Abstract
Aim
Some retrospective studies have shown a lack of benefit of 5‐fluorouracil (5‐FU) adjuvant chemotherapy in patients with mismatch repair (MMR) deficient colorectal cancer. Our aim was to assess if this molecular marker can predict benefit from 5‐FU adjuvant chemotherapy. A second objective was to determine if MMR status influences short term survival.
Methods
We included 754 patients with a median follow up of 728.5 days (range 1–1097). A total of 260 patients with stage II or III tumours received 5‐FU adjuvant chemotherapy, according to standard clinical criteria and irrespective of their MMR status. A tumour was considered MMR deficient when either BAT‐26 showed instability or there was loss of MLH1 or MSH2 protein expression.
Results
At the end of the follow up period, 206 patients died and 120 presented with tumour recurrence. Sixty six (8.8%) patients had MMR deficient tumours. There were no significant differences in overall survival (MMR competent 72.1%; MMR deficient 78.8%; p = 0.3) or disease free survival (MMR competent 61.3%; MMR deficient 72.3%; p = 0.08). In patients with stage II and III tumours, benefit from 5‐FU adjuvant chemotherapy was restricted to patients with MMR competent tumours (overall survival: chemotherapy 87.1%; non‐chemotherapy 73.5%; log rank, p = 0.00001). Patients with MMR deficient tumours did not benefit from adjuvant chemotherapy (overall survival: chemotherapy 89.5%; non‐chemotherapy 82.4%; log rank, p = 0.4).
Conclusions
Benefit from 5‐FU adjuvant chemotherapy depends on the MMR status of tumours in patients with colorectal cancer. 5‐FU adjuvant chemotherapy improves survival in patients with MMR competent tumours but this benefit from chemotherapy cannot be extended to patients with MMR deficient tumours.
Keywords: colorectal cancer, mismatch repair system, prognosis, treatment, 5‐fluorouracil
Colorectal cancer is the third leading cause of cancer mortality in developed countries, after lung cancer in men and breast cancer in women. In Spain, colorectal cancer is responsible for approximately 3% of overall mortality.1 There are different pathogenic pathways leading to colorectal cancer development,2,3 and approximately 15% of colorectal tumours are caused by inactivation of DNA mismatch repair (MMR) genes such as MLH1, MSH2, MSH6, PMS2, or MSH3. These types of tumours display frameshift mutations and base pair substitutions in microsatellites,4,5 which are repetitive genetic loci (1–5 base pairs repeated 15–30 times). This form of genetic destabilisation is referred to as microsatellite instability (MSI). MMR deficiency is present in most cases of hereditary non‐polyposis colorectal cancer and approximately 15% of sporadic cases. In hereditary cases the cause is mutational inactivation of one of the MMR genes, while in sporadic cases the cause is epigenetic biallelic methylation of the promoter sequences of MLH1. Colon cancers with MMR deficiency have clinical and pathological characteristics that distinguish them from microsatellite stable tumours, they tend to be proximal to the splenic flexure, poorly differentiated, mucinous, show marked lymphocyte infiltration, and frequently have a larger size.5 Moreover, MMR deficient tumours seem to have a better prognosis,6,7,8,9 and although some studies have not found an association with prognosis in patients with MMR tumours, a recent systematic review provides evidences that confirms this relationship between MMR deficient tumours and better prognosis.8
To date, tumour stage at diagnosis has been the only parameter that helps in predicting benefit from adjuvant therapy. A clear benefit of 5‐fluorouracil (5‐FU) based chemotherapy has been shown in stage III tumours, and this benefit could be extended to IIb stage tumours.10 However, evidences from in vitro studies suggest that colon cancer cells with MMR deficiency may have a lesser response to chemotherapeutic agents, particularly to 5‐FU.11 Data from clinical retrospective studies suggest a lack of efficacy of 5‐FU based chemotherapy in patients with MMR deficient colorectal cancer.12,13,14 In these patients, 5‐FU adjuvant chemotherapy does not increase overall and disease free survival.14 In spite of this, no changes in clinical practice have been made according to these findings.
The aim of our study was to define the relationship between MMR status and benefit from adjuvant 5‐FU chemotherapy in colorectal cancer. A second objective was to evaluate whether MMR status influences three year overall survival and disease free survival. To address these issues, we designed a nested prospective investigation within the EPICOLON study, a nationwide multicentre study aimed at establishing the most effective and efficient strategy for identification of patients with hereditary non‐polyposis colorectal cancer.15
Patients and methods
Between November 2000 and October 2001, all newly diagnosed colorectal cancer patients in 25 hospitals were included in the EPICOLON study, a clinical epidemiology survey aimed at establishing the incidence of hereditary non‐polyposis colorectal cancer in Spain.15,16 Ten of these 25 centres agreed to participate in a nested prospective follow up investigation. Exclusion criteria were familial adenomatous polyposis, personal history of inflammatory bowel disease, and patient's refusal to participate in the study. The study was approved by the institutional ethics committee of each participating hospital, and written informed consent was obtained from all patients.
