Abstract
Background
Connective tissue growth factor (CCN2) is upregulated in pancreatic fibrosis and desmoplastic pancreatic tumours. CCN2 interacts with integrin α5β1 on pancreatic stellate cells (PSC) in which it stimulates fibrogenesis, adhesion, migration, and proliferation.
Aim
To determine the structural domain(s) in CCN2 that interact with integrin α5β1 to regulation PSC functions.
Methods
Primary activated rat PSC were tested for their adherence to isoforms of CCN2 comprising modules 1–4 (CCN21–4), modules 3–4 (CCN23–4), module 3 alone (CCN23), or module 4 alone (CCN24). Adhesion studies were performed in the presence of EDTA, divalent cations, anti‐integrin α5β1 antibodies, CCN2 synthetic peptides, or heparin, or after pretreatment of the cells with heparinase, chondroitinase, or sodium chlorate. CCN2 integrin α5β1 binding was analysed in cell free systems. The ability of CCN21–4, CCN23–4, or CCN24 to stimulate PSC migration was evaluated in the presence of anti‐integrin α5β1 or heparin.
Results
PSC adhesion was stimulated by CCN21–4, CCN23–4, or CCN24 and supported by Mg2+ but not Ca2+. CCN24 supported PSC adhesion or migration were blocked by anti‐integrin α5β1 antibodies or by treatment of cells with heparinase or sodium chlorate. A direct interaction between CCN24 and integrin α5β1 was demonstrated in cell free assays. The sequence GVCTDGR in module 4 mediated the binding between CCN24 and integrin α5β1 as well as CCN24 mediated PSC adhesion and migration.
Conclusions
A GVCTDGR sequence in module 4 of CCN2 is a novel integrin α5β1 binding site that is essential for CCN2 stimulated functions in PSC and which represents a new therapeutic target in PSC mediated fibrogenesis.
Keywords: pancreatitis, pancreatic fibrosis, fibrogenesis, connective tissue growth factor
Connective tissue growth factor (CCN2, also termed CTGF) is one of six structurally related molecules that comprise the CCN family.1 CCN proteins regulate cell function (for example, cell cycle progression, division, chemotaxis, differentiation, apoptosis, adhesion, gene regulation, ion transport) by interacting contextually with cell surface receptors, cytokines, growth factors, and proteases.1,2 CCN molecules participate in critical processes such as differentiation, development, angiogenesis, placentation, tumour growth, wound healing, and fibrosis,1,2 the latter of which is the most common pathophysiological condition in which CCN2 has been implicated, often following its transcriptional activation or synergistic interaction with transforming growth factor β (TGF‐β).3
In the pancreas, long term heavy alcohol consumption is associated with acute and chronic pancreatitis, the latter of which involves a significant fibrotic component.4 Several recent reports have begun to link CCN2 overexpression with pancreatitis5,6 and desmoplasia in pancreatic cancer.7,8 Evidence from human clinical specimens and rat models has shown that CCN2 expression is associated with enhanced and concomitant expression of TGF‐β and type collagen I in both acute and chronic pancreatitis.5,6 CCN2 is produced by the remaining acinar, ductal, and fibroblastic cells in diseased tissue and is most abundant in severely damaged tissue adjacent to areas of necrosis. In pancreatic cancer, CCN2 mRNA expression was enhanced and positively correlated with the degree of tumour desmoplasia; CCN2 was implicated in the development of the desmoplastic stroma and was mainly produced by fibroblasts.8 Although development of fibrosis during chronic pancreatitis clearly leads to additional tissue destruction and loss of function, pancreatic cancer patients with elevated pancreatic CCN2 mRNA expression have a better prognosis, possibly because a matrix rich desmoplastic stroma provides a growth disadvantage for pancreatic cancer cells.9
The principal fibrogenic cell type in the pancreas is the pancreatic stellate cell (PSC) which are localised around the acini and ducts in normal tissue.10 When cultured, PSC undergo an activation process by which they become α smooth muscle actin expressing myofibroblast‐like cells that are contractile, migratory, and proliferative, and produce high levels of collagen types I and III, laminin, and fibronectin (FN). These phenotypic changes are also proposed to occur during fibrosing pancreatic injury in vivo, resulting in deposition of a high density interstitial extracellular matrix (ECM) that severely compromises pancreatic function.11,12 We recently performed a detailed analysis of the production of and response to CCN2 by PSC and discovered a central role for integrin α5β1 as a novel CCN2 receptor that mediated adhesion, migration, mitogenesis, and fibrogenesis.13 As integrins have emerged as receptors for several CCN proteins,2 CCN2‐integrin interactions may offer a platform for developing new antifibrotic treatments. We now report that a unique region in the carboxy terminal domain of CCN2 plays a central role in functionally engaging integrin α5β1 on activated PSC, highlighting this region of CCN2 as a potential therapeutic target.
Materials and methods
Reagents
Collagenase P and DNase I were purchased from Roche (Indianapolis, Indiana, USA). Heparinase I, chondroitinase ABC, bovine serum albumin (BSA; protease free and γ‐globulin free), mouse IgG, protease XIV, and sodium chlorate (NaClO3) were from Sigma (St Louis, Missouri, USA). Heparin was from USB (Cleveland, Ohio, USA). CyQUANT dye was from Molecular Probes (Eugene, Oregon, USA). FN, Dulbecco's modified Eagle's medium, and fetal bovine serum were purchased from Life Technologies (Grand Island, New York, USA). Purified human integrin α5β1, mouse monoclonal anti‐α5, anti‐β1, rat monoclonal anti‐α5β1 blocking antibodies, and goat polyclonal anti‐α5β1 antibody were purchased from Chemicon Inc. (Temicula, California, USA).
