Abstract
Background and aim
One proposed mechanism whereby hepatic encephalopathy (HE) leads to loss of brain function is dysregulated synthesis of neurosteroids. Mitochondrial synthesis of neurosteroids is regulated by “peripheral benzodiazepine binding sites” (PBBS). Expressed in the brain by activated glial cells, PBBS can be measured in vivo by the specific ligand [11C](R)‐PK11195 and positron emission tomography (PET). Recently, it has been suggested that PBBS expressing glial cells may play a role in the general inflammatory responses seen in HE. Therefore, we measured PBBS in vivo in the brains of patients with minimal HE using [11C](R)‐PK11195 PET.
Methods
Five patients with minimal HE and biopsy proven cirrhosis of differing aetiology were assessed with a neuropsychometric battery. Regional expression of PBBS in the brain was detected by [11C](R)‐PK11195 PET.
Results
All patients showed brain regions with increased [11C](R)‐PK11195 binding. Significant increases in glial [11C](R)‐PK11195 binding were found bilaterally in the pallidum, right putamen, and right dorsolateral prefrontal region. The patient with the most severe cognitive impairment had the highest increases in regional [11C](R)‐PK11195 binding.
Conclusion
HE is associated with increased cerebral binding of [11C](R)‐PK11195 in vivo, reflecting increased expression of PBBS by glial cells. This supports earlier experimental evidence in rodent models of liver failure, suggesting that an altered glial cell state, as evidenced by the increase in cerebral PBBS, might be causally related to impaired brain functioning in HE.
Keywords: hepatic encephalopathy, glia, peripheral benzodiazepine binding sites, PK11195, positron emission tomography
Chronic hepatic encephalopathy (HE) is a characteristically reversible neuropsychiatric disorder without overt structural brain damage, which occurs on a background of chronic liver disease. Minimal HE is characterised by a subtle impairment of cognitive function, leading to deficits in attention and impaired reaction times in driving or operating machinery, whereas confusion, stupor, and coma are the manifestations of overt disease.1
One change observed consistently in the brain of mice with induced hyperammonaemia2 or in post‐mortem brain tissues of patients with portal‐systemic encephalopathy3 is a significant increase in expression of “peripheral benzodiazepine binding sites” (PBBS). Originally discovered as additional binding sites for certain benzodiazepines, such as diazepam, PBBS are a heteromeric complex that is largely, although not exclusively, localised in the outer mitochondrial membrane.4 PBBS were however found to be structurally and functionally unrelated to the central benzodiazepine receptor, which is associated with gamma‐aminobutyric acid (GABA) regulated channels. Subsequently, the isoquinoline PK11195, a specific high affinity ligand, has been used to pharmacologically characterise PBBS.5,6 Therefore, we will refer to PBBS either as “PK11195 binding (sites)” or “(R)‐PK11195 binding”, where the data are obtained using the R enantiomer, for which a slightly higher affinity for PBBS has been reported.7
Unlike in peripheral organs and cell types, normal brain parenchyma has only minimal binding of PK11195. However, as soon as brain disease gives rise to active tissue pathology, de novo expression of PK11195 binding sites is observed. At least three cellular sources have been discussed: (i) astrocytes,3,8 (ii) invading blood borne cells of mononuclear macrophage lineage, if the blood‐brain barrier is disrupted,9 or (iii) activated microglia, the intrinsic population of the normally dormant brain macrophages, if the blood‐brain barrier has remained intact.10,11,12 A role for PBBS in the pathogenesis of HE has been hypothesised, based on their regulatory effect on mitochondrial cholesterol transport and thus the altered synthesis of neurosteroids, which may be responsible for the neuronal inhibition observed in HE.6,13
Additionally, recent evidence points to systemic inflammatory immune responses in minimal HE as important mediators or at least enhancers of ammonia toxicity.14,15 The fact that glial cells, notably microglia, the dominant immune effector cell of the brain, can rapidly express PBBS de novo in response to even the most subtle pathological stimuli is thus of added significance.10,11,12,15 The exclusively non‐neuronal PBBS directly regulate many immune functions, such as release of cytokines and reactive oxygen intermediates, and are strongly regulated even in pathological conditions that are not characterised by overt tissue damage or inflammatory signs, such as recruitment of blood borne immune cells.11 There is therefore the intriguing possibility that PBBS expressing glial cells are local participants in the general inflammatory responses seen in HE. Labelled with carbon‐11, (R)‐PK11195 is a ligand for measurement of PK11195 binding for PBBS in vivo by positron emission tomography (PET). To address the fundamental question of whether there is a change in PBBS expression in the brains of patients with minimal HE, we report measurement of cerebral [11C](R)‐PK11195 binding in five patients with HE and biopsy proven cirrhosis of differing aetiology. We relate the regional pattern of increased [11C](R)‐PK11195 binding to the underlying severity of liver disease and psychometric test analysis.
