Abstract
Lymphangioleiomyomatosis (LAM) is a progressive and often fatal interstitial lung disease characterized by a diffuse proliferation of abnormal smooth muscle cells in the lungs. LAM is of unusual interest biologically because it affects almost exclusively young women. LAM can occur as an isolated disorder (sporadic LAM) or in association with tuberous sclerosis complex. Renal angiomyolipomas, which are found in most tuberous sclerosis patients, also occur in 60% of sporadic LAM patients. We previously found TSC2 loss of heterozygosity in 7 of 13 (54%) of angiomyolipomas from sporadic LAM patients, suggesting that LAM and TSC could have a common genetic basis. In this study, we report the identification of somatic TSC2 mutations in five of seven angiomyolipomas from sporadic LAM patients. In all four patients from whom lung tissue was available, the same mutation found in the angiomyolipoma was present in the abnormal pulmonary smooth muscle cells. In no case was the mutation present in normal kidney, morphologically normal lung, or lymphoblastoid cells. Our data demonstrate that somatic mutations in the TSC2 gene occur in the angiomyolipomas and pulmonary LAM cells of women with sporadic LAM, strongly supporting a direct role of TSC2 in the pathogenesis of this disease.
Lymphangioleiomyomatosis (LAM) is a devastating interstitial lung disease that occurs almost exclusively in young women (1–4). The average age at onset of symptoms is approximately 30 years. Exacerbations of LAM can occur during pregnancy and amelioration of symptoms can sometimes be achieved with therapies that decrease endogenous estrogen production (reviewed in ref. 2), suggesting that steroid hormones may play a role in the pathogenesis of LAM. Lung transplantation is the only effective therapy for end-stage LAM (5).
LAM affects at least 4.6% of women with tuberous sclerosis complex (TSC) (6) and is the third leading cause of TSC-related death (7). TSC is a tumor suppressor gene syndrome associated with seizures, mental retardation, and benign tumors of the brain, heart, kidney, lung, and skin (8). Renal angiomyolipomas (benign tumors composed of fat, smooth muscle, and dysmorphic blood vessels) are the characteristic renal lesions of TSC (9), occurring in approximately 70% of TSC patients.
LAM also occurs in women who do not have neurologic, dermatologic, or retinal signs or symptoms of TSC (sporadic LAM). Sporadic LAM is rare, with <1,000 women currently known to be affected in the United States. The pathologic features of sporadic and TSC-associated pulmonary LAM are identical (10). Sixty percent of women with sporadic LAM have renal angiomyolipomas (11, 12), leading to speculation that LAM and TSC are related diseases (13, 14).
There are two TSC genes: TSC1 on chromosome 9q34 (15) and TSC2 on chromosome 16p13 (16). Loss of heterozygosity (LOH) in TSC-associated angiomyolipomas and other tumors indicates that TSC1 and TSC2 function as tumor suppressor genes (17–20).
We previously found LOH in the TSC2 region of chromosome 16p13 in 7 of 13 (54%) of angiomyolipomas from sporadic LAM patients (21). This suggested a possible genetic relationship between TSC and LAM. However, examination of DNA from peripheral blood lymphocytes or lymphoblastoid cells of LAM patients (12 cases) and cultures of lung cells taken at the time of transplantation for LAM (8 cases) did not reveal any TSC2 mutations (22).
In this study, we report TSC2 mutational analysis of renal angiomyolipoma DNA from seven sporadic LAM patients. Using single-strand conformation polymorphism analysis (SSCP) of all 41 exons of TSC2, we found mutations in five of the seven angiomyolipomas (Table 1 and Fig. 1). We then analyzed all other available tissues from each of these five patients. The same mutation found in the angiomyolipoma was present in microdissected pulmonary LAM cells in all four available cases. The mutations were not detected in any normal tissues, which consisted of normal kidney (four cases), normal lung (three cases), or lymphoblastoid cells (four cases). We also analyzed the microdissected pulmonary cells for LOH in the TSC1 and TSC2 chromosomal regions. In two cases, TSC2 LOH was found in the LAM smooth muscle cells, supporting a two-hit model for the pathogenesis of pulmonary LAM. We conclude that somatic TSC2 mutations are likely to play a direct role in the pathogenesis of sporadic pulmonary LAM.
Table 1.
TSC2 mutations in LAM-associated angiomyolipomas
| Patient | TSC1LOH | TSC2LOH | Nucleotide alteration* | Effect of mutation | Location |
|---|---|---|---|---|---|
| 367 | − | + | |||
| 487 | − | + | G→T at 1096 | Glu→Stop at 366 | Exon 10 |
| 489 | − | − | |||
| 490 | − | + | 2061-2073 del | Ser fs→Stop at 693 | Exon 18 |
| 491 | − | − | 529→532 del | Leu fs→Stop at 180 | Exon 5 |
| 621 | − | + | G→A at 1832 | Arg→Gln at 611 | Exon 16 |
| 651 | − | + | G→A at 1832 | Arg→Gln at 611 | Exon 16 |
+, LOH was present in the angiomyolipoma; −, LOH was not present.