The integrity of the MMR system was evaluated by MSI testing and immunostaining for MSH2 and MLH1 proteins. Both tests were performed in all patients. To avoid variability in the quality of results, MSI testing and immunostaining were centralised in two separate centres. Tumour MMR deficiency was defined when either BAT‐26 showed MSI or immunohistochemistry showed loss of MLH1 or MSH2 protein expression. Analysis of germline mutations in both MLH1 and MSH2 genes was performed following a method described previously.15
Adjuvant chemotherapy was administered according to standard clinical criteria17,18,19,20 regardless of the MMR status of the tumours. Adjuvant chemotherapy with 5‐FU was given to patients with stage II and III tumours following standard schedules and doses. Oncologists that decided adjuvant treatment were blinded to MMR tumour status.
Tumour MSI analysis
Tissue samples from tumour and normal colonic mucosa were obtained from each patient and DNA was extracted from them. In those cases in which fresh tissue was not available, archival formalin fixed, paraffin embedded samples were used. Genomic DNA was isolated using the QiaAmp Tissue Kit (Qiagen, Courtaboeuf, France).
MSI status was assessed using the BAT‐26 mononucleotide marker, based on its high sensitivity as a marker for tumour instability.21,22,23,24 Primers were fluorescently labelled and analysed on an ABI 310 Genetic Analyser using GeneScan Analysis software (Applied Biosystems, Foster City, California, USA).
Tumour MSH2 and MLH1 protein expression
One block of formalin fixed, paraffin embedded tumour tissue was selected per case. Before immunostaining, antigen retrieval was performed by immersing sections in a 10 mmol/l concentration of citrate buffer, pH 6.0, and boiling in a pressure cooker for five minutes. Sections were then incubated for 20 minutes at room temperature with mouse monoclonal antibodies against MLH1 protein (clone G168‐15, dilution 1:40; PharMingen, San Diego, California, USA) and MSH2 protein (clone FE11, dilution 1:35; Oncogene Research Products, Boston, Massachusetts, USA). Ultra‐Vision streptavidin‐biotin peroxidase detection kit (Dako, Carpinteria, California, USA) was used as secondary detection system. The peroxidase reaction was developed using diaminobenzidine tetrachloride as chromogen. Tumour cells were judged to be negative for protein expression only if they lacked staining in a sample in which normal colonocytes and stroma cells were stained. If no immunostaining of normal tissue could be demonstrated, the results were considered ambiguous.
Statistical analysis
Continuous variables are reported as mean (SD) and categorical variables as frequency or percentages. Statistical differences of basal characteristics between groups were analysed using the χ2 test for categorical data applying Yates' correction when required and the Mann‐Whitney U test for quantitative data.
The primary outcomes were overall survival and disease free survival. Both analyses were performed in the whole series as well as in the subset of patients with stage II and III tumours to specifically evaluate the effect of MMR status on the benefit from adjuvant chemotherapy. Overall survival was defined as the time from study entry to death and disease free survival was defined as the time from study entry to death from any cause or the first relapse. Data on overall and disease free survival were censored at 1100 days from the date of diagnosis. Survival curves were generated according to the Kaplan‐Meier method and univariate survival distributions were compared with the use of the log rank test. Intervals of confidence were calculated using the standard error of survival calculated according to the Greenwood method.
A multivariant analysis of hazard risk of death or tumour recurrence, adjusted for tumour, node, metastases (TNM) stage of disease, age, and sex, was performed using Cox proportional hazards regression in a stepwise manner to test the effect of MSI, adjuvant chemotherapy, and interaction term between MMR status and chemotherapy. Hazard ratios and 95% confidence interval (95% CI) for death were computed using Cox survival modelling.
All reported p values are two sided, and p values of less than 0.05 were considered to indicate significance. All calculations were performed using SPSS 10.0 software.
Results
Influence of mismatch repair status on colorectal cancer prognosis
We followed 754 patients. Median follow up was 728.5 days (range 1–1097). Sixteen patients (2.1%) were not operated on due to advanced disease. The rest were surgically treated. Characteristics of the patients at diagnosis, according to MMR status, are shown in table 1. Seven patients had germline mutations in MLH1 (n = 5) or MSH2 (n = 2) genes. Thirty two patients (4.2%) were lost to follow up but they were not excluded from the study and their data were included in the survival analysis until the date of loss. At the end of the follow up period, 206 patients had died (27.3%), with a median of 300 days after inclusion (range 1–953). Twenty patients died in the postoperative period (the first 30 days post‐surgery) and 135 patients died due to tumour progression. Causes of death of the remaining patients were: late post‐surgical complications (12 patients), complications of chemotherapy (10 patients), and other causes (26 patients). Tumour recurrence was seen in 120 (15.9%) patients, at a mean of 438.1 (215.1) days after surgery (median 420.5 (range 23–1015)). Of the 754 patients, 66 (8.8%) had tumours with MMR deficiency.
