Abstract
Aims
Macrophage migration inhibitory factor (MIF) is implicated in tumorigenesis. This study was conducted to determine whether MIF expression is associated with gastric pathology and whether MIF expression is increased in malignant gastric cells in vitro.
Materials and methods
Patients with a normal gastric mucosa, Helicobacter pylori infected gastritis, intestinal metaplasia, and gastric adenocarcinoma were included. Immunohistochemistry and enzyme linked immunosorbent assay (ELISA) were used to determine MIF expression in gastric epithelial cells and MIF levels in serum, respectively. Five gastric cancer cell lines (AGS, MKN‐45, MKN‐28, MGC‐803, and SGC‐7901) and one non‐malignant gastric cell line (GES‐1) were cultured for 24 hours. MIF protein in the supernatant and MIF mRNA in cultured cells were measured by ELISA and reverse transcription‐polymerase chain reaction, respectively.
Results
The percentage of MIF expressing epithelial cells was low in normal mucosa (12%) but substantially higher in gastritis (52%), intestinal metaplasia (66%), and gastric cancer (96%) (p<0.001, ANOVA). Serum MIF levels were low in patients with a normal mucosa (576 (82) pg/ml) but higher in patients with gastritis (2100 (349) pg/ml), intestinal metaplasia (4498 (253) pg/ml), and gastric cancer (9737 (1249) pg/ml) (p<0.001, ANOVA). There was a correlation between epithelial MIF expression and serum MIF levels (r = 0.776, p<0.001). In vitro, expression of MIF protein and mRNA was increased in malignant cells compared with non‐malignant cells.
Conclusions
Epithelial and serum MIF expression was progressively increased in H pylori induced gastritis, intestinal metaplasia, and gastric cancer, suggesting that MIF is involved in gastric carcinogenesis and may be a valuable biomarker for the early detection of gastric cancer.
Keywords: macrophage migration inhibitory factor, Helicobacter pylori infection, intestinal metaplasia, gastric cancer
Gastric cancer is second to lung cancer as a leading cause of cancer deaths worldwide, accounting for 12% of total cancer deaths.1 Among the multiple risk factors leading to the occurrence of gastric cancer, gastric inflammation induced by Helicobacter pylori infection is the foremost important aetiological cause of this malignant disease.2,3H pylori causes gastric mucosal damage through various mechanisms, such as producing virulence factors, promoting inflammatory and immune responses, and releasing chemokines, cytokines, and reactive oxygen species.4 Potential mechanisms linking H pylori induced inflammation with gastric cancer include release of bacterial virulence factors, loss of p53 function, and reactivation of telomerase.5
Among the factors involved in gastric inflammation, macrophage migration inhibitory factor (MIF), a 12.5 kDa cytokine, has increasingly been recognised for its proinflammatory properties in the inflammatory process.6,7 Although identified four decades ago, the biological role and function of MIF have remained elusive until recent identification of the three dimensional structure of this molecule.6 The primary function of MIF is to inhibit the migration of macrophages, a crucial mediator of chronic inflammation that releases numerous bioactive products of cytokines, growth factors, and nitric oxides.3,7 To date, MIF is known for its multiple functions, including catalytic activities, lymphocyte immunity, endocrine functions, signal modulation, and proinflammatory ability.7
A substantial increase in MIF expression has been observed in H pylori induced gastritis. Xia et al reported that the percentage of MIF+ epithelial cells and numbers of MIF mRNA+ inflammatory cells, MIF+ T cells, and MIF+ macrophages were significantly higher in H pylori positive patients with gastritis than in H pylori negative patients, suggesting that MIF plays a significant role in H pylori induced gastric inflammation.8 These investigators also found that H pylori stimulated MIF release in monocytes, which is likely to be achieved through its cag pathogenicity island. Moreover, H pylori induced MIF increased proliferation of gastric epithelial cells which was blocked by a specific anti‐MIF antibody.9 Elevation of MIF expression was also observed in other cancer sites, including the lung,10 liver,11 breast,12 colon,13 and prostate.14 Recent studies have suggested that MIF may participate in carcinogenesis through inactivation of p53,15 enhancement of angiogenesis,10,16,17 as well as a Rho dependent pathway.17
The development of gastric cancer is a multistep process from normal mucosa to chronic gastritis, precancerous lesions, including gastric atrophy, intestinal metaplasia, dysplasia, and finally to invasive cancer.18 Clinical investigation of MIF in association with the development of gastric cancer has been limited, and it is not known how H pylori induced gastritis progresses to gastric cancer. We have previously demonstrated that H pylori infection is associated with increased MIF expression in gastric epithelial and inflammatory cells.8 Here we further hypothesised that MIF expression is also increased in precancerous and cancerous lesions of the stomach, and therefore conducted this study to determine whether increased severity of gastric pathology is associated with increased gastric epithelial MIF expression and serum MIF levels. In addition, we also compared in vitro expression of MIF protein and MIF mRNA between non‐malignant and malignant gastric cell lines with different differentiation.