*No mutation was found in the angiomyolipomas from patients 367 and 489.
Figure 1.
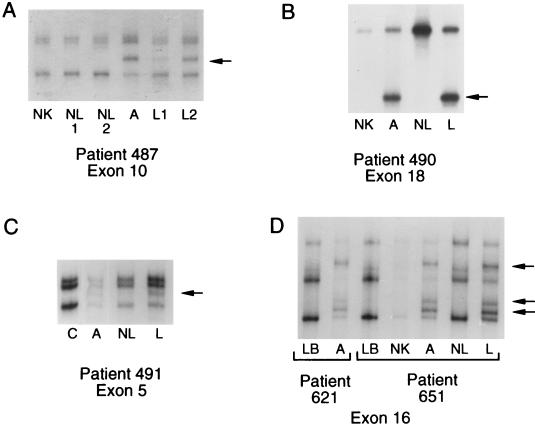
Examples of SSCP analyses. The variant band is indicated with an arrow. (A) Patient 487. The variant band in the angiomyolipoma (A) was present in microdissected LAM cells from two separate paraffin blocks (L1 and L2), but not in normal kidney (NK) or in two regions of normal lung (NL1 and NL2). The variant band was more intense in specimen L2, which contained more actin-positive cells than specimen L1. (B) Patient 490. The variant band in the angiomyolipoma (A) and microdissected LAM cells (L) was not present in normal kidney (NK), or microdissected normal lung (NL). (C) Patient 491. The variant band in the angiomyolipoma (A) was present in microdissected LAM cells (L) and faintly present in the microdissected specimen that consisted primarily of smooth muscle actin-negative cells (NL). This band was absent from the lymphoblastoid cells (Fig. 2C) and from control lymphoblastoid cells, an example of which is shown in the lane labeled C. The four lanes are from the same gel. (D) Patients 621 and 651. The angiomyolipomas from patients 621 and 651 had the same exon 16 mutation. The variant bands in the angiomyolipomas (A) were not present in the lymphoblastoid (LB) cells of either patient. For patient 651, the variant bands were present in the microdissected LAM cells (L) but not in normal kidney or normal lung.
Materials and Methods
Patients.
We studied paraffin-embedded tissue specimens and lymphoblastoid cell lines of seven patients with their informed consent. This study was approved by the Institutional Review Board of Fox Chase Cancer Center. None of the patients in this study had dermatologic or neurologic signs or symptoms of TSC.
DNA Specimens and Laser Capture Microdissection.
DNA was extracted from paraffin-embedded tissue specimens as described previously (20). For the pulmonary LAM and normal lung samples, we used laser capture microdissection (PixCell 100; Arcturus Engineering, Mountain View, CA). To identify the abnormal smooth muscle cells, adjacent tissue sections were stained with muscle-specific actin (BioGenex, San Ramon, CA) and hematoxylin and eosin. The hematoxylin and eosin slide was used for the microdissection. Regions containing primarily normal lung were identified morphologically. Because of the diffuse nature of LAM, it was not possible to identify regions that were entirely normal in the lung biopsy from patient 491. Approximately 500 nuclei of normal lung and LAM cells were captured. The DNA was extracted by overnight incubation in 30 μl of extraction buffer (5% Tween 20, 2 mg/ml proteinase K, 0.5 M Tris·HCl, pH 8.9, 20 mM EDTA, and 10 mM NaCl).
SSCP.
SSCP was used to search for mutations in each of the 41 exons of the TSC2 gene. The primers and PCR conditions have been reported previously (24). A single round of amplification using 35 cycles was performed. PCR was performed with [32P]dGTP in the reaction mix. The PCR products were run on MDE gels (FMC Bioproducts). To maximize the detection of variant bands, each PCR product was run on two gels: one without glycerol and one with 5% glycerol. All reactions were repeated at least twice for confirmation. For the LAM cells and normal lung, the results were confirmed using separately microdissected specimens.
DNA Sequencing.
Samples in which variant bands were detected were reamplified and sequenced. In some cases, the variant band was also excised from the gel, reamplified, and sequenced. Sequence variations were compared with those in the on-line TSC variation database (http://expmed.bwh.harvard.edu/ts/).
Results
Somatic TSC2 Mutations Occur in LAM-Associated Angiomyolipomas.