Table 1 Characteristics of the patients.
| MMR competent (n = 688) | MMR deficient (n = 66) | p Value | |
|---|---|---|---|
| Age (y) (mean (SD) | 70.0 (11.1) | 68.8 (14.5) | 0.4 |
| Sex (n (%)) | 0.0001 | ||
| Males | 427 (62) | 22 (34) | |
| Females | 261 (38) | 44 (66) | |
| TNM (n (%)) | 0.06 | ||
| Stage I | 99 (14) | 4 (6) | |
| Stage II | 261 (38) | 35 (53) | |
| Stage III | 191 (28) | 18 (27) | |
| Stage IV | 137 (20) | 9 (14) | |
| Follow up (days) (mean) | 610 | 599 | 0.8 |
| Chemotherapy (n (%)) | 330 (48) | 23 (35) | 0.04 |
| Vital status (n (%)) | |||
| Dead | 192 (28) | 14 (21) | 0.3 |
| Recurrence (n (%)) | 113 (20) | 7 (12) | 0.1 |
p Values were calculated using the χ2 test.
MMR, mismatch repair; TNM, tumour, node, metastases.
Chemotherapy was given to 353 patients; 257 (72.8%) received adjuvant chemotherapy, 24 (6.8%) received neoadjuvant chemotherapy, and 72 (20.4%) palliative chemotherapy. Chemotherapy was 5‐FU based in 320 of these patients (90.6%).
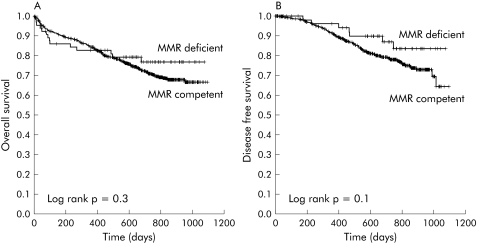
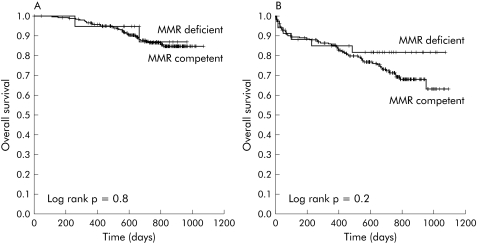
In a pooled analysis not adjusted for the chemotherapy treatment received, there were no differences in overall survival rate (MMR competent 72.1%; MMR deficient 78.8%; χ2, p = 0.3) or disease free survival at the end of follow up (MMR competent 61.3%; MMR deficient 72.3%; χ2, p = 0.08) between patients with MMR competent and MMR deficient tumours (fig 1A, 1B). This lack of difference in survival and disease free survival remained invariable when we excluded patients who died in the postoperative period (data not shown). We did not find differences in overall survival or disease free survival according to MMR status for any of the TNM stages (table 2). In addition, interaction between MMR status and TNM stage, calculated using a Cox proportion hazard model multivariate analysis, did not achieve statistical significance for overall survival (1.27 (95% CI 0.30–5.43); p = 0.743) or disease free survival (1.01 (95% CI 0.31–3.35); p = 0.984).
Figure 1 Overall survival (A) and disease free survival (B) in the whole series of mismatch repair (MMR) deficient and competent tumours.
Table 2 Overall survival and disease free survival according to tumour, node, metastases (TNM) stage.
| MMR competent | MMR deficient | p Value | |
|---|---|---|---|
| Stage I | |||
| Overall survival | 92/99 (93%) | 4/4 (100%) | 0.6 |
| Disease free survival | 82/99 (83%) | 4/4 (100%) | 0.3 |
| Stage II | |||
| Overall survival | 222/261 (85%) | 31/35 (89%) | 0.6 |
| Disease free survival | 196/261 (75%) | 28/35 (80%) | 0.5 |
| Stage III | |||
| Overall survival | 143/191 (75%) | 14/18 (78%) | 0.8 |
| Disease free survival | 117/191 (61%) | 13/18 (73%) | 0.4 |
| Stage IV | |||
| Overall survival | 39/137 (28%) | 3/137 (33%) | 0.7 |
p values were calculated using the log rank value.
MMR, mismatch repair.