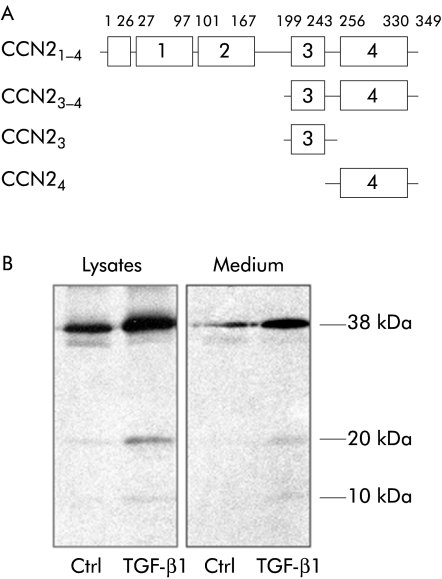
The various forms of CCN2 used in these studies are shown in fig 1A. Intact recombinant 38 kDa human CCN2 containing all four modules (CCN21–4) and a C terminal fragment comprising essentially modules 3 and 4 (CCN23–4) were produced and purified as described previously.14 CCN2 isoforms comprising either module 4 alone (CCN24) or module 3 alone (CCN23) were produced in Escherichia coli using the same approach as we have previously described.15 Synthetic peptides spanning the entire 103 C terminal residues of CCN2 were produced as described previously.16
Figure 1 Structure of connective tissue growth factor (CCN2) isoforms and their production by pancreatic stellate cells (PSC). (A) Recombinant CCN2 isoforms used in these studies. (B) CCN2 isoforms detected in PSC lysates or medium by radioimmunoprecipitation, sodium dodecyl sulphate‐polyacrylamide gel electrophoresis, and autoradiography of samples after labelling of cells with [35S]cysteine/methionine for six hours in the presence or absence of transforming growth factor β (TGF‐β1) 20 ng/ml. The major immunoreactive proteins (10, 20, 38 kDa) are indicated.
Isolation and culture of PSC
Rat PSC were isolated from normal male Sprague‐Dawley rats by in situ collaganese perfusion,13 as approved by the Institutional Animal Care and Use Committee of the Children's Research Institute, Columbus, Ohio, USA. Activated PSC were split every three days at a ratio of 1:3 and used at passages 2–4. CCN2 levels in cell lysates or conditioned media were assessed by immunoprecipitation with anti‐CCN2 antiserum after six hours of labelling of cells with [35S]cysteine/methionine in the presence or absence of 20 ng/ml TGF‐β.
Cell adhesion and migration assays
Activated PSC were used in adhesion and migration assays as described previously.13 Briefly, for assessment of cell adhesion, 96 well plates (Costar, Corning, New York, USA) were precoated with CCN2 proteins or control cell adhesion molecules, prior to addition of 50 μl PSC (2.5×105 cells/ml), that were preincubated or coincubated with the various test substances, as detailed in the figure legends. Adherent cells were stained using CyQUANT GR dye. For PSC migration studies, PSC were suspended in cell culture inserts (2.5×104 cells/insert) that were contained within individual wells of a 12 well culture plates. The ability of PSC to migrate to the underside of the insert (8 μm pore size) in response to the presence of CCN2 proteins or FN was assessed after a six hour incubation. PSC on the upper and lower surfaces of the membrane were counted in 10 random 400× fields.13
Cell free CCN2‐integrin α5β1 binding assays
Complexes were allowed to form in solution between human integrin α5β1 and each CCN2 isoform, prior to immunoprecipitation with anti‐CCN2 antibody and protein A beads, essentially as described previously.13 Immune complexes were separated on 8% sodium dodecyl sulphate‐polyacrylamide gels, transferred to nitrocellulose, and then treated with goat antihuman α5β antibody followed by horseradish peroxidase linked mouse antigoat IgG. Immunoreactive proteins were detected by chemiluminescence.
Binding of integrin α5β1 to individual CCN2 isoform was further tested in a solid phase assay.13 Briefly, microtitre wells (Dynex Technology, Chantilly, Virginia, USA) were precoated with CCN2 proteins or FN and, after extensive blocking and washing, were incubated with 1 μg/ml integrin α5β1. The plate was developed using antihuman α5β1 monoclonal antibody, as described previously.13
Statistical analysis
Values represent mean (SD) of measurements from at least four different PSC isolations. Statistical analysis of the data was performed using SPSS 11.5 for Windows. The Student's t test was used for paired data that were normally distributed. A p value of <0.05 was considered significant.
Results
Multiple CCN2 isoforms were detected in PSC lysates and conditioned medium, levels of which were enhanced by treatment of cells with TGF‐β (fig 1B). The size of the 38 kDa, 20 kDa, and 10 kDa proteins detected correspond to those of, respectively, CCN21–4, CCN23–4, and CCN24 (see fig 1A) that have previously been characterised in several in vitro and in vivo systems and that arise from limited CCN2 proteolysis.14,15,16,17
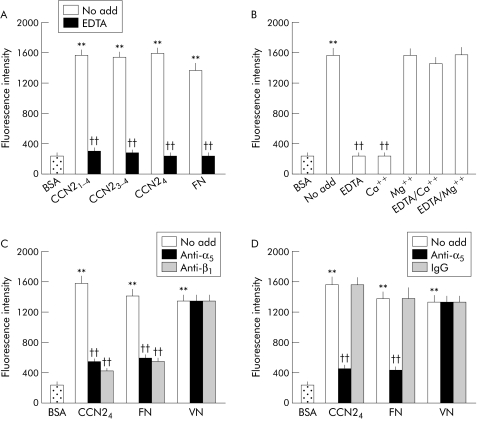
Adhesion of PSC was stimulated by CCN21–4, CCN23–4, and CCN24 and was divalent cation dependent, as shown by the ability of EDTA to block cell adhesion to all CCN2 isoforms (fig 2A). CCN24 mediated PSC adhesion was supported by Mg2+ but not by Ca2+ (fig 2B). There was however no effect on PSC adhesion in the presence of both EDTA and Ca2+, likely reflecting their chelation with one another. Mg2+, which itself supported PSC binding, was able to block the inhibitory effect of EDTA (fig 2B).