Patients and methods
Patients and gastric biopsies
Fifty patients referred for upper endoscopy because of dyspeptic and reflux symptoms were included in the present study. There were 15 patients with a normal gastric mucosa, 15 with chronic antral gastritis, and 20 with intestinal metaplasia in the gastric antrum. Four biopsies were obtained from each patient, including three from the gastric antrum and one from the body. One antral biopsy was used for an inhouse rapid urease test and the other biopsies were fixed in formalin and embedded in paraffin for histological examinations and immunohistochemistry. Serum samples were prepared from each patient at endoscopy. In addition, 40 patients with histologically confirmed adenocarcinoma in the gastric antrum were included in the study. Gastric antral biopsy specimens and serum samples were also collected from these patients. The clinical characteristics of these patients are summarised in table 1. This project was approved by the ethics committee of Guangzhou Medical College, China.
Table 1 Clinical characteristics of the patients.
| Group | No | Age (mean (SD)) | Sex (M/F) | H pylori status (positive/negative) |
|---|---|---|---|---|
| Normal mucosa | 15 | 47.1 (16.2) | 7/8 | 0/15 |
| Chronic antral gastritis | 15 | 43.0 (15.5) | 6/9 | 15/0 |
| Antral intestinal metaplasia | 20 | 60.0 (15.2) | 13/7 | 14/6 |
| Antral gastric adenocarcinoma | 40 | 63.3 (12.4) | 28/12 | 22/18 |
| Overall | 90 | 56.5 (16.3) | 54/36 | 50/40 |
Detection of H pylori infection and histological examinations
The inhouse rapid urease test solution was freshly prepared everyday by dissolving 0.5 g of urea in 10 ml of distilled water and mixing with 10 drops of 0.1% phenol red as a pH indicator. This test has been shown to have a sensitivity of 99% and a specificity of 100% for the detection of H pylori.19 Sections (4 μm thick) were cut from paraffin embedded tissues and stained with haematoxylin and eosin, and Helicobacter‐like organisms were microscopically examined. Patients were classified as H pylori positive if both the rapid urease test and histology showed positive results or if histological examination showed positive results at the gastric antrum and body. Patients were classified as H pylori negative if all tests were negative. Patients with positive results by the rapid urease test only, or with positive histological results in only a single site of either the gastric antrum or body, were excluded from analysis. For patients with gastric cancer, we used histology and a serological test to determine H pylori infection, as described previously.20 The updated Sydney system was adapted to assess the presence of intestinal metaplasia and the activity and severity of gastric inflammation.21 Metaplastic epithelium was recognised morphologically by the presence of goblet cells, absorptive cells, and cells resembling colonocytes, and intestinal metaplasia was defined if it was present at any grade in the stomach. The presence of gastritis was determined when there was infiltration of moderate or marked number (grade 2 or 3 in severity) of chronic inflammatory cells (that is, mononuclear cells) and/or infiltration of polymorphic neutrophils (grade 1, 2, or 3 in activity).