Influence of MMR status on prognosis of patients with locally advanced non‐metastatic colorectal cancer
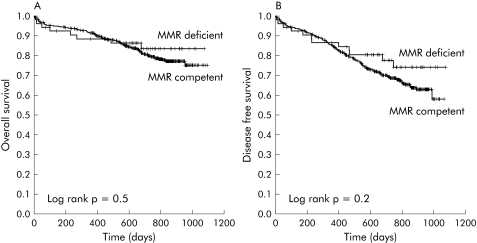
We followed 505 patients with stage II or III disease. Median follow up time was 721 days (range 1–1096). Characteristics of patients according to MMR status can be seen in table 3. Eighteen patients (3.6%) were lost to follow up. At the end of follow up, 95 (18.8%) patients had died and 110 (21.8%) had tumour recurrence. A total of 125 patients with stage II (42.2%) and 135 patients with stage III (64.6%) disease received chemotherapy (p = 0.0001). Patients who received chemotherapy were younger (65.5 (10.2) v 76.1 (9.3) years old; p = 0.0001) and there were no differences regarding sex, days of follow up, or percentage of patients lost to follow up between patients who received adjuvant chemotherapy and patients who did not. Adjuvant chemotherapy was more frequently administered to stage II and stage III cancer patients with MMR competent colorectal tumours (stage II treated patients: MMR competent 44%; MMR deficient 29% (p = 0.08); stage III treated patients: MMR competent 66%; MMR deficient 50%; p = 0.2), albeit the indication for treatment was decided by physicians blind to the MMR status of the tumours (table 3). There were no differences in survival or disease free survival between stage II and III patients according to MMR status (table 3; fig 2A, 2B). In stage II or III, adjuvant chemotherapy was based on 5‐FU in all but nine patients. In these nine patients, 5‐FU was contraindicated due to a previous thrombosis event. All nine patients had stable tumours and were excluded from the benefit of 5‐FU chemotherapy analysis.
Table 3 Characteristics of stage II and III patients with mismatch repair (MMR) competent versus MMR deficient tumours.
| MMR competent (n = 452) | MMR deficient (n = 53) | p Value | |
|---|---|---|---|
| Age (y) | 70.8 (10.7) | 69.2 (14.2) | 0.3 |
| Sex (n (%)) | 0.02 | ||
| Males | 267 (59) | 19 (36) | |
| Females | 185 (41) | 34 (64) | |
| Adjuvant 5‐FU chemotherapy (%) | 241 (53) | 19 (36) | 0.02 |
| Vital status (n (%)) | |||
| Dead | 87 (19) | 8 (15) | 0.4 |
| Recurrence (n (%)) | 103 (23) | 7 (13) | 0.4 |
| Survival (days) (95% CI) | 940 (910–970) | 940 (853–1028) | 0.5 |
| Disease free survival (days) (95% CI) | 884 (854–915) | 970 (897–1042) | 0.1 |
p values were calculated using the log rank value.
MMR, mismatch repair; 5‐FU, 5‐fluorouracil; 95% CI, 95% confidence interval.
Figure 2 Overall survival (A) and disease free survival (B) in patients with stage II and III disease, according to mismatch repair (MMR) status (deficient or competent).
Patients treated with adjuvant chemotherapy showed better overall survival (chemotherapy 999 days (95% CI 972–1026); non‐chemotherapy 865 days (95% CI 819–915); p = 0.0001) and disease free survival (chemotherapy 861 days (95% CI 830–901); non‐chemotherapy 779 days (95% CI 727–831); p = 0.003) (table 4). These differences were significant in both stage II and III disease (data not shown).
Table 4 Univariate survival analysis according to treatment received.
| No | Probability of survival (95% CI) | p Value | Probability of disease free survival (95% CI) | p Value | |
|---|---|---|---|---|---|
| All patients | 505 | ||||
| Adjuvant CT | 260 | 87.3 (83.2–91.4) | 0.0001 | 73.8 (68.3–79.3) | 0.0025 |
| Non adjuvant CT | 245 | 74.7 (69.1–80.3) | 66.1 (60.1–72.1) | ||
| MMR competent | 452 | ||||
| Adjuvant CT | 241 | 87.1 (82.8–91.4) | 0.0001 | 73.9 (68.2–79.6) | 0.0004 |
| Non adjuvant CT | 211 | 73.5 (67.4–79.6) | 64.0 (57.4–70.6) | ||
| MMR deficient | 53 | ||||
| Adjuvant CT | 19 | 89.5 (75.4–103.6) | 0.4 | 73.7 (53.5–93.9) | |
| Non‐adjuvant CT | 34 | 82.4 (69.3–95.5) | 79.4 (65.5–93.3) | 0.9 |
p values were calculated using the χ2 test.
95% CI, 95% confidence interval; CT, chemotherapy; MMR, mismatch repair.
