C‐UBT (p<0.001).
Analysis of the spectrum effect was not performed for the factors peptic ulcer disease and atrophic gastritis of the corpus because of small cell sizes. With regard to age and sex, there were no statistical or clinical differences between the sensitivities and specificities for the 25 and 15 mg 13C‐UBT, being always above 93% (table 3).
Table 3 Spectrum effect of the urea breath tests (UBTs): age and sex.
| Factor | Performance | 25 mg‐13C‐UBT | p Value | 15 mg‐13C‐UBT | p Value | 10 mg‐13C‐UBT | p Value |
|---|---|---|---|---|---|---|---|
| Age<50 y | Sensitivity (95%CI) | 100% (83.7–100) | – | 100% (83.7–100) | 1 | 87% (66.4–97.1) | 1 |
| Age⩾50 y | 100% (84.4–100) | 96.7% (82.7–99.4) | 91.3% (71.9–98.7) | ||||
| Age<50 y | Specificity (95%CI) | 100% (83–100) | – | 100% (84.4–100) | – | 100% (86.7–100) | – |
| Age⩾50 y | 100% (90.4–100) | 100% (86.7–100) | 100% (86.2–100) | ||||
| Male | Sensitivity (95%CI) | 100% (82.2–100) | – | 100% (85–100) | – | 100% (73.4–100) | 0.14 |
| Female | 100% (85.6–100) | 100% (87.5–100) | 85.3% (68.9–95) | ||||
| Male | Specificity (95%CI) | 100% (85.6–100) | – | 100% (81.3–100) | 0.52 | 100% (83.7–100) | – |
| Female | 100% (89.3–100) | 93.3% (77.9–99) | 100% (88.3–100) |
95% CI, 95% confidence interval.
For the 10 mg‐13C‐UBT there was no significant difference between sensitivities according to age (87% (95% CI 66.4–97.1) for age <50 years v 91.3% (95% CI 71.9–97.1) for age ⩾50 years; differences between sensitivities: p = 1) or sex (100% (95% CI 73.4–100) for males v 85.3% (95% CI 68.9 to 95) for females; differences between sensitivities: p = 0.14).
Further analysis of the15‐13C‐UBT following treatment
As the test with the best performance and the lowest dose of 13C‐urea was the 15 mg‐13C‐UBT, we enrolled 222 more consecutive dyspeptic patients to be tested with this dose. Demographic characteristics are shown in table 4.
Table 4 Demographic characteristics of the patients enrolled for the second part of the study.
| Characteristic | Total (n = 321) | 15 mg‐ 13C‐UBT | p Value | |
|---|---|---|---|---|
| Patients enrolled in the first part of the study (n = 99‡) | Patients enrolled in the second part of the study (n = 222) | |||
| Sex (M/F) | 146/175 | 41/58 | 105/117 | 0.33* |
| Age (y) (median (IQR)) | 51 (38–63) | 50.5 (35–63) | 46 (37–63) | 0.90† |
| BMI (kg/m2) (median (IQR)) | 24.50 (21.40–26.8) | 24.61 (21.96–26.30) | 24.46 (21.70–26.31) | 0.88† |
| Patients with PUD (%; 95% CI) | 6 (1.9; 0.9–4.0) | 1 (1.0; 0.2–5.4) | 5 (2.3; 1.0–5.2) | 0.67* |
| H pylori infected patients (%; 95% CI) | 158 (49.2; 43.8–54.7) | 51 (51.5; 41.8–61.1) | 107 (48.2; 41.7–54.7) | 0.71* |
IQR, interquartile range; 95% CI, 95% confidence interval; BMI, body mass index; PUD, peptic ulcer disease; UBT, urea breath test.
‡One patient was not included as the breath sample before treatment was not available.
*χ2 test for differences between the two groups; †Mann‐Whitney test for differences between the two groups.
There was no significant differences with regard to sex, age, BMI, prevalence of peptic ulcer disease, or H pylori infection between these new patients and those randomised to be tested with the 15 mg‐13C‐UBT in the first part of the study.
ROC analysis was performed again using all of the 321 patients tested with the 15 mg‐13C‐UBT (99+222 patients enrolled in the first and second parts of the study, respectively), and the results are shown in table 4. A cut off value greater than 4.5 would result in a sensitivity of 96.8% and a specificity of 100%.