Figure 2 Connective tissue growth factor (CCN2) dependent pancreatic stellate cell (PSC) adhesion is mediated by interactions of module 4 with integrin α5β1. (A) Microtitre wells were precoated at 4°C for 16 hours with phosphate buffered saline (PBS) or 2 μg/ml CCN21–4, CCN23–4, CCN24, or fibronectin (FN) and then blocked with PBS containing 1% bovine serum albumin (BSA) for one hour. Rat activated PSC (2.5×105 cells/ml) were preincubated in serum free medium for 30 minutes in vehicle buffer (no add) or EDTA (5 mM) prior to addition to individual wells at 50 μl/well. After incubation at 37°C for 20 minutes, adherent cells were washed, fixed, and stained by CyQUANT GR dye and quantified by measuring fluorescence intensity at an excitation of 485 nm and an emission of 530 nm. (B) PSC adhesion assays were performed using CCN24 following preincubation of the cells for 30 minutes with EDTA (5 mM) or with addition of Ca2+ (10 mM) or Mg2+ (10 mM) either alone or in combination. (C) PSC were preincubated with 25 μg/ml anti‐integrin α5 or anti‐integrin β1 monoclonal antibodies for 30 minutes prior to adding the cells to the wells that had been precoated with CCN24 (2 μg/ml), FN (2 μg/ml), or vitronectin (VN 4 μg/ml). (D) Microtitre wells were coated with CCN24, FN, or VN, as indicated, above prior to addition of PSC that had been preincubated at 37°C for 30 minutes with vehicle buffer (no add), 25 μg/ml monoclonal anti‐α5β1, or 25 μg/ml normal mouse IgG. Data are means (SD) of quadruplicate determinations and are representative of three experiments. **p<0.01 versus control; ††p<0.01 versus “no add” group.
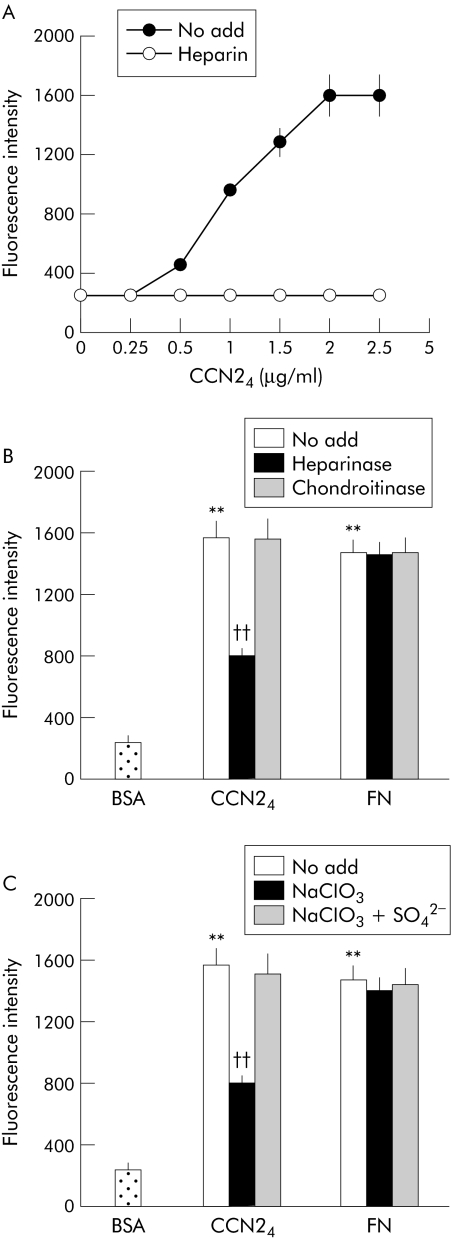
As CCN24 appeared to be fully active in these assays, these data demonstrated that module 4 contributed fundamentally to PSC binding. Since CCN21–4 was previously shown to interact with integrin α5β1,13 we next tested whether this integrin was involved in adhesion of PSC to CCN24. Antibodies to the individual integrin α5 or β1 subunits or to integrin α5β1 itself were effective in blocking CCN24 mediated PSC adhesion whereas normal IgG had no effect (fig 2C, D). The same pattern of binding was seen for FN, a well characterised ligand of integrin α5β1, but not for vitronectin which does not bind to integrin α5β1. Cell adhesion in response in CCN24 was dose dependent, reaching a plateau at coating concentrations of 2 μg/ml; this effect was heparin dependent, as shown by the inability of CCN24 to support PSC adhesion when the assays were performed in the presence of soluble heparin (fig 3A). Treatment of the cells with heparinase or sodium chlorate (an inhibitor of sulfation of heparin sulphate proteoglycans (HSPG)) reduced the ability of PSC to adhere to CCN24 by approximately 50% whereas adhesion to FN was not affected (fig 3B,C). Binding of PSC to CCN24 was not diminished by chondroitinase treatment, while the chlorate induced block was reversed by addition of sodium sulphate to the medium. Overall, these data suggest that heparin or heparin‐like molecules such as cell surface HSPG contribute to the regulation of cell adhesion by CCN24.