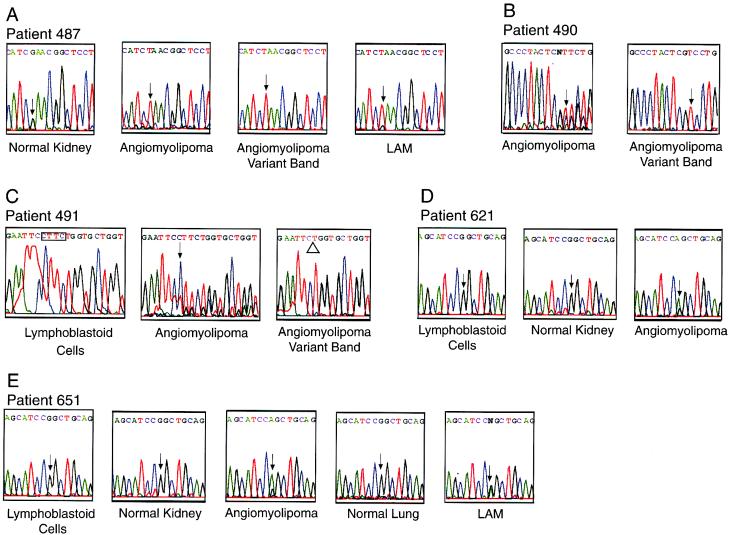
Variant bands were identified by SSCP in five angiomyolipomas (Fig. 1). Direct sequencing of the exons containing variant bands was used to identify DNA sequence variations, which are summarized in Table 1. In the angiomyolipoma from patient 487, a nonsense mutation was present in exon 10 (Figs. 1A and 2A). In the angiomyolipoma from patient 490, a 13-bp deletion was present in exon 18 (Figs. 1B and 2B). In the angiomyolipoma from patient 491, a 4-bp deletion was present in exon 5 (Figs. 1C and 2C). Each change was demonstrated in PCR products from the tumor itself (“angiomyolipoma”) and in the variant band that was excised from the gel (“angiomyolipoma variant band”) (Fig. 2). All three of these changes are predicted to cause premature protein truncation. These three mutations have not been previously reported in TSC patients or tumors.
Figure 2.
Examples of DNA sequencing results. (A) Patient 487. Wild-type sequence is present in normal kidney DNA. Overlapping peaks representing the wild-type (G) and mutant (T) bases are present in the angiomyolipoma (arrow). The variant band in DNA from the angiomyolipoma was cut from the SSCP gel, reamplified, and sequenced (angiomyolipoma variant band). Only the mutant sequence, containing the T, is present. Overlapping peaks are seen at the same position in the microdissected L2 LAM cells (arrow). The mutant peak is predominant in both the LAM and the angiomyolipoma, consistent with the LOH results. (B) Patient 490. A 13-bp deletion in one copy of TSC2 results in overlapping peaks beginning at position 2061 in the angiomyolipoma (arrow). DNA from the angiomyolipoma variant band that was cut from the gel shows the mutant sequence, with the arrow indicating the first affected base. (C) Patient 491. A 4-bp deletion in one copy of TSC2 results in overlapping peaks beginning at position 529 in the angiomyolipoma (arrow). The angiomyolipoma variant band shows the deletion, indicated by the open triangle. The deletion, indicated by the four outlined bases, is not present in lymphoblastoid cells. (D) Patient 621. Wild-type sequence is seen in lymphoblastoid cells and normal kidney. Overlapping peaks of wild-type sequence (G) and mutant (A) are present in the angiomyolipoma (arrow). (E) Patient 651. Wild-type sequence is seen in lymphoblastoid cells, normal kidney, and normal lung. Overlapping peaks of wild-type sequence (G) and mutant (A) are present in the angiomyolipoma and LAM cells (arrows).
In the angiomyolipomas from patients 621 and 651, identical variant bands were found in exon 16 (Fig. 1D). DNA sequencing identified a missense change at position 1832, resulting in a change from arginine to glutamine at codon 611 (Fig. 2 D and E). This change has been previously found in seven unrelated TSC patients. It has been verified as a mutation, and not a polymorphism, by three separate groups, each of which showed that this change was not present in the unaffected parents of TSC patients carrying the mutation (24–26).
Normal kidney tissue was available from four patients whose angiomyolipomas had TSC2 mutations (Table 2). In no case was the TSC2 mutation present in the adjacent normal kidney by either SSCP or sequencing. The variant band was also not evident on long exposures of the SSCP gels (results not shown).
Table 2.
Tissues in which TSC2 mutations were identified
| Patient | Mutation | Angiomyolipoma | Normal kidney | Pulmonary LAM | Normal lung | Lymphoblastoid cells |
|---|---|---|---|---|---|---|
| 487 | G1096T | + | − | + | − | NA |
| 490 | del 2061-73 | + | − | + | − | − |
| 491 | del 529-32 | + | NA | + | NA* | − |
| 621 | G1832A | + | − | NA | NA | − |
| 651 | G1832A | + | − | + | − | − |
+, the specific mutation listed was present in this tissue; −, the mutation was not present; NA, the tissue was not available for analysis.