One hundred and forty four of 158 H pylori infected patients agreed to be treated; 110 patients were followed up as 29 patients refused to perform a follow up breath test and five patients moved to other cities. Ninety five of 110 patients were eradicated (eradication rate 86.4% (95% CI 78.7–91.6)). ROC analysis in these patients established that a cut off value of 3.4–5.2 would result in a sensitivity of 100% and a specificity of 98.9% (table 5).
Table 5 Diagnostic accuracy of the 15 mg‐13C‐ urea breath test (UBT) according to receiver operating characteristic analysis.
| DOB best cut offs | TP | FN | TN | FP | Sensitivity (95% CI) | Specificity (95% CI) | LR+ve (95% CI) | LR−ve (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| 15 mg 13C‐UBT | |||||||||
| Before treatment (n = 321) | >4.5 | 153 | 5 | 163 | 0 | 96.8% (92.8–100) | 100% (97.7–100) | ∞ (42.05–∞) | 0.032 (∞–∞) |
| After treatment (n = 110) | 3.40–5.2 | 15 | 0 | 94 | 1 | 100% (79.6–100) | 98.9% (94.3–99.8) | 95 (∞–∞) | 0.000 (0.0–∞) |
DOB, difference over baseline; TP, true positive; FN, false negative; TN, true negative; FP, false positive; LR+ve, likelihood ratio for a positive test; LR−ve, likelihood ratio for a negative test; 95% CI, 95% confidence interval.
As in the first part of the study, analysis of the spectrum effect was not performed for the factors peptic ulcer disease and atrophic gastritis of corpus because of small cell sizes. For the remaining factors (sex and age), there were no clinical and/or statistical differences in sensitivity or specificity, being always more than 96% before or after treatment (table 6).
Table 6 Spectrum effect of the 15 mg‐13C‐urea breath test: age and sex.
| Sensitivity (95% CI) | p Value | Specificity (95%CI) | p Value | |
|---|---|---|---|---|
| Before treatment (n = 321) | ||||
| Age <50 y | 100% (93.8–100) | 0.07 | 100% (95.2–100) | – |
| Age ⩾50 y | 95% (88.7–98.3) | 100% (95.8–100) | ||
| Male | 97.4 (90.8–99.6) | 1 | 100% (94.8–100) | – |
| Female | 96.3 (89.7–99.2) | 100 (96.1–100) | ||
| After treatment (n = 110) | ||||
| Age <50 y | 100% (48–100) | – | 100% (90.2–100) | 1 |
| Age ⩾50 y | 100% (69–100) | 98.3% (90.9–99.7) | ||
| Male | 100% (58.9–100) | – | 100% (92.2–100) | 1 |
| Female | 100% (62.9–100) | 98% (89.1–99.7) | ||
95% CI, 95% confidence interval.
Discussion
Over the years the dose of urea has been progressively reduced from the 350 mg (5 mg/kg) initially used in the study by Graham and colleagues,5 and at the present at least three different doses are used. A dose of 125 mg has been validated in the USA by Klein and colleagues19 who showed that sensitivity and specificity values as high as 100% could be reached with this dose. Logan and colleagues20 used 100 mg in their proposal of a standard diagnostic protocol and this dosage has received a great deal of attention in Europe. Furthermore, since the tests employing a standard dose of 75 mg (approximately 1 mg//kg) have proved to be as accurate as those using the higher dosage, the 75 mg dose has been increasingly adopted in several research studies.3 The 75 mg dose is included in many commercial kits for 13C‐UBT as it decreases the cost of the test.1
Various studies have also suggested the possibility of further reducing the dose of labelled urea. Bielanski and Konturek7 assessed the performance of 38 mg of 13C‐urea administered as a solid capsule, reporting a sensitivity of 97% and a specificity of 95%. Three other trials involving more than 300 patients studied in the pretreatment setting indicated that a dose of 50 mg of 13C‐urea, dispensed as a liquid solution or as a solid capsule, had sensitivity and specificity values greater than 95%.8,9,10 One of these studies also evaluated the performance of 50 mg in the post‐treatment setting, reporting sensitivity and specificity values of 100%.10
In this study, we first evaluated the sensitivity and specificity of three breath tests, using 25, 15, and 10 mg of 13C‐urea, respectively, before eradication of H pylori. For the 25 and 15 mg doses, ROC analysis showed sensitivity and specificity values greater than 95%. For the 10 mg dose, specificity was excellent but sensitivity was less than 90%. Although the sample size was not very high (100 patients randomly evaluated for each UBT), the range of confidence intervals was not large, suggesting good estimation of sensitivity and specificity for each test.