Subjects and methods
Subjects
Five patients with HE and biopsy proven cirrhosis (mean (SD) age 60.4 (15.4) years) were recruited to the study. The patient cohort comprised two patients with alcohol related cirrhosis, one with autoimmune chronic active hepatitis/cirrhosis, one with post‐viral cirrhosis secondary to hepatitis C virus (HCV) infection, and one with a dual HCV and alcohol related cirrhosis aetiology. The patient with autoimmune chronic active hepatitis/cirrhosis (table 1, patient 1) had a surgically placed portocaval shunt of more than 20 years' duration and a history of well documented minimal HE. This patient was taking maintenance oral prednisolone at 4–5 mg daily with intermittent courses of azathioprine over this 20 year period. All patients were clinically stable at the time of the study and had been abstinent from alcohol for a minimum of six months prior to the PET examination. None was receiving psychoactive medication and none was thiamine deficient. Patients with a prior history of alcohol excess had started thiamine supplementation at the time of first diagnosis.
Table 1 Summary of the clinical characteristics for each patient with hepatic encephalopathy.
| Age (y) | Sex | Aetiology | Disease duration (y) | Child grade | Bilirubin (μmol/l) | Albumin (g/l) | NH3 (μmol/l) | Varices | Surgical shunt (y) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | ||||||||||
| 1 | 57 | F | Autoimmune | 22 | B | 72 | 34 | 91 | − | 20* |
| 2 | 45 | F | Alcohol | 4 | A | 8 | 41 | 286 | − | 2† |
| 3 | 78 | F | Hepatitis C | 14 | A | 22 | 37 | 75 | + | − |
| 4 | 47 | M | Hepatitis C/alcohol | 3 | A | 16 | 44 | 181 | − | − |
| 5 | 75 | M | Alcohol | 10 | C | 31 | 24 | 57 | + | − |
| Control group | ||||||||||
| n = 10 | 62.7 (12.7)‡ | 6F/4M |
*Surgical portocaval anastomosis.
†Transjugular intrahepatic portosystemic stent shunt.
‡Mean (SD).
Blood was drawn for estimation of standard biochemical and haematological parameters of liver function and for serum electrolytes. A Pugh's score and Child's grade (as modified by Pugh),16 reflecting the severity of hepatic dysfunction, were calculated for each subject (table 1). Functionally, three were Child's grade A, one Child's grade B, and one was classified as having Child's grade C disease (table 1).
A full neurological, psychometric, and electrophysiological assessment was performed on each patient. Mental state was assessed using West Haven criteria.17 Psychometric performance was assessed under standardised conditions, using a battery of four tests comprising number connection tests (NCT) A and B,18 the digit symbol subtest of the Wechsler adult intelligence scale,19 and the digit copying subscore of the Kendrick battery.20 Results are shown in table 2.
Table 2 Results of four psychometric tests for individual patients. The normal range derives from a data set obtained from a cohort of healthy age matched controls.
| Patient No | NCT A (s) | NCT B (s) | Digit symbol | Digit copying |
|---|---|---|---|---|
| 1 | 32 | 100 | 76 | 128 |
| 2 | 55 | Not completed | 50 | 154 |
| 3 | 59 | Not completed | 45 | 118 |
| 4 | 31 | 90 | 67 | 143 |
| 5 | 72 | Not completed | 19 | 80 |
| Normal range | 15–37 s | 31–81 s | 55–90 | 124–208 |
NCT, number connection tests.
