Short abstract
Use of an anti‐ghrelin Spiegelmer could be an innovative new approach to inhibit the biological actions of circulating ghrelin
Keywords: ghrelin, obesity, antagonism, NOX‐B11, food intake, rat
The article by Kobelt and colleagues1 in this issue of Gut, further explores a novel mechanism to interfere with the action of ghrelin on its receptor, growth hormone secretagogue receptor 1 (GHS‐R) (see page 788). Ghrelin is a 28 amino acid peptide localised immunocytochemically in parietal cells of the human stomach2 where it is released and stimulates growth hormone release, food intake, and adiposity. Human plasma has relatively low levels of ghrelin3 although, somewhat counter intuitively, in anorectic patients the fasting level of ghrelin, including the active form (n‐octanoyl modification at serine 3), is significantly higher.4 The main impact of this work is that the investigators have demonstrated the efficacy of and dose related inhibition of ghrelin stimulated food intake in rats. They did this by using a biological approach to bind to ghrelin and prevent the interaction of ghrelin with its receptor in vivo. These data support the results of an earlier study whereby the same approach inhibited ghrelin stimulated growth hormone release in vivo.5
Biological, as opposed to small molecule, manipulation of receptors and enzymes has gained acceptance in experimental basic science and clinically. Examples of these types of approaches are monoclonal antibodies, with high specificity and affinity for proteins, and antisense technology to prevent gene expression. Modulation of gene expression has shown promise in clinical trials—for example, antisense treatment to reduce a cellular adhesion molecule has been shown to be effective in inflammatory bowel disease.6 In this article by Kobelt and colleagues,1 the approach taken has been to use a single stranded oligonucleotide “mirror image” (that is, Spiegelmer), to inhibit the effects of exogenous ghrelin. Spiegelmers are cousins to aptamers, which are small nucleic acid molecules that directly inhibit proteins. These are selected from a nucleic acid library for their affinity to the target molecule and then amplified. However, aptamers are composed of the natural sugar moiety consisting of l‐ribose and are therefore rapidly broken down by nucleases. Spiegelmers differ from aptamers in that the sugar moiety is based on the mirror image, d‐ribose. This enhances the half life because they are more resistant to breakdown than the naturally occurring nucleic acids. The process of making Spiegelmers that have high binding affinity to ligands was pioneered in the late 1990s.7,8 Synthesis of a Spiegelmer involves first the synthesis of the enantiomer, in the case of peptides using the d‐ rather than l‐amino acid. Next, a large collection of nucleic acids is screened and an enhancement process (SELEX) is applied to identify an aptamer that binds to the non‐natural enantiomer. Then the selected l‐enantiomer of the aptamer is synthesised and the resulting Spiegelmers have high binding affinity to their ligands.
The Spiegelmer used in the present study, NOX‐B11, has an IC50 of 5 nM, calculated in an in vitro system using ghrelin to stimulate intracellular calcium release.5 The bioactive form of ghrelin has an n‐octanoyl modification at the serine‐3 and the NOX‐B11 binds to the N terminal portion at ghrelin preventing this modification at serine‐3.5 This is thought to explain its high affinity to, and inhibition of, bioactive ghrelin. However, similar to other biologicals, Spiegelmers must be administered intravenously because they are not orally bioavailable. To prolong the half life in plasma, the Spiegelmer is also PEGylated. This prolonged half life accounts for the dosing regimen in the report by Kobelt and colleagues1 where NOX‐B11 was given intravenously 12 hours prior to administration of the natural ligand ghrelin. However, pharmacokinetic data are not presented in this study at 12 hours after administration of the NOX‐B11 Spiegelmer to demonstrate sufficient plasma levels that would be expected to block exogenous ghrelin. This would be helpful to support a pharmacokinetic:pharmacodynamic correlation. Finally, although PEGylation is advantageous in terms of increasing the half life, it would be expected to be disadvantageous in terms of loss of potency. This is because the attachment of the large molecule and negative charge would be expected to interfere with binding. Thus it would be interesting to know the potency (IC50) for both the PEGylated form and Spiegelmer alone.