As expected, median values for DOB in H pylori infected patients increased significantly with the amount of labelled urea administered. Further analysis of the 15 mg‐13C‐UBT on 321 patients analysed before treatment confirmed the estimates obtained in the first part of the study, with sensitivity and specificity values of 96.8% and 100%, respectively. The UBT using 15 mg of 13C‐urea proved to be effective in assessing eradication, with a sensitivity of 100% and a specificity of 98.6%.
We have also assessed the spectrum effect of the devices, considering different factors using ROC analysis. The spectrum effect reflects the inherent variation in test performance among population subgroups. Subgroup variation is not a bias but is clinically relevant information to be identified and reported.18 For the 25‐ and 15 mg‐13C‐UBTs performed before treatment as well as for the 15 mg‐13C‐UBT after treatment, estimates of sensitivity and specificity within the strata of age and sex were not clinically or statistically different from those in the source population. For the 10 mg 13C‐UBT it was found that sensitivity for patients aged ⩾50 years was higher than that found in patients aged less than 50 years (91.3 v 87%) and also higher than that found in the overall population (91.3% v 89.1%). It was also found that sensitivity for males was higher than that reported for females (100% v 85.3%) and also higher than that found in the overall population (100% v 89.1%). These differences were not statistically significant but might be important in clinical practice if this dose were routinely used in breath testing. We are unable to explain the biological reasons for these differences.
The principal advantages of this study were that it was prospective and designed to fulfil both the STARD criteria15 and guidelines for studies on testing for H pylori.13 However, there was a potential drawback—namely, the low frequency of patients with atrophic gastritis of the corpus, which makes it impossible to evaluate the performance of the device in this subset of patients. It is well known that detection of infection with 13C‐UBT in patients with atrophic gastritis of the corpus is often unsatisfactory,21,22 and it might be argued that it is very likely that the same happens when low doses of labelled urea are used. European and US guidelines for the management of dyspepsia recommend non‐invasive testing followed by treatment for H pylori in infected individuals.23,24 This strategy has been shown to be effective and reduces the costs associated with managing dyspepsia.25 For this reason the need for an inexpensive non‐invasive test with good accuracy is increasing. In this study we found that with a UBT of 1/6 (on average) of the dose of labelled urea typically used in commercial kits, we were able to obtain good results in terms of accuracy in both the pre and post‐treatment setting. Although a proper economic analysis is beyond the aims of this study, it suggests that a low cost low dose test may be possible, making the breath test more accessible in developing countries.
In conclusion, we found that UBTs using 25 or 15 mg of 13C‐urea were both accurate in the diagnosis of H pylori infection in untreated patients. We also found that the UBT using 15 mg of 13C‐urea was accurate in the follow up of infected patients after treatment.
Abbreviations
UBT - urea breath test
DOB - difference over baseline
STARD - standards for reporting of diagnostic accuracy
LR+ve - LR−ve, likelihood ratio for a positive or negative test
ROC - receiver operating characteristic
BMI - body mass index
IQR - interquartile range
Footnotes
Conflict of interest: None declared.