Figure 3 Role of cell surface heparan sulphate proteoglycan in connective tissue growth factor module 4 (CCN24) mediated pancreatic stellate cell (PSC) adhesion. (A) Cell adhesion assays were performed on CCN24 precoated microtitre wells using PSC that were treated with vehicle buffer (no add) or heparin (2 μg/ml) prior to plating. (B) Microtitre wells were coated with 2 μg/ml CCN24 or fibronectin (FN) prior to addition of PSC that had been pretreated at 37°C for 30 minutes with vehicle buffer (no add), 2 units/ml heparinase I, or chondroitinase ABC. (C) PSC were cultured in complete medium containing 10 mM NaClO3 for 48 hours in the presence or absence of 10 mM Na2SO4 prior to addition to CCN24 precoated wells. Data are means (SD) of quadruplicate determinations and are representative of three experiments. **p<0.01 versus control; ††p<0.01 versus “no add” group.
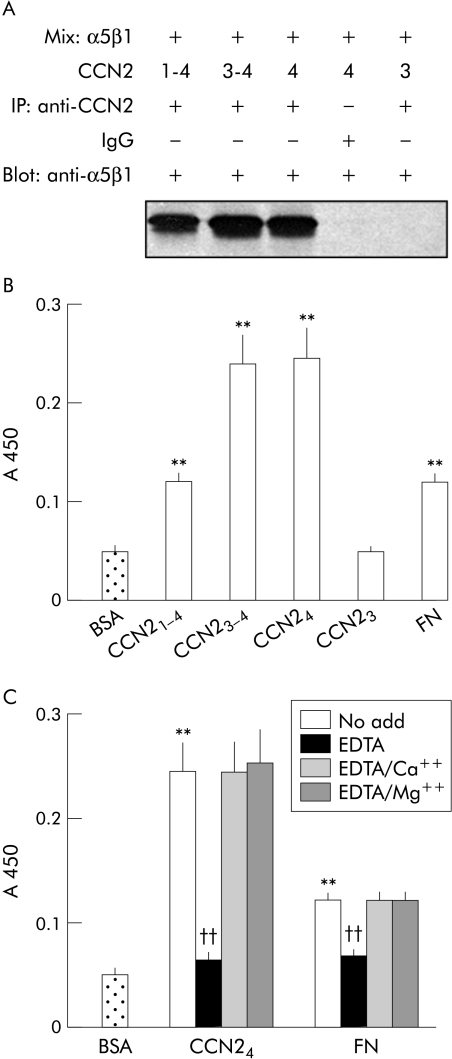
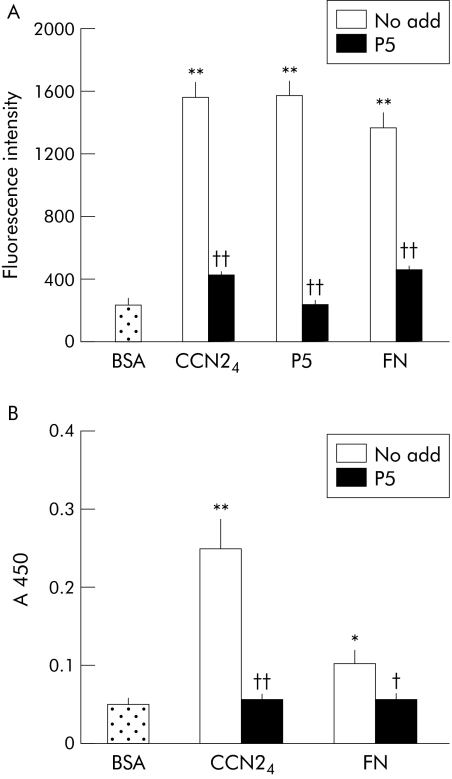
To verify that an integrin α5β1 binding site was located in module 4 of CCN2, a series of cell free binding assays were performed. We have previously shown that CCN21–4 is capable of engaging integrin α5β1 in either solution based or solid phase cell free assays.13 We found that this effect was mimicked by either CCN23–4 or CCN24 (the latter being divalent cation dependent) but not by CCN23 (fig 4A–C). These data thus pointed to the presence of an integrin α5β1 binding site in module 4 of CCN2. When overlapping synthetic peptides from module 4 of CCN2 were analysed, we found that a peptide of the sequence GVCTDGR (corresponding to residues 285–291) was able to support PSC adhesion (fig 5A). Significantly, this peptide was also capable of blocking PSC adhesion to either CCN24 or FN (fig 5A) as well as blocking the interaction between integrin α5β1 and either CCN24 or FN in a cell free system (fig 5B). Other peptides from module 4 were ineffective in these assays (data not shown). Overall, these data demonstrate the presence of an integrin α5β1 binding motif within amino acids 285–291 of CCN2.