No morphologically normal lung was present in the specimen from patient 491. The variant band was present in microdissected smooth muscle actin-negative cells, but at a greatly reduced intensity compared with the LAM cells.
Both TSC2 Alleles Are Inactivated in LAM-Associated Angiomyolipomas.
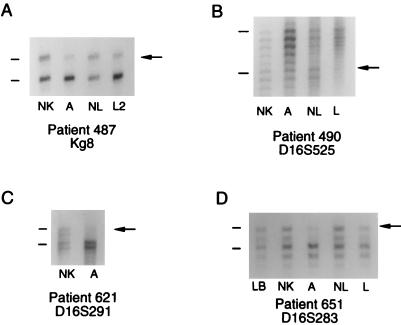
Four of the five angiomyolipomas in which TSC2 mutations were identified also had TSC2 LOH (patients 487, 490, 621, and 651). The LOH results for patients 487 and 490 have been reported previously (21). The angiomyolipoma from patient 621 had LOH at the chromosome 16p13 marker D16S291 (Fig. 3C). The markers kg8, D16S283, and D16S525 were not informative in patient 621. The angiomyolipoma from patient 651 had LOH at the markers D16S283 (Fig. 3D), kg8, and D16S525. Patient 651 was not informative at D16S291. Chromosome 9q34 LOH was not found in any of the angiomyolipomas.
Figure 3.
Examples of chromosome 16p13 LOH analyses. The upper band of each allele is indicated with a line and the lost allele is indicated with an arrow. (A) Patient 487. Two alleles of the marker kg8 are present in normal kidney (NK) and normal lung (NL). Decreased intensity of the upper allele was found in the angiomyolipoma (A) and microdissected LAM specimen (L2) consistent with LOH. (B) Patient 490. Two alleles of the marker D16S525 are present in normal kidney and normal lung. Each allele is represented by a ladder of three or four bands, the highest of which is indicated with a line. Decreased intensity of the lower allele was found in the angiomyolipoma (A) and microdissected LAM specimen (L) consistent with LOH. (C) Patient 621. Two alleles of the marker D16S291 are present in normal kidney. Each allele is represented by two bands. Decreased intensity of the upper allele was found in the angiomyolipoma (A) consistent with LOH. (D) Patient 651. Two alleles of the marker D16S283 are seen in lymphoblastoid cells (LB), normal kidney, and normal lung. Each allele is represented by a ladder of three bands. Decreased intensity of the upper allele was seen in the angiomyolipoma (A) consistent with LOH. Decreased intensity of the upper allele was also seen in the LAM cells (L), suggestive of LOH.
In the angiomyolipoma from patient 491, which does not have TSC2 LOH (21), a single TSC2 mutation was identified. No mutations were found in two of the angiomyolipomas: one from patient 367, that had TSC2 LOH (21), and one from patient 489, that did not have LOH (21).
TSC2 Mutations Occur in Pulmonary LAM Cells, but Not in Normal Lung.
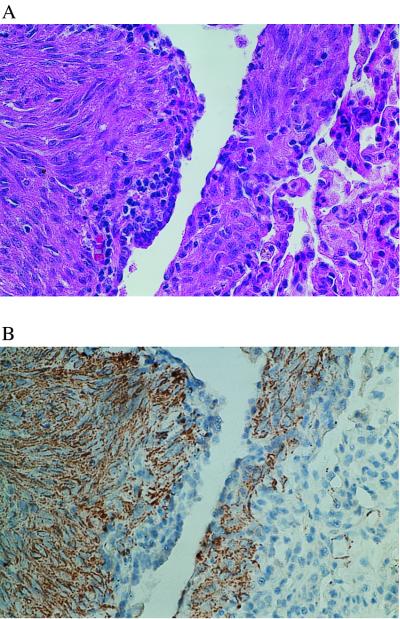
We next asked whether the mutations identified in the angiomyolipomas were present in pulmonary LAM cells. Using immunohistochemistry for smooth muscle actin, we found that the abnormal smooth muscle cells of LAM were invariably in close proximity to other cells that were smooth muscle actin negative. In two cases (patients 487 and 490), foci containing primarily smooth muscle actin-positive cells were present (Fig. 4). In the other two cases (patients 491 and 651), the foci of LAM contained at most 50% smooth muscle actin-positive cells, and these cells were tightly intermixed with smooth muscle actin-negative cells.
Figure 4.
Patient 487. Adjacent sections of the lung biopsy stained with hematoxylin and eosin (A) and immunostained with muscle-specific actin (B). Regions with primarily actin-positive cells are evident. Within these regions, actin-negative cells are also present. Microdissection from this region represented the L2 specimen in Fig. 1A.