Electroencephalograms (EEG) were performed using conventionally placed electrodes and mean cycle frequency was obtained. Capillary ammonia concentrations from a finger prick were measured from the pulp of the index finger, using a blood ammonia checker II (Kyoto Daiichi Kagaku Co, Ltd, Kyoto, Japan). All patients underwent baseline T1 weighted volumetric magnetic resonance imaging (MRI) on the same day of the PET study with [11C](R)‐PK11195 to rule out significant structural brain pathology. Additionally, patients were characterised within one week of the PET study by in vivo cerebral 1H magnetic resonance spectroscopy (MRS) and 31P MRS. Methods of data acquisition and analysis have previously been reported.21,22,23 Exclusion criteria for PET study were the presence of focal brain lesions or severe atrophy detected by MRI, presence of a past history of neurological or psychiatric disorders, or episodes of acute HE or gastrointestinal bleeding in the previous three months.
The cohort of control subjects enrolled in the PET study comprised 10 aged matched healthy volunteers (mean (SD) age 62.7 (12.7) years; range 41–80 years). Also, except for the thalamus, there was no region in the brain in which a significant age related increase in baseline binding of [11C](R)‐PK11195 was observed.24 Each subject underwent an extensive clinical, neurological, and psychometric investigation and a T1 weighted volumetric MRI scan on the same day of the PET study with [11C](R)‐PK11195, to rule out systemic and neurological diseases.
The study conformed to the guidelines set out in the Declaration of Helsinki of 1975 and prior ethics approval was obtained from the Ethics Committee of the Imperial College School of Medicine, London. All subjects provided written informed consent.
PET study
The PET study was performed on a CTI/Siemens ECAT 953B PET scanner operated in three dimensional acquisition mode. [11C](R)‐PK11195 was injected as a bolus, 30 seconds after the acquisition scan started. Mean tracer dose was 360 (30) MBq with a specific activity of 37 (1) GBq/mmol. Dynamic data were collected over 60 minutes as 18 temporal frames. Attenuation correction factors were determined using a 15 minute transmission scan acquired before the dynamic scan. Scatter correction was achieved using a dual energy window method.25 Data were reconstructed with a ramp filter at Nyquist cut off, producing an image resolution of 5.8 mm (full width half maximum) at the centre of the field of view. A three dimensional T1 weighted MRI scan for the purpose of co‐registration was acquired on a 1.0T Philips HPQ MRI scanner (voxel size 1×1×1.3 mm; 128 contiguous slices; repetition time (TR) 35 ms, echo time (TE) 6 ms, flip angle 35°) on the same day of the PET scan.
Regional binding of [11C](R)‐PK11195, expressed as binding potential (BP), a measure of specific binding of the tracer, was calculated using a basis function implementation of a simplified reference tissue model.26,27,28 Selection of an anatomically defined reference region may introduce errors since an a priori assumption that the chosen reference region is devoid of specific binding has to be made. This is particularly true under conditions where global changes in binding may occur, such as in metabolic encephalopathy. Therefore, cluster analysis27,29 was employed as an alternative approach for extraction of a normal ligand kinetic to serve as the reference input function, as previously described.28,30 For each patient the appropriate ligand kinetic was selected by χ2 test (p<0.05) comparison against a population input kinetic previously created from the ligand kinetics of the normal cortex in healthy control patients.28,30
For calculation of regional mean BP values, the following anatomical volumes of interest were defined on the individuals' volumetric MRI prior to spatial co‐registration with the regional [11C](R)‐PK11195 binding potential map31: right and left temporal gyrus (superior, inferior, and middle), insula, inferior parietal lobule, anterior and posterior cingulate gyrus, dorsolateral prefrontal cortex, as well as right and left pallidum, putamen, and thalamus. The cerebellum, seen within the restricted field of view (10.65 cm) of the PET camera only to a varying extent, was excluded from formal analysis.
Statistical analysis
The Student's t test was used to determine the significance of the differences in regional mean [11C](R)‐PK11195 binding between normal control subjects and patients. Z values were calculated to test for significant increases in [11C](R)‐PK11195 binding in the brains of individual patients, compared with normal control brain. As [11C](R)‐PK11195 binding changes are unidirectional (that is, only increases) a z value of 1.6 in a one tailed z test represents a level of significance of p<0.05.32 Potential error due to multiple comparisons was examined using the Hochberg correction and p‐plot graphical method for estimation of the number of “true” null hypotheses.33 Analysis of the set of p values revealed a widespread pattern of statistically significant changes. The estimated number of “true” null hypotheses was <3. This being the theoretical default minimum of “true” null hypotheses, it indicates that correction for multiple comparisons would not be meaningful for this data set.