Figure 4 Integrin α5β1 binds to connective tissue growth factor module 4 (CCN24) directly in cell free systems. (A) Integrin α5β1 2 μg were individually added to 4 μg CCN21–4, CCN23–4, CCN24, or CCN23 in 1 ml of NP40 buffer and mixed at 4°C for two hours prior to treatment with immunoprecipitating polyclonal rabbit anti‐CCN2 antibody or normal IgG. Samples were separated on 8% sodium dodecyl sulphate‐polyacrylamide gels, and transferred onto a nitrocellulose membrane which was immunoblotted with anti‐human α5β1 before detection using enhanced chemiluminescence. (B) Microtitre wells were precoated with 2 μg/ml of CCN21–4, CCN23–4, CCN24, CCN23, or fibronectin (FN) at 4°C for 16 hours. The wells were blocked and then incubated with 1 μg/ml integrin α5β1 in blocking solution. The plate was developed by addition of antihuman α5β1 monoclonal antibody followed by link antibody and horseradish peroxidase conjugated streptavidin. The colour reaction was developed using horseradish peroxidase substrate measured at A450. BSA, bovine serum albumin. (C) Microtitre wells were precoated with CCN24 (2 μg/ml) or FN (2 μg/ml) at 4°C for 16 hours, and the ability of integrin α5β1 to subsequently bind to the plates was detected by ELISA following pretreatment of the integrin α5β1 with either 5 mM EDTA alone or in combination with 10 mM Ca++ or 10 mM Mg++. Data are means (SD) of quadruplicate determinations and are representative of three experiments. **p<0.01 versus control; ††p<0.01 versus “no add” group.
Figure 5 A synthetic connective tissue growth factor module 4 (CCN24) peptide, GVCTDGR, contains an integrin α5β1 binding site. (A) Cell adhesion assays were performed on 96 well plates that had been coated at 2 μg/ml with CCN24, P5 (a synthetic peptide comprising the CVCTDGR, corresponding to residues 285–291 of CCN2) or fibronectin (FN). BSA, bovine serum albumin. (B) Microtitre wells were coated with CCN24 (2 μg/ml) or FN (2 μg/ml) at 4°C for 16 hours and then incubated with 1 μg/ml integrin α5β1 alone or after its preincubation with 35 μM P5 for one hour. CCN24 binding by integrin α5β1 was quantified by ELISA. Data are means (SD) of quadruplicate determinations and are representative of three experiments. *p<0.05, **p<0.01 versus control; †p<0.05, ††p<0.01 versus the “no add” group.
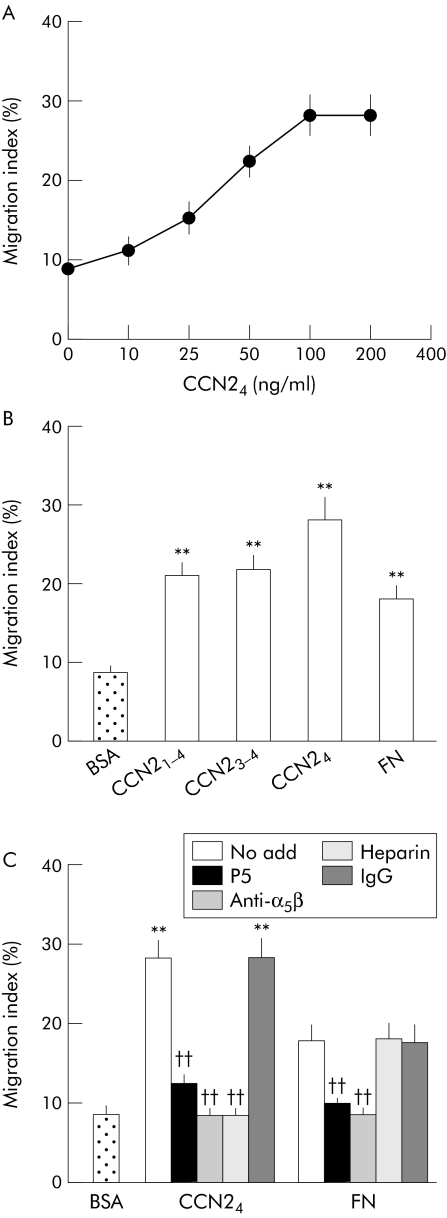
As we have recently shown that that CCN21–4 is chemotactic for activated PSC,13 we next determined whether module 4 of CCN24 was involved in stimulating PSC migration. CCN24 was found to stimulate dose dependent migration of PSC in vitro, with a maximal response at 100 ng/ml (fig 6A). The migratory response of PSC to CCN24 was similar to that of CCN21–4, CCN23–4, or FN (fig 6B). PSC migration in response to either CCN24 or FN was blocked when the cells were treated with the P5 peptide or an anti‐integrin α5β1 monoclonal antibody, whereas treatment of the cells with soluble heparin caused PSC adhesion to be blocked only in the case of CCN24 (fig 6C).
Figure 6 Connective tissue growth factor module 4 (CCN24) induces integrin α5β1 dependent pancreatic stellate cell (PSC) migration. (A) PSC migration assays were performed by placing the cells in culture inserts (2.5×105 cells/insert) followed by incubation in a 12 well companion plate for six hours in the absence or presence of desired concentrations of CCN24 in the lower chamber. (B) PSC migration assays were performed in the presence of 100 ng/ml of CCN21–4, CCN23–4, CCN24, or 100 ng/ml fibronectin (FN) in the lower chamber. (C) Cell migration assays were performed following 30 minutes of preincubation of PSC with P5 (35 μM), anti‐α5β1 (25 μg/ml), mouse IgG (25 μg/ml), or heparin (2 μg/ml). Data are means (SD) of quadruplicate determinations and are representative of three experiments. **p<0.01 versus control; ††p<0.01 versus the “no add” group.