We used laser capture microdissection to enrich for the abnormal smooth muscle actin-positive cells from paraffin-embedded pulmonary biopsy specimens (Fig. 4). A similar number of cells from a region with morphologically normal lung was also microdissected. DNA from these specimens was used for SSCP and sequence analysis.
In all four cases from which pulmonary LAM tissue was available (patients 487, 490, 491, and 651), the TSC2 mutation found in the angiomyolipoma was present in microdissected pulmonary LAM cells (Table 2 and Figs. 1 A–D and 2 A and E). In all three cases from which morphologically normal lung tissue was available, there was no evidence of the mutation by either SSCP or sequencing.
For patient 487, two separate paraffin blocks containing pulmonary LAM were available. Microdissections were performed on slides from both blocks, resulting in two normal lung specimens (NL1 and NL2) and two LAM specimens (L1 and L2). The variant SSCP band was present in both LAM cell specimens and absent in both normal lung specimens (Fig. 1A). Our ability to isolate smooth muscle actin-positive LAM cells differed between the two blocks. In the L1 block, the LAM cells were tightly intermingled with actin-negative cells. In the L2 block, foci consisting primarily of actin-positive cells were present, an example of which is shown in Fig. 4. The L2 LAM specimen had a stronger variant band by SSCP than the L1 LAM cells (Fig. 1A), suggesting that the L2 specimen containing a higher proportion of cells with the mutation.
In one case (patient 491), the disease was more advanced, and no morphologically normal lung was present. We therefore microdissected cells from regions that were predominantly actin negative. The variant band was present in this specimen (Fig. 1C, lane labeled NL), but at a greatly reduced intensity compared with the specimen consisting primarily of LAM cells (Fig. 1C, lane labeled L). The lymphoblastoid cells from patient 491 had no evidence of the mutation by either SSCP or sequencing (Fig. 2C).
Molecular Mechanism of Pulmonary Smooth Muscle Proliferation in LAM Involves “Two Hits” in TSC2.
For three patients (487, 490, and 651) whose angiomyolipomas had TSC2 LOH, pulmonary LAM tissue was available for LOH analysis. LOH at the chromosome 16p13 marker kg8 was detected in the L2 microdissected LAM cells from patient 487 (Fig. 3A). The same allele was lost in the LAM cells as in the angiomyolipoma. None of the other three markers in the TSC2 chromosomal region (D16S525, D16S283, and D16S291) was informative in this patient. LOH at the marker D16S525 was found in the microdissected LAM cells from patient 490 (Fig. 3B). The other markers were not informative.
LOH was not detected in the L1 specimen from patient 487 or in the LAM cells from patient 651 (Fig. 3D), both of which were more heavily contaminated with smooth muscle actin-negative cells than the patient 487 L2 specimen or the patient 490 LAM cells. In the LAM cells from patient 651, a decrease in intensity of the upper allele at the marker D16S283 was observed relative to the NL cells (Fig. 3D). Similar intensity differences were seen at the markers kg8 and D16S525. For each marker, the less intense allele was the same allele that was “lost” in the angiomyolipoma. The differences in intensity were not strong enough to be considered LOH. However, they were consistently present. This may indicate that a subpopulation of cells in the specimen has LOH.
Discussion
The relationship between sporadic LAM and TSC has been debated in the medical literature for almost 30 years (e.g., refs. 13, 14, 27, and 28). The recent National Heart Lung and Blood Workshop on lymphangioleiomyomatosis concluded, “there is no evidence that LAM is a form of tuberous sclerosis (29).” Our data demonstrate, for the first time, the genetic basis of the relationship between LAM and TSC. This relationship appears to be an unusual or perhaps novel mechanism for a disease involving tumor suppressor gene mutations.
We examined DNA from seven sporadic LAM angiomyolipomas for mutations in the TSC2 gene. TSC2 mutations were identified in five of the seven tumors. The same mutation found in the angiomyolipoma was present in microdissected pulmonary LAM cells in all four of the patients from whom this tissue was available. In distinct contrast to TSC patients in whom germline mutations are present in both tumor and normal tissue, the mutations in the sporadic LAM angiomyolipomas were not detected in any of the normal tissues. We also found TSC2 LOH in the pulmonary LAM cells from two of the patients.
We base four conclusions on these data. First, TSC2 somatic mutations are likely to play a direct role in the pathogenesis of angiomyolipomas in sporadic LAM patients. TSC2 LOH was present in four of the five angiomyolipomas in which we identified TSC2 mutations. Therefore, these four angiomyolipomas have inactivation of both alleles of TSC2, consistent with the Knudson tumor suppressor gene model (30).