Effect of MMR status on survival according to chemotherapy treatment
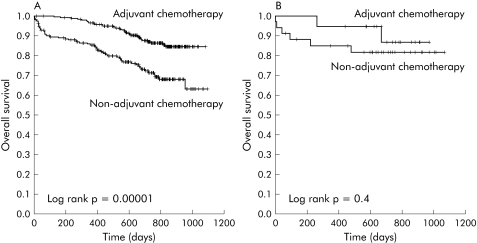
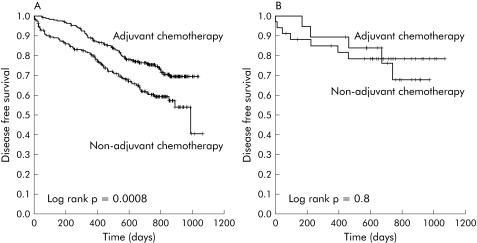
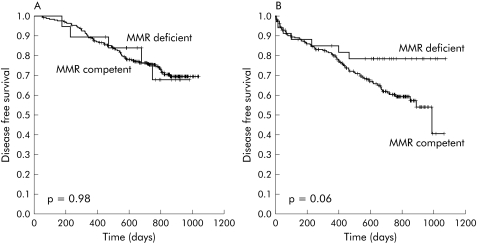
Patients with MMR competent tumours who received adjuvant chemotherapy had a higher probability of survival (adjuvant chemotherapy 87.1 % (95% CI 82.8–91.4); non‐adjuvant chemotherapy 73.5% (95% CI 67.4–79.6); p = 0.0001) and disease free survival (adjuvant chemotherapy 73.9 % (95% CI 68.2–79.6); non‐adjuvant chemotherapy 64.0% (95% CI 57.4–70.6); p = 0.0004) (table 4; figs 3A, 4A). In contrast, adjuvant chemotherapy did not improve the probability of survival (adjuvant chemotherapy 89.5% (95% CI 75.4–103.6); non‐adjuvant chemotherapy 82.4% (95% CI 69.3–95.5); p = 0.4) or the probability of disease free survival (adjuvant chemotherapy 73.7 % (95% CI 53.5–93.9); non‐adjuvant chemotherapy 79.4% (95% CI 65.5–93.3); p = 0.9) in MMR deficient patients (table 4; figs 3B, 4B). These trends were consistently maintained in subgroup analysis according to disease stage. Thus treatment was not associated with better overall survival or disease free survival in stage II or stage III patients with MMR deficient tumours.
Figure 3 Overall survival of patients with mismatch repair (MMR) competent tumours (A) or MMR deficient tumours (B) regarding treatment received.
Figure 4 Disease free survival of patients with mismatch repair (MMR) competent tumours (A) or MMR deficient tumours (B) according to the treatment received.
Effect of chemotherapy on survival according to MMR status
Patients who received adjuvant chemotherapy showed the same probability of survival (MMR competent 87.1% (95% CI 82.8–91.4); MMR deficient 89.5% (95% CI 75.4–103.6); p = 0.8) and disease free survival (MMR competent 73.9% (95% CI 68.2–79.6); MMR deficient 73.7% (95% CI 53.5–93.9); p = 0.8) independently of their MMR status (table 5; figs 5A, 6A). In patients who did not receive adjuvant chemotherapy, the probability of survival (MMR competent 73.5% (95% CI 67.4–79.6); MMR deficient 82.4% (95% CI 69.3–95.5); p = 0.3) and disease free survival (MMR competent 64.0% (95% CI 57.4–70.6); MMR deficient 79.4% (95% CI 65.5–93.3); p = 0.06) were better when tumours were MMR deficient, but the differences did not reach statistical significance (table 5; figs 5B, 6B).
Table 5 Univariate survival analysis according to microsatellite status.
| n | Probability of survival (95% CI) | p Value | Probability of disease free survival (95% CI) | p Value | |
|---|---|---|---|---|---|
| All patients | 505 | ||||
| MMR competent | 452 | 80.7 (77.0–84.4) | 0.5 | 69.2 (64.9–73.5) | 0.2 |
| MMR deficient | 53 | 84.9 (75.1–94.7) | 77.3 (65.8–88.8) | ||
| Chemotherapy | 260 | ||||
| MMR competent | 241 | 87.1 (82.8–91.4) | 0.8 | 73.9 (68.2–79.6) | 0.8 |
| MMR deficient | 19 | 89.5 (75.4–103.6) | 73.7 (53.5–93.9) | ||
| Non‐chemotherapy | 245 | ||||
| MMR competent | 211 | 73.5 (67.4–79.6) | 0.3 | 64.0 (57.4–70.6) | 0.06 |
| MMR deficient | 34 | 82.4 (69.3–95.5) | 79.4 (65.5–93.3) |
p values were calculated using the χ2 test.
95% CI, 95% confidence interval; CT, chemotherapy; MMR, mismatch repair.
Figure 5 Overall survival of patients that received (A) or did not receive (B) adjuvant chemotherapy, according to their mismatch repair (MMR) status (deficient or competent).
Figure 6 Disease free survival of patients that received (A) or did not receive (B) adjuvant chemotherapy, according to their mismatch repair (MMR) status (deficient or competent).