Results
All patients were clinically normal on examination but showed slowing of the alpha rhythm in the EEG below the reference range of 8.9 cycles per seconds and/or impaired performance in at least two of the four psychometric tests compared with the reference range for normal healthy volunteers. None had structural brain abnormalities on T1 weighted MRI, apart from hyperintensity in the basal ganglia in three of the five patients (patient Nos 1, 2, and 4), a finding common in chronic liver disease (fig 1).34 All patients had cerebral metabolite abnormalities on 1H MRS (increased glutamine/glutamate and reduced choline resonances) and on 31P MRS (reduced phosphomonoester, phosphodiester, and β‐nucleoside triphosphate resonances), which have previously been described in patients with hepatic encephalopathy.21,22,23
Figure 1 Magnetic resonance imaging (MRI) and [11C](R)‐PK11195 positron emission tomography (PET) images of patients with hepatic encephalopathy. All images follow the radiological convention: the left side of the image corresponds to the subject's right side. (A, B) Transverse and coronal orientation of T1 weighted MRI images (first and third) and co‐registered [11C](R)‐PK11195 images overlaid on the MRI (second and fourth) of patient No 1, showing MRI hyperintensity in the basal ganglia and normal [11C](R)‐PK11195 binding (only constitutive binding in the thalamus). (C, D) [11C](R)‐PK11195 PET images of patient No 2, (E, F) patient No 3, and (G, H) patient No 4. Increases in [11C](R)‐PK11195 binding sites were localised in the frontal lobe, particularly in the anterior cingulate cortex (F, G) and in the white matter along fibre tracts such as in the corpus callosum (C) or following projections connecting the frontal cortex with subcortical structures (E). (H) A sagittal view of patient No 4, demonstrating the spatial pattern of increase in [11C](R)‐PK11195 binding involving the basal ganglia and frontal lobe (arrows). In patient No 2 (D), MRI hyperintensity in the basal ganglia overlapped with the regions with an increase in [11C](R)‐PK11195. cc, corpus callosum; ac, anterior cingulate; wmt, white matter tract. The colour scale is calibrated for binding potential values from 0 to 1. White indicates values >1.
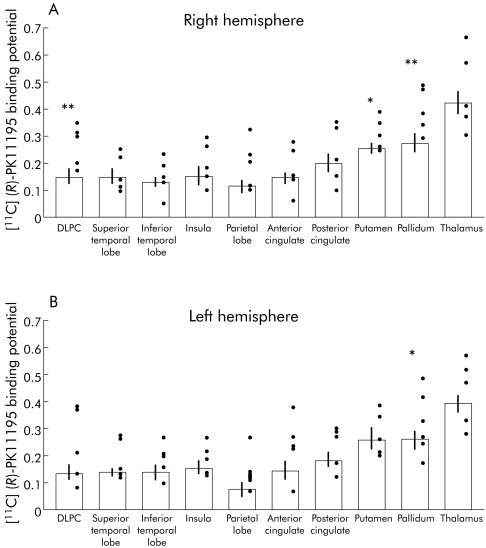
With regard to the PET study, group analysis of the patients revealed increases in [11C](R)‐PK11195 binding bilaterally in the pallidum (right BP: HE 0.39 (0.08), controls 0.27 (0.07), p<0.01; left: HE 0.35 (0.10), controls 0.25 (0.07), p<0.05), in the right putamen (BP: HE 0.31 (0.06), controls 0.25 (0.04), p<0.05) and the right dorsolateral prefrontal region (BP: HE 0.27 (0.08), controls 0.15 (0.06), p<0.01) (fig 2). Individual analysis demonstrated marked heterogeneity in the regional pattern of increased [11C](R)‐PK11195 binding (fig 1, 3). Three patients (patient Nos 2, 3, and 5) showed widespread increase in [11C](R)‐PK11195 signal, which was particularly pronounced in the pallidum and the frontal cortex (fig 3).
Figure 2 Mean [11C](R)‐PK11195 binding potential values for all volumes of interest drawn for the right (A) and left (B) hemispheres. Mean values for control subjects are expressed as empty histograms with the standard deviation bar on the top. Black dots represent individual patient values. DLPC, dorsolateral prefrontal cortex. *p<0.05, **p<0.01.