Immunohistochemistry of MIF in the gastric epithelium
Immunohistochemistry staining was used to examine antral biopsy specimens for all patients for the assessment of MIF expression in the gastric epithelium.8 Briefly, sections cut from paraffin embedded tissues were deparaffinised and dehydrated for 10 minutes. These sections were treated in 3% hydrogen peroxide for five minutes and incubated with mouse antihuman MIF monoclonal antibody (1:1500 dilution) for one hour, followed by a 30 minute treatment with biotinylated goat antimouse immunoglobins (1:300 dilution). Streptavidin‐horseradish peroxidase (R&D Systems, Inc., Minneapolis, Minnesota, USA) was then applied for staining. Finally, these sections were developed with diaminobenzidene‐hydrogen peroxidase substrate (DAB; Dako Corporation Carpinteria, California, USA), and lightly counterstained with haematoxylin. All antibodies and streptavidin‐horseradish peroxidase were obtained from Dako A/S (Glostrup, Denmark). The percentage of MIF positive epithelial cells over the total epithelial cells counted (>300) in five randomly selected fields in the gastric mucosa was calculated and used as a labelling index (LI) of MIF expression.8
Enzyme linked immunosorbent assay for the detection of MIF in serum
Serum MIF was measured with an enzyme linked immunosorbent assay (ELISA) adapted from the R&D protocol (R&D Systems). Briefly, 100 μl binding solution (0.05 M Na2CO3 buffer, pH 9.6) containing the capture antibody, mouse antihuman MIF antibody (2 μg/ml; R&D Systems) was added to each well of an ELISA plate. The plate was then incubated overnight at 4°C. After three washes using 0.05% Tween 20 in phosphate buffered saline, the plate was blocked with 1% bovine serum albumin for two hours at room temperature. The plate was washed twice and then 100 μl of each sample or recombinant human MIF (R&D Systems) at different concentrations were added in triplicate to corresponding wells for two hours at room temperature. The plate was then washed three times and 100 μl of the detection antibody, biotinylated antihuman MIF antibody (0.2 μg/ml; R&D Systems), were added and the plate was incubated for two hours at room temperature. After washing, 100 μl of streptavidin‐horseradish peroxidase (R&D System) at a dilution of 1:200 was added to each well and the plate was incubated for 20 minutes at 37°C. After another four washings, 100 μl of a 1:1 mixture of reagents A (H2O2) and B (tetramethylbenzidine) were added and the plate was incubated for 20 minutes at 37°C. Finally, the reaction was terminated by adding 50 μl of H2SO4. Optical density was read using a microtitre plate reader (Bio‐Rad, NovaPath, USA) set at 450 nm. A standard curve was generated by correlating the original concentrations of the recombinant human MIF and the corresponding optical densities. MIF concentrations in serum samples were then calculated according to the standard curve. The sensitivity of the assay was 15.125 pg/ml of MIF.
Expression of MIF protein and mRNA in non‐malignant and malignant gastric cell lines
Six gastric epithelial cell lines (GES‐1, AGS, MKN‐45, MKN‐28, MGC‐803, and SGC‐7901) were used to determine expression of MIF protein and mRNA. Their biological characteristics have been reported previously22,23,24 and are summarised in table 2. Cells were cultured at 37°C in an atmosphere of 5% CO2 in RPMI‐1640 supplemented with 10% fetal bovine serum, penicillin, and streptomycin (Gibco BRL, Life Technologies, New York, USA) in 25 ml culture flasks for 24 hours. Supernatant was collected into a 1.5 ml Eppendorf tube and centrifuged at 2000 rpm for three minutes at room temperature. The concentration of MIF in the supernatant was measured in triplicate by the ELISA method described above.