Discussion
CCN2 is a recently described profibrotic factor that is produced in fibrosing tissues and organs and has fibrogenic properties in vivo and in vitro. Accumulating evidence suggests that CCN2 plays a role in acute and chronic pancreatitis as well as in pancreatic cancer. Of particular interest is the apparent involvement of CCN2 in driving excessive production of ECM proteins that are characteristic of pancreatic fibrosis and tumour desmoplasia. Based on our recent observations13 as well as those in this report, we have shown that CCN2 is capable of stimulating responses in PSC (mitogenesis, chemotaxis, adhesion, proliferation, DNA synthesis, fibrogenesis) that are typically associated with the activated phenotype of this normally quiescent cell type, suggesting that CCN2 plays a key role in PSC mediated fibrogenesis. Moreover, CCN2 mRNA expression, along with that of FN, TGF‐β1, and collagen I, is produced as a function of PSC activation in vitro, while CCN2 levels and promoter activity were enhanced following stimulation by TGF‐β1, alcohol, or acetaldehyde.13 CCN2 also mediates TGF‐β stimulated collagen I production in PSC. Thus through its production by PSC themselves or by its production by other cell types such as ductular epithelium, acinar cells, fibroblasts, and endothelial cells,5,6 CCN2 likely participates in autocrine and paracrine signalling pathways in response to pancreatic injury and, furthermore, is subject to limited proteolytic processing which generates bioactive C terminal isoforms. CCN2 proteolysis is promoted by matrix metalloproteases (MMPs), several of which are produced by PSC.18,19,20,21,22 Although the significance of CCN2 processing has yet to be fully established, it likely has a major impact of the bioavailability and distribution of the protein. This is highlighted by the fact that CCN2 N terminal fragments in body fluids have been implicated as markers of fibrotic disease.23,24,25 On the other hand, C terminal fragments, which are bioactive and fibrogenic, may persist in tissues and act as matrix associated stimulants of fibrogenic pathways in target cells such as PSC.
Chronic pancreatitis and pancreatic cancer are associated with alterations in levels of various integrins and their ECM ligands.26,27 PSC are believed to play a central role in the regulation of ECM levels as they not only regulate synthesis of ECM constituents such as FN or collagen but also produce MMPs, as well as tissue inhibitors of MMPs which collectively regulate the balance between ECM degradation and synthesis.20,21,22 None the less, the manner in which PSC function is regulated by the interplay between ECM constituents, integrins, and other matrix molecules such as matricellular proteins (of which CCN2 is an example) has not been previously studied. While integrins have become recognised as receptors for various CCN proteins, the interaction is complex and involves a high degree of specificity with respect to the location of the CCN binding domain, the integrin partner, the cell type in question, and the functional readout. With respect to PSC, identification of integrin α5β1 as the principal CCN2 receptor was unexpected yet this integrin was responsible for mediating several important biological properties of CCN2 in PSC, including adhesion and migration.13 Interestingly, we have shown that hepatic stellate cells (HSC) also produce integrin α5β but they appear not to exploit this integrin as an adhesion receptor for CCN2 (unpublished data). In addition, mutant CCN24 proteins in which the integrin αvβ3 site has been targeted show an unchanged ability to support PSC adhesion (unpublished data) while HSC binding is highly compromised.28 Overall, the data suggest that CCN2 mediated cell adhesion involves principally integrin α5β1 for PSC and integrin αvβ3 for HSC. Inhibition of CCN2 mediated PSC adhesion by Ca2+ is consistent with previously published data showing that ligand binding by integrin α5β1 is not supported by millimolar concentrations of Ca2+ and is likely attributable to induction of an inactive integrin conformation by high concentrations of calcium.29,30 In contrast, Mg2+ favours integrin activation and supports ligand‐integrin α5β1 interactions,29 as was observed in our studies with CCN2.
The most significant findings now reported here are that a novel sequence in module 4 of CCN2 contains the principal integrin α5β1 binding site and that an isoform of CCN2 that contains module 4 alone (CCN24) is able to support PSC adhesion and migration. Although it was previously shown that module 4 of CCN2 binds to integrin αvβ328 and that module 4 of CCN1 binds to integrin αMβ2,31 the binding domains are clearly distinct from those used by CCN2 to engage integrin α5β1. Whereas RGD motifs within integrin ligands are often used for binding their cognate integrin receptors, this motif is absent from CCN proteins. Even so, it is of interest that the GVCTDGR integrin α5β1 binding sequence in module 4 contains a reverse RGD motif. While the precise role of this motif requires further study, a DGR sequence in fibroblast growth factor 2 was shown to be involved in its ability to bind to integrin αvβ3 on endothelial cells.32 Whatever the precise role of the DGR sequence in residues 285–291, our data suggest that this domain is pivotal for integrin α5β1 dependent functions of CCN2 in PSC.
Although integrin α5β1 supported either FN or CCN24 mediated PSC adhesion or migration, responses of the cells were distinct in that the effects of CCN24 were HSPG dependent whereas those of FN were not. These data are consistent with the observation that CCN24 is heparin binding, a property that is attributed to the presence of one or more heparin binding domains in module 4.14,15,16 Dependency on cell surface HSPG of several CCN2 bioactivities have been reported.16,28,33 While the heparin binding properties of CCN2 may affect its bioavailability through interactions with HSPG in ECM, the ability of CCN2 to stimulate its fibrogenic target cells via integrins in vivo will likely be strongly influenced by their concomitant expression of HSPG. These data suggest that additional studies regarding HSPG production by PSC as a function of their activation may give further insight regarding the ability of these cells to interact with and respond to CCN2.