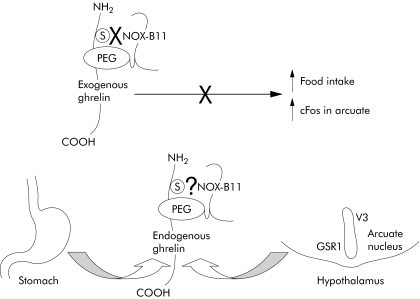
The data presented in fig 1 demonstrate that NOX‐B11 is effective in reversing ghrelin stimulated feeding in <4 hours to a cumulative intake similar to controls treated with vehicle/vehicle. However, the response to ghrelin alone in the control group was statistically significant only at the one hour period; subsequently the investigators speculated that there was a “rebound effect” such that feeding was lower in ghrelin treated animals. Thus only the efficacy of NOX‐B11 for one hour can be concluded from this feeding study due to the control group responses. Little evidence suggests that addition of NOX‐B11 inhibits the actions of endogenous ghrelin in this model or that it reduces basal short term food intake. There are several potential reasons for this that the investigators discuss, primary among them is the lack of ability to penetrate the CNS. The arcuate nucleus in the hypothalamus is thought to be a site of action of endogenous ghrelin to stimulate food intake. However, recent data suggest that arcuate neurones also synthesise ghrelin in addition to GHS‐R.9 Thus it is not surprising that a large PEGylated molecule would not inhibit endogenously released ghrelin expressed within the brain, although it would be expected to bind to circulating ghrelin. The relative importance of hypothalamic versus peripherally released ghrelin in the control of food intake needs to be investigated further. If locally released ghrelin within the hypothalamus is critical for regulation of food intake, in addition to peripherally released ghrelin, then this would limit the usefulness of agents that act primarily on ghrelin within the vascular compartment.
Figure 1 NOX‐B11 is a PEGylated ghrelin Spiegelmer that binds to the serine on the amino terminus of ghrelin. In the paper by Kobelt and colleagues,1 NOX‐B11 prevented both exogenous ghrelin induced increased food intake in the first hour and, in separate experiments, ghrelin evoked activation of neurones in the arcuate nucleus (presumably those that express the receptor growth hormone secretagogue receptor 1 (GSR1)). Endogenous ghrelin is released from the stomach and it is not known whether NOX‐B11 interferes with the activity of the circulating peptide to prevent its site of action in the hypothalamus. NOX‐B11 would not be expected to cross the blood‐brain barrier and inhibit the local release of ghrelin in the arcuate nucleus where it presumably acts nearby. Thus although these pharmacodynamic effects of NOX‐B11 in vivo are encouraging, the extent to which NOX‐B11 can modify disease states in which endogenous levels of ghrelin are elevated is not clear.
Aside from the caveat of its ability to reverse endogenous ghrelin, it is encouraging that there are dose related effects of 1, 10, and 30 nmol doses of NOX‐B11 on ghrelin stimulated food intake. However, the response was statistically significant only in the first hour after intraperitoneal administration of ghrelin. Therefore, reversal of this effect by NOX11B should be interpreted with caution in terms of a potential role in obesity for the Spiegelmer. Short term food intake changes may be only partially related to obesity and the work presented here does not address the adiposity or decreased fat utilisation that would be expected after ghrelin administration. Indeed, genetically modified mice which have the ghrelin gene ablated do not exhibit differences in food intake or obesity, suggesting that multiple redundant mechanisms exist.
The article by Kobelt and colleagues1 demonstrates that cfos is induced in neurones of the arcuate nucleus after systemic administration of ghrelin, which is consistent with an earlier report.10 A dose of 30 nmol NOX‐B11 prevented the expected increase in cfos positive cells in response to ghrelin. Although suggestive of a reversal of ghrelin stimulated cfos induction, the data should be interpreted with some caution as the dose administered was 10‐fold higher (30 nmol) than that used to reverse food intake. In addition, the non‐active l‐Spiegelmer was not included as a control, to demonstrate selectivity of the response, as it was in the food intake studies. As noted above, it is unlikely that inhibition of cfos induction is a direct effect of NOX‐B11 on the arcuate nucleus, even though this area is less protected by the blood‐brain barrier. This is due to the large size of the Spiegelmer.