Table 2 Sources and biological characteristics of the gastric epithelial cell lines.
| Cell line | Biological characteristics | Source |
|---|---|---|
| GES‐1 | An SV40 human fetal gastric epithelial cell line, non‐malignant | Beijing Institute for Cancer Research, Beijing, China |
| AGS | A human gastric cancer cell line with wild‐type p53, poorly differentiated | American Type Culture Collection, USA |
| MKN‐45 | A human gastric cancer cell line with wild‐type p53, poorly differentiated | The RIKEN (Institute of Physical and Chemical Research) Cell Bank, Japan |
| MKN‐28 | A human gastric cancer cell line with mutant p53, moderately differentiated | The RIKEN (Institute of Physical and Chemical Research) Cell Bank, Japan |
| MGC‐803 | A human gastric mucous adenocarcinoma cell line, with mutant p53, poorly differentiated | Kind gift from Prof DM Fan of Xijing Hospital, Xi'an, China. |
| SGC‐7901 | A human gastric cancer cell line with mutant p53, moderately differentiated | Kind gift from Prof SD Xiao, Shanghai Second Medical University, China |
Semiquantitative reverse transcription‐polymerase chain reaction (RT‐PCR) was used to determine MIF mRNA expression in cultured gastric epithelial cells. In brief, total RNA was isolated from cultured gastric cells using RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse transcribed using Moloney murine leukaemia virus reverse transcriptase. PCR was carried out using QuantiTECT SYBR PCR kit (Qiagen). The following primers were used to amplify a 185 base pair fragment of MIF: sense primer, 5′‐GTT CCT CTC CGA GCT CAC CCA GCA GC‐3′; antisense primer, 5′‐GCA GCT TGC TGT AGG AGC GGT TCT G‐3′. Primers for the amplification of human β‐actin mRNA were as follows: sense primer, 5′‐ATG GAA TTC CCG TGG AAC AAG AAT GAG ATC AG‐3′; antisense primer, 5′‐CGT CAT ACT CCT GCT TGC TGA TCC ACA TCA GC‐3′. The amplification profile for MIF was 15 minutes of activation, 28 cycles of 45 seconds of denaturation at 95°C, and two minutes of annealing and extension at 63°C (G2002‐667). For β‐actin, the amplification profile was 15 minutes of activation, 35 cycles of one minute of denaturation at 94°C, one minute of annealing at 56°C, and one minute of extension at 72°C. PCR products were resolved on a 1.7% agarose gel, stained with ethidium bromide, and analysed by densitometry. Expression of MIF mRNA was measured as a ratio of MIF intensity over β‐actin intensity in the corresponding band.
Statistic analysis
Data are expressed as mean (SD). Independent sample t test and the Mann‐Whitney U test were used to determine differences in MIF expression in gastric epithelial cells and serum MIF concentrations between the different groups, as well as differences in MIF expression in different gastric cell lines. One way analysis of variance (ANOVA) was used to determine the MIF expression difference in gastric epithelial cells and serum MIF concentrations among patients with different pathological changes. Bivariate correlation was used to assess the association between gastric epithelial MIF expression and serum MIF concentration. Data analysis was performed using SPSS software (version 13.0; SPSS Inc., Chicago, Illinois, USA). All analyses were two tailed and an alpha level of significance was set at p<0.05.
Results
MIF expression in normal, inflammatory, metaplastic, and cancerous gastric epithelial cells
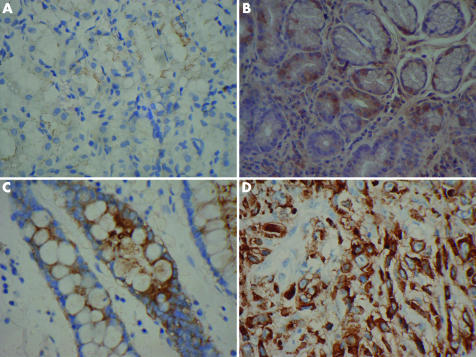
Using the immunostaining technique, MIF+ gastric epithelial cells were identified, and are shown in fig 1A–D. MIF expression was weak or undetectable in normal gastric epithelial cells but significantly increased in gastric inflammation, especially in intestinal metaplasia and gastric cancer (fig 1A–D).