In the liver, sinusoidal accumulation of integrins and integrin ligands has been reported during fibrotic disease.34,35 In addition, a variety of integrins are expressed by HSC during the activation process, and α and β1 integrin subunits are more highly expressed by HSC in human fibrotic liver.34,35,36,37,38 Furthermore, binding of some integrins (including integrin αvβ3, the principal CCN2 receptor) by their respective ligands is linked to the differentiated function and survival of HSC.39,40 These data, coupled with the observation that liver fibrosis in mice can be blocked by RGD peptides,41 highlight integrins as possible targets in fibrotic pathways, especially those that are driven by CCN2. There is growing optimism that anti‐CCN2 strategies will offer new leads in the development of novel therapies for fibrosis.42 However, mechanisms of CCN2 action in key fibrogenic cell types such as PSC in the pancreas, HSC in the liver, or mesangial cells in the kidney need to be thoroughly investigated so that rational CCN2 based therapeutic approaches can be developed. For example, mechanisms of injury and fibrosis are clearly different between the liver and pancreas, especially with respect to the role of pancreatic enzymes and ductal obstruction and these may influence CTGF receptor expression and signalling pathways in stellate cells. Interestingly, the GVCTDGR sequence was shown not to be involved in supporting adhesion of HSC cells, which instead preferentially use integrin αvβ3 as a CCN2 receptor.28 This difference highlights the fact that antifibrotic modalities may need to be developed for CCN2 on an organ by organ basis, taking into account the specific integrin subtypes that are used by CCN2 for binding to its respective target cells. Based on our observations, targeting of integrin α5β1 on PSC and/or of its binding domain in module 4 of CCN2 provide a new platform for the development of novel antifibrotic strategies in pancreatic fibrosis.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Acknowledgements
We thank Zhen‐Yue Tong for providing CCN23. This work was supported by National Institutes of Health grant R01 AA12817 awarded to DRB.
Abbreviations
BSA - bovine serum albumin
CCN2 - connective tissue growth factor
ECM - extracellular matrix
FN - fibronectin
HSC - hepatic stellate cell
HSPG - heparan sulphate proteoglycan
MMPs - matrix metalloproteases
PSC - pancreatic stellate cell
TGF‐β - transforming growth factor β
Footnotes
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Brigstock D R. The connective tissue growth factor/cysteine‐rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev 199920189–206. [DOI] [PubMed] [Google Scholar]
- 2.Lau L F, Lam S C. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res 199924844–57. [DOI] [PubMed] [Google Scholar]
- 3.Rachfal A W, Brigstock D R. Structural and functional properties of CCN proteins. Vitam Horm 20057069–103. [DOI] [PubMed] [Google Scholar]
- 4.Saluja A K, Bhagat L. Pathophysiology of alcohol‐induced pancreatic injury. Pancreas 200327327–331. [DOI] [PubMed] [Google Scholar]
- 5.di Mola F F, Friess H, Martignoni M E.et al Connective tissue growth factor is a regulator for fibrosis in human chronic pancreatitis. Ann Surg 199923063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.di Mola F F, Friess H, Riesle E.et al Connective tissue growth factor is involved in pancreatic repair and tissue remodeling in human and rat acute necrotizing pancreatitis. Ann Surg 200223560–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.di Mola F F, Di Sebastiano P, Gardini A.et al Expression of connective tissue growth factor (CTGF) in pancreatic cancer. Tumori 20038958–59. [PubMed] [Google Scholar]
- 8.Wenger C, Ellenrieder V, Alber B.et al Expression and differential regulation of connective tissue growth factor in pancreatic cancer cells. Oncogene 1999181073–1080. [DOI] [PubMed] [Google Scholar]
- 9.Hartel M, Di Mola F F, Gardini A.et al Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg 200428818–825. [DOI] [PubMed] [Google Scholar]
- 10.Apte M V, Haber P S, Applegate T L.et al Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 199843128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider E, Schmid‐Kotsas A, Zhao J.et al Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol 2001281C532–C543. [DOI] [PubMed] [Google Scholar]
- 12.Apte M V, Wilson J S. Stellate cell activation in alcoholic pancreatitis. Pancreas 200327316–320. [DOI] [PubMed] [Google Scholar]
- 13.Gao R, Brigstock D R. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: Integrin α5β1 as a novel CCN2 receptor. Gastroenterology 20051291019–1030. [DOI] [PubMed] [Google Scholar]
- 14.Ball D K, Moussad E E, Rageh M A.et al Establishment of a recombinant expression system for connective tissue growth factor (CTGF) that models CTGF processing in utero. Reproduction 2003125271–284. [DOI] [PubMed] [Google Scholar]
- 15.Ball D K, Rachfal A W, Kemper S A.et al The heparin‐binding 10 kDa fragment of connective tissue growth factor (CTGF) containing module 4 alone stimulates cell adhesion. J Endocrinol 2003176R1–R7. [DOI] [PubMed] [Google Scholar]
- 16.Brigstock D R, Steffen C L, Kim G Y.et al Purification and characterization of novel heparin‐binding growth factors in uterine secretory fluids. Identification as heparin‐ regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem 199727220275–20282. [DOI] [PubMed] [Google Scholar]