In one angiomyolipoma without TSC2 LOH (patient 491), a single TSC2 mutation was identified and a mutation in the remaining TSC2 allele was not found. In one angiomyolipoma with LOH (patient 367) and one without LOH (patient 489), no TSC2 mutations were identified. TSC2 mutations not detected by SSCP, such as mutations in the noncoding regions of the gene, large deletions, deletions of entire exons, or promotor silencing, may be present in these tumors.
It is certainly possible that other genes are mutated in the angiomyolipomas in which TSC2 mutations were not found. The TSC1 gene is an important candidate to consider. We have now evaluated 24 angiomyolipomas from sporadic LAM patients for TSC1 and TSC2 LOH (ref. 21, this report, and unpublished data). We have not detected TSC1 LOH in any of these angiomyolipomas, whereas TSC2 LOH occurs in approximately 60%. TSC2 LOH is also significantly more frequent than TSC1 LOH in sporadic angiomyolipomas from patients without LAM (23) and in angiomyolipomas from patients with TSC (20). Taken together, these findings may simply indicate that TSC2 is more frequently the target of mutational inactivation than TSC1. It is also possible that angiomyolipomas resulting from TSC2 inactivation are more likely to be large and/or symptomatic than those resulting from TSC1 inactivation, and therefore are more often surgically resected. All of the tissues in this study were from surgical specimens.
Second, we conclude that TSC2 mutations are likely to play a direct role in the pathogenesis of pulmonary LAM. The same mutation found in the angiomyolipoma was present in all four microdissected pulmonary LAM specimens. This finding diminishes the likelihood that a circulating factor such as a growth factor is primarily responsible for smooth muscle proliferation in LAM, as has been postulated previously (29).
Third, we conclude that the Knudson two-hit tumor suppressor gene model is likely to apply to pulmonary LAM cells as well as to angiomyolipoma cells. We found chromosome 16p13 LOH in the pulmonary LAM cells from patients 487 and 490. There are no previous reports of LOH analyses of sporadic pulmonary LAM cells. There has been one report of TSC2 LOH analysis of a case of TSC-associated LAM (31). LOH was not detected in this report. Microdissection or any other method to isolate the abnormal smooth muscle cells was not used.
Fourth, we conclude that the TSC2 mutations in the angiomyolipomas and pulmonary LAM cells arose somatically. The mutations were not present in normal lung, normal kidney, or lymphoblastoid cells. This explains our previous inability to detect TSC2 mutations in the peripheral blood of sporadic LAM patients (22).
A model to account for the presence of TSC2 mutations in the angiomyolipoma and pulmonary LAM cells, but not in other tissues, is clearly required. We propose two potential mechanisms for the pathogenesis of sporadic LAM that would be consistent with our data. Either of these mechanisms would be, to our knowledge, novel for a disease associated with tumor suppressor gene mutations.
First, sporadic LAM could result from somatic mosaicism for TSC2 mutations. Mosaicism appears to be common in tumor suppressor gene syndromes (32), including TSC (33–35). Typically, a patient with somatic mosaicism carries the mutation in some tissues, but not others. Sporadic LAM patients could have TSC2 mutations only in selected kidney and lung cells, and not in surrounding cells within the normal kidney or lung. In this case, the mutations might not be evident by SSCP or sequencing in the normal kidney or lung. The cell type that gives rise to angiomyolipomas and LAM is not known. If this model were correct, however, one might expect multiple independent tumor foci to develop, whereas most sporadic LAM patients have a single angiomyolipoma (12).
A second model to explain our data would involve the migration or spread of smooth muscle cells from the angiomyolipoma to the lung. Angiomyolipomas are histologically benign neoplasms. However, in patients with sporadic, solitary renal angiomyolipomas, it is not unusual to find angiomyolipoma cells in perirenal lymph nodes (reviewed in ref. 36), suggesting that these cells are capable of spreading beyond the primary tumor.
Spread of angiomyolipoma smooth muscle cells to the lungs could explain the occasional recurrences of LAM in the donor lung after lung transplantation (37–39). Curiously, however, in two cases the recurrent LAM cells appeared to be of donor, rather than recipient, origin (37, 39). Both of these cases involved female patients who received a male donor lung. In each, the LAM cells appeared to contain Y chromosomes, as demonstrated by in situ hybridization. We have found that LAM cells (which are smooth muscle actin positive) are nearly always tightly intermixed with other cells that are smooth muscle actin negative. Since the actin staining and Y chromosome detection in these studies were performed on separate slides, it is possible that the Y chromosome-containing cells were not, in fact, the abnormal smooth muscle cells of LAM, and instead represented smooth muscle actin-negative cells derived from the donor.