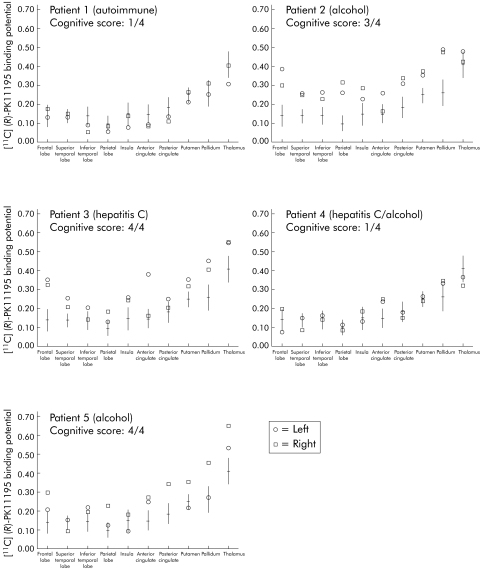
Figure 3 Overview of individual patient mean [11C](R)‐PK11195 binding potential and standard deviation for each region of interest. The aetiology of each patient's disease is indicated in parentheses. The degree of cognitive impairment (cognitive score) is expressed as the number of impaired psychometric tests out of the 4 tested in the battery. Patient Nos 2, 3, and 5 showed a widespread increase in mean [11C](R)‐PK11195 binding potential (expression of increased peripheral benzodiazepine binding sites) throughout the cortical and subcortical regions, with predominance in frontal areas and basal ganglia. These patients (Nos 2, 3 and 5) showed significant cognitive impairment.
The observation appeared to be unrelated to the aetiology of the disease or other clinical characteristics, apart from the severity of cognitive decline. The three patients (patient Nos 2, 3 and 5) with significantly increased [11C](R)‐PK11195 binding were those impaired in the majority of the psychometric tests administered. In contrast, the two patients who were impaired in one test (that is, NCT B) had either normal regional mean [11C](R)‐PK11195 binding in all regions (patient No 1) or increased [11C](R)‐PK11195 binding only in the anterior cingulate cortex (patient No 4) (fig 3).
Discussion
Increased PK11195 binding has been shown in experimental animal studies2 and in post‐mortem brain samples from patients with HE.35 Our study is the first attempt to localise and quantify expression of PK11195 binding sites in vivo in patients with HE.
Regional pattern
Although increases in [11C](R)‐PK11195 binding were widespread in some HE patients, group analysis suggested the presence of a regional pattern, whereby the highest level of [11C](R)‐PK11195 binding was found in the basal ganglia, dorsolateral prefrontal regions, and anterior cingulate gyrus. Involvement of the pallidum in liver disease is widely known and T1 weighted MRI signal hyperintensities are well documented and have been ascribed to manganese deposition.36 Abnormal MRI signal intensities in the pallidum correlate with poor motor performance in tasks involving speed, while cognitive decline is associated with measures of cortical atrophy.37 In our study, three out of the five patients showed T1 weighted MRI signal hyperintensities in the pallidum and only one matched with a significant increase in [11C](R)‐PK11195 binding, indicating that increases in PBBS appear to be unrelated to MRI signal alteration.
The regionality detected by [11C](R)‐PK11195 PET is, instead, in keeping with other neuroimaging observations, such as decreased glucose metabolism and cerebral blood flow in the frontal‐limbic‐basal ganglia circuits that correlate with neuropsychological deficits.38,39 It is thus possible that widespread increased [11C](R)‐PK11195 binding with a superimposed regional pattern involving the basal ganglia and frontal regions is one pathological correlate of the cognitive impairment found in HE.
Clinical correlations
In our study, neither duration or severity of liver disease, expressed as Child‐Pugh score, nor blood ammonia levels correlated with [11C](R)‐PK11195 binding. However, we found instead that patients with the highest [11C](R)‐PK11195 binding were the cognitively most impaired on psychometric testing.