Interaction between MMR status and chemotherapy calculated in a Cox proportion hazard model multivariate analysis, adjusted by sex, age, and TNM stage was statistically significant, thus suggesting differences between the MMR groups regarding chemotherapy effectiveness (table 6).
Table 6 Multivariate analysis of overall survival and disease free survival in patients with locally advanced non‐metastatic colorectal cancer.
| Overall survival (Hazard ratio* (95% CI)) | p Value | Disease free survival (Hazard ratio* (95% CI)) | p Value | |
|---|---|---|---|---|
| MMR status (deficient/competent) | 1.02 (0.49–2.13) | 0.961 | 1.22 (0.67–2.22) | 0.508 |
| Adjuvant chemotherapy (yes/no) | 1.88 (1.13–3.12) | 0.015 | 1.54 (1.04–2.29) | 0.031 |
| MMR status×chemotherapy | 1.81 (1.13–2.92) | 0.014 | 1.68 (1.15–2.44) | 0.007 |
*Adjusted for age, sex, and tumour, node, metastases (TNM) stage (Cox proportional hazards regression).
95% CI, 95% confidence interval; MMR, mismatch repair.
Discussion
In this prospective study, we showed that MMR status in colorectal cancer may predict adjuvant chemotherapy response. Thus while in general, patients with stage II or III disease who received 5‐FU based adjuvant chemotherapy had a better overall survival and disease free survival, this benefit was found only in patients with MMR competent tumours. Patients with MMR deficient tumours did not have a better survival or disease free survival when they received 5‐FU adjuvant chemotherapy. The results remained unchanged when we stratified according to TNM stage. Conversely, when we analysed the efficacy of treatment received according to MMR status, we found that patients with MMR deficient tumours who did not receive adjuvant chemotherapy had a slightly better survival and disease free survival than patients with MMR competent tumours, even though the analysis failed to reach statistical significance.
Several studies have evaluated the benefit of adjuvant chemotherapy according to MMR status of colorectal tumours. In vitro studies have shown that DNA MMR deficiency might be responsible for tumour resistance to 5‐FU, and MMR deficient colon cancer cells are not as readily killed by this agent as mismatch competent cells.11,25 This is probably due to the fact that chemosensitivity may be related to 5‐FU incorporation into tumour DNA by MMR proteins.26 Several clinical studies have also addressed this issue. While some did not find any differences in chemotherapy response between patients with MMR deficient and MMR competent tumours,12,27,28 others found a better prognosis in patients with MMR deficient tumours that received adjuvant chemotherapy.29,30 Potential drawbacks of these studies could be related to patient selection and the nature of the comparisons performed. The study of Elsaleh and colleagues29 showed a significant difference in mortality between patients with MSI and microsatellite stable tumours. The study was a retrospectively selected series of patients with stage III colon cancer diagnosed over a period of nine years, probably with important differences in clinical management over time. Hemminki and colleagues30 did not take into account patients who did not receive adjuvant chemotherapy, and the good prognosis showed in treated patients could be related to the intrinsic good prognosis of MSI tumours. Those studies did not investigate if MMR status could predict adjuvant chemotherapy benefit. Three studies have shown data suitable for examining the benefit of adjuvant chemotherapy in stage II or III colorectal cancer according to MMR status of the tumours.12,13,14 Two of these showed the benefit of chemotherapy in patients with microsatellite stable tumours and no benefit in patients with MSI tumours13,14; the other study did not show any significant differences.12 A pooled analysis,8 that included the studies of Ribic and colleagues14 and Barrat and colleagues,12 showed a lack of benefit from adjuvant 5‐FU in patients with MMR deficient colorectal cancer.
The hypothesis that patients with MMR deficient tumours could have a different prognosis is clearly plausible given the significant biological differences between MMR deficient and MMR competent tumours. Different studies addressing this issue have shown contradictory results but we should bear in mind that the number of reported tumours was rather small in most of them. A recent systematic review that pooled the results of published studies showed that MMR deficient tumours have a better prognosis, regardless of tumour stage.8 This improvement in prognosis has been shown in both hereditary and sporadic MMR deficient tumours.6,9,28,31,32,33 It is not known if this better prognosis is due to the intrinsic carcinogenic features of these tumours that confers a less aggressive pattern of growth or if it is due to a better response to chemotherapy agents. In our cohort, patients with colorectal cancer exhibiting MMR defects showed the same rate of overall survival and disease free survival as patients with stable tumours. This is probably due to the short follow up period reported (24 months). The study is still ongoing and we should eventually be able to report on a longer follow up. In contrast with other reports that suggest more advanced stages at diagnosis in MMR deficient colorectal tumours,6,9,28 we saw no differences between patients with stable or unstable colorectal cancer.