References
- 1.Savarino V, Vigneri S, Celle G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut 199945I18–I22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braden B, Caspary W F. Detection of Helicobacter pylori infection: when to perform which test? Ann Med 20013391–97. [DOI] [PubMed] [Google Scholar]
- 3.Vaira D, Vakil N. Blood, urine, stool, breath, money and Helicobacter pylori. Gut 200148287–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parente F, Bianchi Porro G. The 13C‐urea breath test for non‐invasive diagnosis of Helicobacter pylori infection: which procedure and which measuring equipment? Eur J Gastroenterol Hepatol 200113803–806. [DOI] [PubMed] [Google Scholar]
- 5.Graham D Y, Klein P D, Evans D J., Jret al Campylobacter pylori detected non‐invasively by the 13C‐urea breath test. Lancet 1987I1174–1177. [DOI] [PubMed] [Google Scholar]
- 6.Basset C, Holton J, Gatta L.et al Helicobacter pylori infection: anything new should we know? Aliment Pharmacol Ther 2004201–10. [DOI] [PubMed] [Google Scholar]
- 7.Bielanski W, Konturek S J. New approach to 13C‐urea breath test: capsule‐based modification with low‐dose of 13C‐urea in the diagnosis of Helicobacter pylori infection. J Physiol Pharmacol 199647545–553. [PubMed] [Google Scholar]
- 8.Wong W M, Wong B C, Li T M.et al Twenty‐minute 50 mg 13C‐urea breath test without test meal for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther 2001151499–1504. [DOI] [PubMed] [Google Scholar]
- 9.Liao C C, Lee C L, Chiang T C.et al The 13C‐urea breath test to detect Helicobacter pylori infection: a validated simple methodology with 50 mg 13C‐urea. Aliment Pharmacol Ther 200216787–792. [DOI] [PubMed] [Google Scholar]
- 10.Gatta L, Vakil N, Ricci C.et al A rapid, low‐dose, 13C‐urea tablet for the detection of Helicobacter pylori infection before and after treatment. Aliment Pharmacol Ther 200317793–798. [DOI] [PubMed] [Google Scholar]
- 11.Talley N J, Stanghellini V, Heading R C.et al Functional gastroduodenal disorders. Gut 199945II37–II42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon M F, Genta R M, Yardley J H.et al Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 1996201161–1181. [DOI] [PubMed] [Google Scholar]
- 13.Working party of the European Helicobacter pylori Study Group Technical annex: tests used to assess Helicobacter pylori infection. Guidelines for clinical trials in Helicobacter infection. Gut 199741S10–S18. [PubMed] [Google Scholar]
- 14.Malfertheiner P, Megraud F, O'Morain C.et al Current concepts in the management of H pylori infection‐The Maastricht 2‐2000 Consensus Report. Aliment Pharmacol Ther 200216167–180. [DOI] [PubMed] [Google Scholar]
- 15.Bossuyt P M, Reitsma J B, Bruns D E.et al Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative, BMJ 200332641–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcombe R G, Altman D G. Proportion and their differences. In: Altman DG, Machin D, Trevor NB, eds. Statistics with confidences, 2nd edn. London: BMJ Books, 200045–56.
- 17.Altman D G. Diagnostic tests. In: Altman DG, Machin D, Trevor NB, eds. Statistics with confidences, 2nd edn. London: BMJ Books, 2000105–119.
- 18.Mulherin S A, Miller W C. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Ann Intern Med 2002137598–602. [DOI] [PubMed] [Google Scholar]
- 19.Klein P D, Malaty H M, Martin R F.et al Noninvasive detection of Helicobacter pylori infection in clinical practice: the 13C urea breath test. Am J Gastroenterol 199691690–694. [PubMed] [Google Scholar]
- 20.Logan R P H, Dill S, Bauer F E.et al The European 13C‐urea breath test for detection of Helicobacter pylori: Eur J Gastroenterol Hepatol 19913915–921. [Google Scholar]
- 21.Kokkola A, Rautelin P, Puolakkainen P.et al Diagnosis of Helicobacter pylori infection in patients with atrophic gastritis: comparison of histology, 13C‐urea breath test, and serology. Scand J Gastroenterol 200035138–141. [DOI] [PubMed] [Google Scholar]
- 22.Lahmer E, Vaira D, Figura N.et al Role of noninvasive tests (13C‐urea breath test and stool antigen test) as additional tools in diagnosis of Helicobacter pylori infection in patients with atrophic body gastritis. Helicobacter 20049436–442. [DOI] [PubMed] [Google Scholar]
- 23.Talley N, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol 20051002324–2337. [DOI] [PubMed] [Google Scholar]
- 24. Dyspepsia: Managing dyspepsia in adults in primary care, NICE guidelines http://www.nice.org.uk/page.aspx?o = 218514 (last accessed 10 January 2006)
- 25.Lassen A T, Hallas J, Schaffalitzky de Muckadell O B. Helicobacter pylori test and eradicate versus prompt endoscopy for management of dyspeptic patients: 6.7 year follow up of a randomised trial. Gut 2004531758–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]