Pathophysiological relevance of increased PK11195 binding
The apparent link between upregulation of [11C](R)‐PK11195 binding and cognitive decline, the key symptom for minimal HE, supports the hypothesis that PBBS in the brain could indeed participate in the pathogenic mechanism of HE. This further implies that glial cell dysfunction is pivotal in this process, as the binding sites for PK11195 binding are exclusively non‐neuronal and, given an intact blood‐brain barrier, have been shown to be expressed only by activated or reactive glia (microglia and astrocytes). One theory, the “neurosteroid hypothesis”, suggests that by virtue of the regulatory influence of PBBS on the transport of cholesterol across the mitochondrial membrane,40,41 higher expression of PBBS increased the synthesis of neurosteroids, such as pregnenolone. Neurosteroids in turn, are potent positive allosteric modulators of GABA and/or glutamate neurotransmission.3 Several experimental studies have confirmed that conditions inducing hyperammonaemia result in an increase in PK11195 binding and brain pregnenolone synthesis and are concomitant with the clinical presentation of HE.42
Although our study does not resolve the issue of the relative contribution of the different glial subtypes to the increased [11C](R)‐PK11195 signal in vivo, binding sites of this ligand have been shown to be expressed only by glial cells in a reactive or dysregulated state. There is now a substantial body of evidence that the responses in a wide range of disease conditions of both astrocytes and microglia are accompanied by profound functional changes, such as expression of cytokines, their respective receptors, and other molecules characteristic of an inflammatory tissue response, such as reactive oxygen intermediates. These changes are often referred to as “glial inflammation or neuroinflammation” as they largely occur without any recruitment of blood borne immune cells seen in classical inflammation.43 Microglia and the regulatory influence that astrocytes exert over them are increasingly recognised as the cellular link between the peripheral immune system and the local immune system in the brain.43 Long overlooked, microglial responses to a large variety of pathological stimuli (including subtle toxic ones) are now known to set in early and rapidly.43 They are regularly found well before overt pathological changes are noted, such as in cortical spreading depression, a condition that has been referred to as “a pathology without pathology” and even in behavioural changes, such as in repetitive movements, that may be otherwise viewed as physiological.44,45
In conclusion, in the brains of patients with HE that we studied, abnormally high [11C](R)‐PK11195 binding in the frontal lobe was detected in those patients with the poorest performance on psychometric testing. This was independent of underlying aetiology of liver disease and most likely reflects glial activation with concomitant upregulation of PBBS. The observation is in keeping with the hypothesis that PBBS may have a key role in the pathogenesis of hepatic encephalopathy via neurosteroid synthesis. Given the fact that activated, but not resting, microglia (which represent the brain's intrinsic immune effector cells) are an important source of de novo (R)‐PK11195 binding, our observation also supports more recent hypotheses that in HE, like in many other primarily neurological conditions, a local inflammatory mechanism within the brain itself may act synergistically with the toxic effects of a noxious agent, which in the case of HE would primarily be ammonia.14,15
Acknowledgements
This study was supported by the UK Department of Health, the British Medical Research Council (G9900178), Deutsche Forschungsgemeinschaft, and Philips Medical Systems Inc (Cleveland, Ohio, USA). Dr Adrian Lim (Consultant Radiologist, Hammersmith Hospitals NHS Trust) reported the images of the brain. Dr Camilla Buckley and Dr Shahid Khan, Gastroenterology Unit, Hammersmith Hospital, London, helped with patient recruitment. We also thank Professor Graeme M Bydder (Magnetic Resonance Unit), Imperial College London, for his valuable insight and useful discussions on study design. AC was supported by a Fellowship from the European Community (BMH4/CT98/5100) in the Training and Mobility of Researchers Programme in Biomedicine. DF was supported by a fellowship from the European Association for the Study of the Liver and the St Mary's Hospital Trustees, London.
Abbreviations
HE - hepatic encephalopathy
PBBS - peripheral benzodiazepine binding sites
PET - positron emission tomography
GABA - gamma‐aminobutyric acid
HCV - hepatitis C virus
NCT - number connection tests
EEG - electroencephalogram
MRI - magnetic resonance imaging
MRS - magnetic resonance spectroscopy
BP - binding potential
Footnotes
Conflict of interest: None declared.
References
- 1.Schenker S, Brady C E. Pathogenesis of hepatic encephalopathy. In: Conn HO, Bircher , J , eds. Hepatic encephalopathy: syndromes and therapies. Bloomington: Medi‐Ed Press, 199443–61.