The investigators conclude that the use of an anti‐ghrelin Spiegelmer could be an innovative new approach to inhibit the biological actions of circulating ghrelin. The day when Spiegelmers are used in therapy for disorders due to high levels of ghrelin, such as the Prader‐Willi syndrome, may be quite far away. Although the prolonged half life similar to other forms of biologics may make intravenous administration more palatable to patients, route of administration still remains a significant hurdle for patients without severe morbidity. Development of antibodies to biopolymer drugs may limit the duration of their usefulness and may prevent their utility although, other Spiegelmers, conjugated with bovine serum albumin and administered to rabbits over a six week period, resulted in much lower titres than that expected to true antigens. Although encouraging, the data have to be interpreted with caution in light of the immunogenic responses typical of other biological treatments in patients over a long duration. High costs would be expected in synthesising Spiegelmers, especially if there is reduced in vivo potency due to PEGylation. This could limit the viability of developing the therapy for an orphan indication, such as Prader‐Willi Syndrome, which has an incidence of 1:10 000–1:15 000. Finally, to consider the potential utility of NOX‐B11 to reduce food intake in obese patients who may have higher circulating levels of ghrelin is probably unlikely, due to the route of administration of the Spiegelmer. Although acceptance of biologicals as therapy for diseases that are not life threatening is shifting (for example, tumour necrosis factor α antibody therapy in psoriasis) it is not clear whether systemic administration of biologicals using this approach for obesity would become acceptable.
In conclusion, the data presented are intriguing and further support a role of ghrelin as a peripherally acting orexigenic agent. The utility of NOX‐B11 is evidenced by inhibition of the response to exogenous ghrelin in two separate experiments on different dependent variables, food intake and brain cfos levels. However, the ultimate test of utility of a potential “antagonist” is to demonstrate the role of the endogenous ligand in normal and pathological physiology. Thus although this represents a significant step forward as an experimental tool to inhibit exogenous ghrelin administration, a higher goal and challenge would be to demonstrate its ability to inhibit endogenous ghrelin.
Footnotes
Conflict of interest: None declared.
References
- 1.Kobelt P, Helmling S, Stengel A.et al Anti‐ghrelin Spiegelmer NOX‐B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut 200655788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka‐Shintani M, Watanabe M. Distribution of ghrelin‐immunoreactive cells in human gastric mucosa: comparison with that of parietal cells. J Gastroenterol 200540345–349. [DOI] [PubMed] [Google Scholar]
- 3.Rose K, Bougueleret L, Baussant T.et al Industrial‐scale proteomics: from liters of plasma to chemically synthesized proteins. Proteomics 200442125–2150. [DOI] [PubMed] [Google Scholar]
- 4.Nakai Y, Hosoda H, Nin K.et al Plasma levels of active form of ghrelin during oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol 2003149R1–R3. [DOI] [PubMed] [Google Scholar]
- 5.Helmling S, Maasch C, Eulberg D.et al Inhibition of ghrelin action in vitro and in vivo by an RNA‐Spiegelmer. Proc Natl Acad Sci U S A 200410113174–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanahan W R., Jr ISIS 2302, an antisense inhibitor of intercellular adhesion molecule 1. Expert Opin Investig Drugs 199981417–1429. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher T N, Mayr L M, Minor D L., Jret al Identification of D‐peptide ligands through mirror‐image phage display. Science 19962711854–1857. [DOI] [PubMed] [Google Scholar]
- 8.Klussmann S, Nolte A, Bald R.et al Mirror‐image RNA that binds D‐adenosine. Nat Biotechnol 1996141112–1115. [DOI] [PubMed] [Google Scholar]
- 9.Mondal M S, Date Y, Yamaguchi H.et al Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept 200512655–59. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Saint‐Pierre D H, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y ‐ synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 200232547–51. [DOI] [PubMed] [Google Scholar]