In summary, our prospective study confirms previous retrospective reports suggesting that adjuvant 5‐FU based chemotherapy may not be useful in stage II and III MMR deficient colorectal cancer and a revision in the management of this subgroup should be reconsidered. Thus stage II patients with MMR deficient tumours should probably be treated only with surgery due to their excellent prognosis and lack of efficacy of 5‐FU chemotherapy. In stage III patients with MMR deficient tumours, other chemotherapeutic agents should be tested. The topoisomerase I inhibitor irinotecan could be one such agent, considering that it has been shown to be effective in MMR deficient colon cancer cells.34 Selection of colorectal cancer patients for 5‐FU treatment will have to be based on the biology of the tumour, in addition to its stage. Our study supports the importance of the molecular characteristics of tumours in the tailoring of adjuvant therapy. These results support the concept that MSI analysis or immunohistochemistry should be routinely conducted in order to design rational adjuvant chemotherapy for colorectal cancer.
Abbreviations
MMR - mismatch repair
5‐FU - 5‐fluorouracil
MSI - microsatellite instability
TNM - tumour, node, metastases
Appendix
STUDY ORGANISATION AND INVESTIGATORS FROM THE GASTROINTESTINAL ONCOLOGY GROUP OF THE SPANISH GASTROENTEROLOGICAL ASSOCIATION WHO PARTICIPATED IN THE STUDY
All participants listed below were fully involved in the study:
Hospital 12 de Octubre, Madrid: Juan Diego Morillas (local coordinator), Raquel Muñoz, Marisa Manzano, Francisco Colina, Jose Díaz, Carolina Ibarrola, Guadalupe López, Alberto Ibáñez; Hospital Clínic, Barcelona: Antoni Castells (local coordinator), Virgínia Piñol, Sergi Castellví‐Bel, Francisco Rodríguez‐Moranta, Francesc Balaguer, Antonio Soriano, Rosa Cuadrado, Maria Pellisé, Rosa Miquel, J Ignasi Elizalde, Josep M. Piqué; Hospital Cristal‐Piñor, Complexo Hospitalario de Ourense: Joaquin Cubiella (local coordinator), Mª Soledad Díez, Mercedes Salgado, Eloy Sánchez, Mariano Vega; Hospital del Mar, Barcelona: Montserrat Andreu (local coordinator), Xavier Bessa, Agustín Panadés, Asumpta Munné, Felipe Bory, Miguel Nieto, Agustín Seoane; Hospital Donosti, San Sebastián: Luis Bujanda (local coordinator), Juan Ignacio Arenas, Isabel Montalvo, Julio Torrado, Ángel Cosme; Hospital General Universitario de Alicante: Rodrigo Jover (local coordinator), Artemio Payá , Juan Carlos Penalva, Cristina Alenda, Mª José Poveda, Bartomeu Massuti, Ana L Yuste; Complejo Hospitalario Universitario, Vigo: Juan Clofent (local coordinator), Jaime Seoane, Antoni Tardío, Eugenia Sanchez; Hospital Universitari Germans Trias i Pujol, Badalona: Xavier Llor (local coordinator), Rosa M Xicola, Marta Piñol, Mercè Rosinach, Anna Roca, Elisenda Pons, José M. Hernández, Miquel A Gassull; Hospital Universitari Arnau de Vilanova, Lleida: Josep M Reñé (local coordinator), Carmen Piñol, Juan Buenestado, Joan Viñas; Hospital Universitario de Canarias: Enrique Quintero (local coordinator), David Nicolás, Adolfo Parra, Antonio Martín.
Footnotes
This work was supported by grants from the Fondo de Investigación Sanitaria (FIS 01/0104) and from the Instituto de Salud Carlos III (RC03/02 and RC03/10). Xavier Llor is a recipient of a Ramon y Cajal grant from Ministerio de Ciencia y Tecnología of the Spanish government. Virgínia Piñol received a research grant from the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS).
Conflict of interest: None declared.
This work was presented in part as a poster at the 2004 Digestive Diseases Week meeting, New Orleans, LA, USA.
References
- 1.Paz‐Valinas L, Atienza M G. Population screening for colorectal cancer: a systematic review. Gastroenterol Hepatol 200427450–459. [DOI] [PubMed] [Google Scholar]
- 2.Lengauer C, Kinzler K W, Vogelstein B. Genetic instabilities in human cancers. Nature 1998396643–649. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Fearon E R, Hamilton S R.et al Genetic alterations during colorectal‐tumor development. N Engl J Med 1988319525–532. [DOI] [PubMed] [Google Scholar]
- 4.Thibodeau S N, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993260816–819. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler J M, Bodmer W F, Mortensen N J. DNA mismatch repair genes and colorectal cancer. Gut 200047148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gryfe R, Kim H, Hsieh E T.et al Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 200034269–77. [DOI] [PubMed] [Google Scholar]
- 7.Parc Y, Gueroult S, Mourra N.et al Prognostic significance of microsatellite instability determined by immunohistochemical staining of MSH2 and MLH1 in sporadic T3N0M0 colon cancer. Gut 200453371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popat S, Hubner R, Houlston R S. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 200523609–618. [DOI] [PubMed] [Google Scholar]
- 9.Samowitz W S, Curtin K, Ma K N.et al Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev 200110917–923. [PubMed] [Google Scholar]
- 10.Clark J W, Willet C ‐ G. Chemotherapy and radiation therapy of colorectal cancer. In: Rustgi AK, ed. Gastrointestinal cancers. Philadelphia: Saunders, 2003453–472.