If our second model is correct, there could be pathogenic parallels between LAM and two other diseases in which histologically benign smooth muscle cells appear to spread: benign metastasizing leiomyoma and disseminated peritoneal leiomyomatosis. Both of these diseases affect primarily young women, as does LAM. Benign metastasizing leiomyoma is a poorly understood disorder typically involving numerous pulmonary smooth muscle tumors (40). In disseminated peritoneal leiomyomatosis, multiple histologically benign smooth muscle tumors arise on the peritoneal and omental surfaces. These individual tumors have been shown to have inactivation of the same X chromosome, consistent with a metastatic process (41). In contrast to LAM, which is often fatal, benign metastasizing leiomyoma and disseminated peritoneal leiomyomatosis generally have benign clinical courses.
The reasons that LAM occurs almost exclusively in women, whereas sporadic angiomyolipomas occur in both men and women (36), remain unknown. This gender specificity may involve the expression of estrogen and progesterone receptors by the smooth muscle cells of angiomyolipomas (42) and/or the modulation of steroid hormone transcription by tuberin, the product of the TSC2 gene (43).
In summary, we identified somatic TSC2 mutations in angiomyolipomas and the abnormal pulmonary smooth muscle cells from women with sporadic LAM. These mutations were not found in normal lung, normal kidney, or lymphoblastoid cells. We also found chromosome 16p13 LOH in the pulmonary LAM cells from two patients. Our data strongly support a direct role of TSC2 mutations in the pathogenesis of sporadic LAM.
Acknowledgments
We are grateful to the patients who contributed blood and tissue specimens for this research and to Drs. Andrew Godwin, Alfonso Bellacosa, and Warren Kruger for critical review of this manuscript. This work was supported by the National Institutes of Health (RO1 HL 60746) and The LAM Foundation (Cincinnati, OH).
Abbreviations
- LAM
lymphangioleiomyomatosis
- TSC
tuberous sclerosis complex
- LOH
loss of heterozygosity
- SSCP
single-strand conformation polymorphism
References
- 1.Taylor J, Ryu J, Colby T, Raffin T. N Engl J Med. 1990;323:1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan E. Chest. 1998;114:1689–1703. doi: 10.1378/chest.114.6.1689. [DOI] [PubMed] [Google Scholar]
- 3.Kalassian K, Doyle R, Kao P, Ruoss S, Raffin T. Am J Respir Crit Care Med. 1997;155:1183–1186. doi: 10.1164/ajrccm.155.4.9105053. [DOI] [PubMed] [Google Scholar]
- 4.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, Cordier J-F. Medicine. 1999;78:321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Boehler A, Speich R, Russi E, Weder W. N Engl J Med. 1996;335:1275–1280. doi: 10.1056/NEJM199610243351704. [DOI] [PubMed] [Google Scholar]
- 6.Castro M, Shepherd C, Gomez M, Lie J, Ryu J. Chest. 1995;107:189–195. doi: 10.1378/chest.107.1.189. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd C, Gomez M, Lie J. Mayo Clin Proc. 1991;66:792–796. doi: 10.1016/s0025-6196(12)61196-3. [DOI] [PubMed] [Google Scholar]
- 8.Gomez M. In: Tuberous Sclerosis Complex. 3rd Ed. Gomez M, Sampson J, Whittemore V, editors. New York: Oxford Univ. Press; 1999. pp. 10–23. [Google Scholar]
- 9.Neumann H, Schwarzkopf G, Henske E. Semin Pediatr Neurol. 1998;5:269–275. doi: 10.1016/s1071-9091(98)80005-3. [DOI] [PubMed] [Google Scholar]
- 10.Chan J, Tsang W, Pau M, Tang M, Pang S, Fletcher C. Histopathology. 1993;22:445–455. doi: 10.1111/j.1365-2559.1993.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein S, Newell J, Adamczyk D, Mortenson R, King T, Lynch D. Am J Respir Crit Care Med. 1995;152:2138–2143. doi: 10.1164/ajrccm.152.6.8520787. [DOI] [PubMed] [Google Scholar]
- 12.Chu S, Horiba K, Usuki J, Avila N, Chen C, Travis W, Ferrans V, Moss J. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 13.Kerr L, Blute M, Ryu J, Swensen S, Malek R. Urology. 1993;41:440–444. doi: 10.1016/0090-4295(93)90504-4. [DOI] [PubMed] [Google Scholar]
- 14.Bonetti F, Chiodera P. Eur Respir J. 1996;9:399–401. doi: 10.1183/09031936.96.09030399. [DOI] [PubMed] [Google Scholar]