- 2.Desjardins P, Bandeira P, Rao V L R.et al Portacaval anastomosis causes selective alterations of peripheral‐type benzodiazepine receptor expression in rat brain and peripheral tissues. Neurochem Int 199935293–299. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth R F. The astrocytic (“peripheral‐type”) benzodiazepine receptor: role in the pathogenesis of portal systemic encephalopathy. Neurochem Int 200036411–416. [DOI] [PubMed] [Google Scholar]
- 4.Anholt R R, Pedersen P L, DeSouza E B.et al The peripheral‐type benzodiazepine receptor. Localisation to the mitochondrial outer membrane. J Biol Chem 1986261776–783. [PubMed] [Google Scholar]
- 5.Benavides J, Cornu P, Dennis T.et al Imaging of human brain lesions with an omega 3 site radioligand. Ann Neurol 198824708–712. [DOI] [PubMed] [Google Scholar]
- 6.Gavish M, Bachman I, Shoukrun R.et al Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev 199951629–650. [PubMed] [Google Scholar]
- 7.Shah F, Hume S P, Pike V W.et al Synthesis of the enantiomers of [N‐methyl‐11C]PK 11195 and comparison of their behaviours as radioligands for PK binding sites in rats. Nucl Med Biol 199421573–581. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann A C, Guilarte T R. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem 2000741694–1704. [DOI] [PubMed] [Google Scholar]
- 9.Myers R, Manjil L G, Cullen B M.et al Macrophage and astrocytes populations in relation to [3H] PK11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab 199111314–322. [DOI] [PubMed] [Google Scholar]
- 10.Banati R B, Myers R, Kreutzberg G W. PK (‘peripheral benzodiazepine')‐binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H] PK11195 binding to activated microglia. J Neurocytol 19972677–82. [DOI] [PubMed] [Google Scholar]
- 11.Banati R B. Visualising microglial activation in vivo. Glia 200240206–217. [DOI] [PubMed] [Google Scholar]
- 12.Mankowski J L, Queen S E, Tarwater P J.et al Elevated peripheral benzodiazepine receptor expression in simian immunodeficiency virus encephalitis. J Neurovirol 2003994–100. [DOI] [PubMed] [Google Scholar]
- 13.Ahboucha S, Desjardins P, Chatauret N.et al Normal coupling of brain benzodiazepine and neurosteroid modulatory sites on the GABA‐A receptor complex in human hepatic encephalopathy. Neurochem Int 200345551–556. [DOI] [PubMed] [Google Scholar]
- 14.Shawcross D L, Davies N A, Williams R.et al Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 200440247–254. [DOI] [PubMed] [Google Scholar]
- 15.Blei A T. Infection, inflammation and hepatic encephalopathy, synergism redefined. J Hepatol 200440327–330. [DOI] [PubMed] [Google Scholar]
- 16.Pugh R N H, Murray‐Lyon I M, Dawson J L.et al Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 197260646–649. [DOI] [PubMed] [Google Scholar]
- 17.Conn H O, Leevy C M, Vlahcevic Z R.et al Comparison of lactulose and neomycin in the treatment of chronic portal‐systemic encephalopathy: a double blind trial. Gastroenterology 197772573–583. [PubMed] [Google Scholar]
- 18.Conn H O. Trail making and number‐connection tests in the assessment of mental state in portal systemic encephalopathy. Am J Dig Dis 197722541–550. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D.Wechsler adult intelligence scale manual. New York: Psychological Corporation, 1955
- 20.Kendrick D C, Gibson A J, Moyes I G A. The revised Kendrick battery: clinical studies. Br J Soc Clin Psychol 197918329–339. [DOI] [PubMed] [Google Scholar]
- 21.Taylor‐Robinson S D, Sargentoni J, Marcus C D.et al Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metab Brain Dis 19949347–359. [DOI] [PubMed] [Google Scholar]
- 22.Taylor‐Robinson S D, Sargentoni J, Oatridge A.et al MR imaging and spectroscopy of the basal ganglia in chronic liver disease: Correlation of T1‐weighted contrast measurements with abnormalities in proton and phosphorus‐31 MR spectra. Metab Brain Dis 199611249–268. [DOI] [PubMed] [Google Scholar]
- 23.Taylor‐Robinson S D, Buckley C, Changani K K.et al Cerebral phosphorus‐31 and proton magnetic resonance spectroscopy in patients with subclinical hepatic encephalopathy. Liver 199919389–398. [DOI] [PubMed] [Google Scholar]
- 24.Cagnin A, Brooks D J, Kennedy A M.et al In‐vivo measurement of activated microglia in dementia. Lancet 2001358461–467. [DOI] [PubMed] [Google Scholar]
- 25.Grootoonk S, Spinks T J, Sashin D.et al Correction for scatter in 3D brain PET using a dual energy window method. Phys Med Biol 1996412757–2774. [DOI] [PubMed] [Google Scholar]
- 26.Lammertsma A A, Hume S P. Simplified reference tissue model for PET receptor studies. Neuroimage 19964153–158. [DOI] [PubMed] [Google Scholar]
- 27.Gunn R N, Lammerstma A A, Cunningham V J. Parametric imaging of ligand‐receptor interactions using a reference tissue model and cluster analysis. In: Carson R, Daule M, Witherspoon P, et al, eds. Quantitative functional brain imaging with positron emission tomography. San Diego: Academic Press, 1998401–406.