- 11.Carethers J M, Chauhan D P, Fink D.et al Mismatch repair proficiency and in vitro response to 5‐fluorouracil. Gastroenterology 1999117123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barratt P L, Seymour M T, Stenning S P.et al DNA markers predicting benefit from adjuvant fluorouracil in patients with colon cancer: a molecular study. Lancet 20023601381–1391. [DOI] [PubMed] [Google Scholar]
- 13.Carethers J M, Smith E J, Behling C A.et al Use of 5‐fluorouracil and survival in patients with microsatellite‐unstable colorectal cancer. Gastroenterology 2004126394–401. [DOI] [PubMed] [Google Scholar]
- 14.Ribic C M, Sargent D J, Moore M J.et al Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003349247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinol V, Castells A, Andreu M.et al Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 20052931986–1994. [DOI] [PubMed] [Google Scholar]
- 16.Pinol V, Andreu M, Castells A.et al Frequency of hereditary non‐polyposis colorectal cancer and other colorectal cancer familial forms in Spain: a multicentre, prospective, nationwide study. Eur J Gastroenterol Hepatol 20041639–45. [DOI] [PubMed] [Google Scholar]
- 17.Moertel C G, Fleming T R, Macdonald J S.et al Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990322352–358. [DOI] [PubMed] [Google Scholar]
- 18.Moertel C G, Fleming T R, Macdonald J S.et al Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995122321–326. [DOI] [PubMed] [Google Scholar]
- 19.Wolmark N, Rockette H, Fisher B.et al The benefit of leucovorin‐modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C‐03. J Clin Oncol 1993111879–1887. [DOI] [PubMed] [Google Scholar]
- 20.Wolmark N, Rockette H, Mamounas E.et al Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C‐04. J Clin Oncol 1999173553–3559. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez‐Garcia I, Moreno V, Navarro M.et al Standardized approach for microsatellite instability detection in colorectal carcinomas. J Natl Cancer Inst 200092544–549. [DOI] [PubMed] [Google Scholar]
- 22.Hoang J M, Cottu P H, Thuille B.et al BAT‐26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res 199757300–303. [PubMed] [Google Scholar]
- 23.Loukola A, Eklin K, Laiho P.et al Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res 2001614545–4549. [PubMed] [Google Scholar]
- 24.Zhou X P, Hoang J M, Li Y J.et al Determination of the replication error phenotype in human tumors without the requirement for matching normal DNA by analysis of mononucleotide repeat microsatellites. Genes Chromosomes Cancer 199821101–107. [DOI] [PubMed] [Google Scholar]
- 25.Aebi S, Fink D, Gordon R.et al Resistance to cytotoxic drugs in DNA mismatch repair‐deficient cells. Clin Cancer Res 199731763–1767. [PubMed] [Google Scholar]
- 26.Tajima A, Hess M T, Cabrera B L.et al The mismatch repair complex hMutS alpha recognizes 5‐fluorouracil‐modified DNA: implications for chemosensitivity and resistance. Gastroenterology 20041271678–1684. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Wu T T, Catalano P J.et al Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 20013441196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halling K C, French A J, McDonnell S K.et al Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999911295–1303. [DOI] [PubMed] [Google Scholar]
- 29.Elsaleh H, Joseph D, Grieu F.et al Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 20003551745–1750. [DOI] [PubMed] [Google Scholar]
- 30.Hemminki A, Mecklin J P, Jarvinen H.et al Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000119921–928. [DOI] [PubMed] [Google Scholar]
- 31.Percesepe A, Benatti P, Roncucci L.et al Survival analysis in families affected by hereditary non‐polyposis colorectal cancer. Int J Cancer 199771373–376. [DOI] [PubMed] [Google Scholar]
- 32.Sankila R, Aaltonen L A, Jarvinen H J.et al Better survival rates in patients with MLH1‐associated hereditary colorectal cancer. Gastroenterology 1996110682–687. [DOI] [PubMed] [Google Scholar]
- 33.Wright C M, Dent O F, Barker M.et al Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg 2000871197–1202. [DOI] [PubMed] [Google Scholar]
- 34.Bras‐Goncalves R A, Rosty C, Laurent‐Puig P.et al Sensitivity to CPT‐11 of xenografted human colorectal cancers as a function of microsatellite instability and p53 status. Br J Cancer 200082913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]