- 15.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, et al. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 16.European Chromosome 16 Tuberous Sclerosis Consortium. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 17.Carbonara C, Longa L, Grosso E, Borrone C, Garre M, Brisigotti M, Migone N. Hum Mol Genet. 1994;3:1829–1832. doi: 10.1093/hmg/3.10.1829. [DOI] [PubMed] [Google Scholar]
- 18.Green A, Johnson P, Yates J. Hum Mol Genet. 1994;3:1833–34. doi: 10.1093/hmg/3.10.1833. [DOI] [PubMed] [Google Scholar]
- 19.Green A, Smith M, Yates J. Nat Genet. 1994;6:193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- 20.Henske E, Scheithauer B, Short M, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh C, Kwiatkowski D. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- 21.Smolarek T, Wessner L, McCormack F, Mylet J, Menon A, Henske E. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astrinidis A, Khare L, Carsillo T, Smolarek T, Au K-S, Northrup H, Henske E. J Med Genet. 2000;37:55–57. doi: 10.1136/jmg.37.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henske E, Neumann H, Scheithauer B, Herbst E, Short M, Kwiatkowski D. Genes Chromosomes Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 24.Au K-S, Rodriguez J, Finch J, Volcik K, Roach E, Delgado M, Rodriguez E, Northrup H. Am J Hum Genet. 1997;62:286–294. doi: 10.1086/301705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchamp R, Banwell A, McNamara P, Jacobsen M, Higgins E, Northrup H, Short P, Sims K, Ozelius L, Ramesh V. Hum Mutat. 1998;12:408–416. doi: 10.1002/(SICI)1098-1004(1998)12:6<408::AID-HUMU7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Jones A, Shyamsundar M, Thomas M, Maynard J, Idziaszczyk S, Tomkins S, Sampson J, Cheadle J. Am J Hum Genet. 1999;64:1305–1315. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valensi Q. Am Rev Respir Dis. 1973;108:1411–1415. doi: 10.1164/arrd.1973.108.6.1411. [DOI] [PubMed] [Google Scholar]
- 28.Capron F, Ameille J, Leclerc P, Mornet P, Barbagellata M, Reynes M, Rochemaure J. Cancer. 1983;52:851–855. doi: 10.1002/1097-0142(19830901)52:5<851::aid-cncr2820520518>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.National Heart Lung and Blood Institute. Am J Respir Crit Care Med. 1999;159:000–000. doi: 10.1164/ajrccm.159.2.9803107. [DOI] [PubMed] [Google Scholar]
- 30.Knudson A. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Yamamoto T, Nanba E, Kitamura Y, Terada T, Akaboshi S, Yuasa I, Ohtani K, Nakamoto S, Takeshita K, et al. Am J Med Genet. 1999;82:368–370. doi: 10.1002/(sici)1096-8628(19990219)82:5<368::aid-ajmg2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Evans D, Wallace A, Wu C, Trueman L, Ramsden R, Strachan T. Am J Hum Genet. 1998;63:727–736. doi: 10.1086/512074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhoef S, Vrtel R, Essen T V, Bakker L, Sikkens E, Halley D, Lindhout D, Ouweland A v d. Lancet. 1995;345:202. doi: 10.1016/s0140-6736(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowska J, Wigowska-Sowinska J, Napierala D, Slomski R, Kwiatkowski D. N Engl J Med. 1999;340:703–707. doi: 10.1056/NEJM199903043400905. [DOI] [PubMed] [Google Scholar]
- 35.Rose V, Au K-S, Pollom G, Roach E, Prashner H, Northrup H. Am J Hum Genet. 1999;64:986–992. doi: 10.1086/302322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eble J N. Semin Diagn Pathol. 1998;15:21–40. [PubMed] [Google Scholar]
- 37.Nine J, Yousem S, Paradis I, Keenan R, Griffith B. J Heart Lung Transplant. 1994;13:714–719. [PubMed] [Google Scholar]
- 38.O'Brien J, Lium J, Parosa J, Deyoung B, Wick M, Trulock E. Am. J. Respir. Crit. Care Med. 1995. [DOI] [PubMed] [Google Scholar]
- 39.Bittmann I, Dose T, Muller C, Dienemann H. Hum Pathol. 1997;28:1420–1423. doi: 10.1016/s0046-8177(97)90233-1. [DOI] [PubMed] [Google Scholar]
- 40.Horiuchi K, Yabe H, Mukai M, Morioka H, Udagawa Y, Nozawa S, Yabe Y. Am J Surg Pathol. 1998;22:897–901. doi: 10.1097/00000478-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Quade B J, McLachlin C M, Soto-Wright V, Zuckerman J, Mutter G L, Morton C C. Am J Pathol. 1997;150:2153–2166. [PMC free article] [PubMed] [Google Scholar]
- 42.Logginidou H, Ao X, Russo I, Henske E. Chest. 2000;117:25–30. doi: 10.1378/chest.117.1.25. [DOI] [PubMed] [Google Scholar]
- 43.Henry K, Yuan X, Koszewski N, Ondo H, Kwiatkowski D, Noonan D. J Biol Chem. 1998;273:20535–20539. doi: 10.1074/jbc.273.32.20535. [DOI] [PubMed] [Google Scholar]