- 28.Banati R B, Newcombe J, Gunn R N.et al The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 20001232321–2337. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J, Haslam J, Taylor C.et al A cluster analysis approach for the characterization of dynamic PET data. In: Myers R, Cunningham V, Bailey D, et al, eds. Quantification of brain function using PET. San Diego: Academic Press, 1996301–306.
- 30.Cagnin A, Myers R, Gunn R N.et al In vivo visualization of activated glia by [11C](R)‐PK11195‐PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain 20011242014–2027. [DOI] [PubMed] [Google Scholar]
- 31.Studholme C, Hill D L G, Hawkes D J. Automated three dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimisation of voxel similarity measures. Med Phys 19972425–35. [DOI] [PubMed] [Google Scholar]
- 32.Snedecor G W, Cochran W G.Statistical methods, 7th Edn. Iowa State: University Press 1980
- 33.Turkheimer F E, Smith C B, Schmidt K. Estimation of the number of “true” null hypotheses in multivariate analysis of neuroimaging data. Neuroimage 200113920–930. [DOI] [PubMed] [Google Scholar]
- 34.Taylor‐Robinson S D, Oatridge A, Hajnal J V.et al MR Imaging of the basal ganglia in chronic liver disease: Correlation of T1‐weighted and magnetisation transfer contrast measurements with liver dysfunction and neuropsychiatric status. Metab Brain Dis 199510175–188. [DOI] [PubMed] [Google Scholar]
- 35.Lavoie J, Layrargues G P, Butterworth R F. Increased densities of peripheral‐type benzodiazepine receptors in brain autopsy samples from cirrhotic patients with hepatic encephalopathy. Hepatology 199011874–878. [DOI] [PubMed] [Google Scholar]
- 36.Spahr L, Butterworth R F, Fontaine S.et al Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 199624116–120. [DOI] [PubMed] [Google Scholar]
- 37.Kulisevsky J, Pujol J, Junque C.et al MRI pallidal hyperintensity and brain atrophy in cirrhotic patients: two different MRI patterns of clinical deterioration? Neurology 1993432570–2573. [DOI] [PubMed] [Google Scholar]
- 38.Lockwood A H, Murphy B W, Donnelly K Z.et al Positron‐emission tomographic localization of abnormalities of brain metabolism in patients with minimal hepatic encephalopathy. Hepatology 1993181061–1068. [PubMed] [Google Scholar]
- 39.Catafau A M, Kulisevsky J, Berna L.et al Relationship between cerebral perfusion in frontal‐limbic‐basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J Nucl Med 200041405–410. [PubMed] [Google Scholar]
- 40.Benavides J, Quarteronet D, Imbault F.et al Labelling of “peripheral‐type” benzodiazepine binding sites in the rat brain by using [3H]PK11195, an isoquinoline carboxamide derivative: kinetic studies and autoradiographic localization. J Neurochem 1983411744–1750. [DOI] [PubMed] [Google Scholar]
- 41.Casellas P, Galiegue S, Basile A S. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int 200240475–486. [DOI] [PubMed] [Google Scholar]
- 42.Itzhak Y, Roig‐Cantisano A, Dombro R S.et al Acute liver failure and hyperammoniemia increase peripheral‐type benzodiazepine receptor binding and pregnenolone synthesis in mouse brain. Brain Res 1995705345–348. [DOI] [PubMed] [Google Scholar]
- 43.Graeber M B. Genetics of neuroinflammation in Alzheimer disease. Neurogenetics 19992135–136. [DOI] [PubMed] [Google Scholar]
- 44.Gehrmann J, Mies G, Bonnekoh P.et al Microglial reaction in the rat cerebral cortex induced by cortical spreading depression. Brain Pathol 1993311–17. [DOI] [PubMed] [Google Scholar]
- 45.Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglial proliferation in the adult murine neocortex. Cereb Cortex 200313845–851. [DOI] [PubMed] [Google Scholar]