- 17.Ball D K, Surveyor G A, Diehl J R.et al Characterization of 16‐ to 20‐kilodalton (kDa) connective tissue growth factors (CTGFs) and demonstration of proteolytic activity for 38‐kDa CTGF in pig uterine luminal flushings. Biol Reprod 199859828–835. [DOI] [PubMed] [Google Scholar]
- 18.Tam E M, Morrison C J, Wu Y I.et al Membrane protease proteomics: Isotope‐coded affinity tag MS identification of undescribed MT1‐matrix metalloproteinase substrates. Proc Natl Acad Sci U S A 20041016917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto G, Inoki I, Fujii Y.et al Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 200227736288–36295. [DOI] [PubMed] [Google Scholar]
- 20.Phillips P A, McCarroll J A, Park S.et al Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 200352275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shek F W, Benyon R C, Walker F M.et al Expression of transforming growth factor‐beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 20021601787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kordes C, Brookmann S, Haussinger D.et al Differential and synergistic effects of platelet‐derived growth factor‐BB and transforming growth factor‐beta1 on activated pancreatic stellate cells. Pancreas 200531156–167. [DOI] [PubMed] [Google Scholar]
- 23.Hinton D R, Spee C, He S.et al Accumulation of NH(2)‐terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care 200427758–764. [DOI] [PubMed] [Google Scholar]
- 24.Roestenberg P, van Nieuwenhoven F A, Wieten L.et al Connective tissue growth factor is increased in plasma of type 1 diabetic patients with nephropathy. Diabetes Care 2004271164–1170. [DOI] [PubMed] [Google Scholar]
- 25.Dziadzio M, Usinger W, Leask A.et al N‐terminal connective tissue growth factor is a marker of the fibrotic phenotype in scleroderma. Q J Med 200598485–492. [DOI] [PubMed] [Google Scholar]
- 26.Shimoyama S, Gansauge F, Gansauge S.et al Altered expression of extracellular matrix molecules and their receptors in chronic pancreatitis and pancreatic adenocarcinoma in comparison with normal pancreas. Int J Pancreatol 199518227–234. [DOI] [PubMed] [Google Scholar]
- 27.Weinel R J, Rosendahl A, Neumann K.et al Expression and function of VLA‐alpha 2, ‐alpha 3, ‐alpha 5 and ‐alpha 6‐integrin receptors in pancreatic carcinoma. Int J Cancer 199252827–833. [DOI] [PubMed] [Google Scholar]
- 28.Gao R, Brigstock D R. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C‐terminal domain to integrin αvβ3 and heparan sulfate proteoglycan. J Biol Chem 20042798848–8855. [DOI] [PubMed] [Google Scholar]
- 29.Mould A P, Akiyama S K, Humphries M J. Regulation of integrin α5β1‐fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+. J Biol Chem 199527026270–26277. [DOI] [PubMed] [Google Scholar]
- 30.Johansson S, Svineng G, Wennerberg K.et al Fibronectin‐integrin interactions. Front Biosci 19972d126–d146. [DOI] [PubMed] [Google Scholar]
- 31.Schober J M, Lau L F, Ugarova T P.et al Identification of a novel integrin αMβ2 binding site in CCN1 (CYR61), a matricellular protein expressed in healing wounds and atherosclerotic lesions. J Biol Chem 200327825808–25815. [DOI] [PubMed] [Google Scholar]
- 32.Rusnati M, Tanghetti E, Dell'Era P.et al αvβ3 integrin mediates the cell‐adhesive capacity and biological activity of basic fibroblast growth factor (FGF‐2) in cultured endothelial cells. Mol Biol Cell 199782449–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao R, Brigstock D R. Low density lipoprotein receptor‐related protein (LRP) is a heparin‐dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res 200327214–220. [DOI] [PubMed] [Google Scholar]
- 34.Reeves H L, Dack C L, Day C P.et al β1‐integrin expression in hepatic stellate cells from normal and diseased human livers. In: Wisse E, Knook DL, Balabaud C, eds. Cells of the hepatic sinusoid: eighth international symposium on cells of the hepatic sinusoid; 1997 1997; Bordeaux, France. Leiden, the Netherlands: The Kupffer Cell Foundation, 1997154–155.
- 35.Richter H B, Franke H, Dargel R. Expression of tenascin, fibronectin, and laminin in rat liver fibrogenesis—a comparative immunohistochemical study with two models of liver injury. Exp Toxicol Pathol 199850315–322. [DOI] [PubMed] [Google Scholar]
- 36.Carloni V, Romanelli R G, Pinzani M.et al Expression and function of integrin receptors for collagen and laminin in cultured human hepatic stellate cells. Gastroenterology 19961101127–1136. [DOI] [PubMed] [Google Scholar]
- 37.Levine D, Rockey D C, Milner T A.et al Expression of the integrin α8β1 during pulmonary and hepatic fibrosis. Am J Pathol 20001561927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imai K, Sato T, Senoo H. Adhesion between cells and extracellular matrix with special reference to hepatic stellate cell adhesion to three‐dimensional collagen fibers. Cell Struct Funct 200025329–336. [DOI] [PubMed] [Google Scholar]
- 39.Kato R, Kamiya S, Ueki M.et al The fibronectin‐derived antiadhesive peptides suppress the myofibroblastic conversion of rat hepatic stellate cells. Exp Cell Res 200126554–63. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Murphy F R, Gehdu N.et al Engagement of αvβ3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem 200427923996–24006. [DOI] [PubMed] [Google Scholar]
- 41.Kotoh K, Nakamuta M, Kohjima M.et al Arg‐Gly‐Asp (RGD) peptide ameliorates carbon tetrachloride‐induced liver fibrosis via inhibition of collagen production and acceleration of collagenase activity. Int J Mol Med 2004141049–1053. [PubMed] [Google Scholar]
- 42.Blom I E, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol 200221473–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.