Figure 1 Macrophage migration inhibitory factor expression in gastric epithelial cells in the gastric antrum. Immunostaining demonstrated weak expression in normal mucosa (A) and strong cytoplasmic expression (brown colour) in inflammatory (B), intestinal metaplastic (C), and cancerous (D) mucosa. Original magnification ×400.
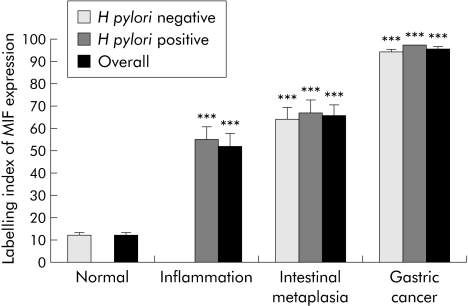
H pylori infection was absent in all patients with a normal mucosa but present in all patients with chronic gastritis. H pylori infection was detected in 70% (14/20) and 55% (22/40) of patients with intestinal metaplasia and gastric cancer, respectively. MIF expression in gastric epithelial cells was significantly higher in H pylori induced gastritis than in normal mucosa (52.12 (5.89)% v 12.19 (1.29)%; p<0.001). MIF expression was dramatically increased in intestinal metaplasia (66.11 (4.33)%; p<0.001) and gastric cancer (95.51 (0.83)%; p<0.001) compared with normal mucosa. Moreover, this increase was independent of H pylori infection (fig 2). In addition, the increase in epithelial MIF expression was significantly associated with the progress of gastric mucosal changes in the antrum (p<0.001, ANOVA).
Figure 2 Epithelial macrophage migration inhibitory factor (MIF) expression in normal, inflammatory, metaplastic, and cancerous gastric antral mucosa. Labelling index (LI) of MIF expression is calculated as the percentage of positively stained cells over the total number of cells counted. Results are means (SEM). All cases with normal mucosa were negative for Helicobacter pylori whereas all cases with inflammation were positive for H pylori. ***p<0.001 compared with normal mucosa.
Serum MIF concentrations in patients with normal, inflammatory, metaplastic, and cancerous gastric mucosa
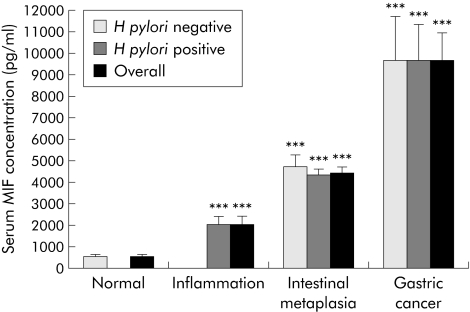
In patients with a normal gastric mucosa, MIF was detected in serum at low levels (576.35 (82.18) pg/ml). However, serum MIF levels were significantly higher in patients with gastric inflammation (2099.91 (348.88) pg/ml; p = 0.001). Furthermore, a dramatic increase in serum MIF concentration was observed in patients with intestinal metaplasia (4497.70 (252.78) pg/ml; p<0.001) and gastric cancer (9737.12 (1249.01) pg/ml; p<0.001) compared with those with a normal mucosa. The increase in serum MIF concentration was significantly associated with progression of gastric pathological changes (p<0.001, ANOVA) (fig 3). Similar to the findings from gastric epithelial cells, serum MIF concentration in patients with intestinal metaplasia and gastric cancer was independent of H pylori infection (fig 3).
Figure 3 Serum macrophage migration inhibitory factor (MIF) concentration in subjects with normal, inflammatory, precancerous, and cancerous gastric mucosa. Results are means (SEM). All cases with a normal mucosa were negative for Helicobacter pylori whereas all cases with inflammation were positive for H pylori. ***p<0.001 compared with the normal mucosa.
Correlation of epithelial MIF expression and serum MIF concentration in patients with normal, inflammatory, and metaplastic gastric mucosa
Overall, there was a strong correlation between LI of MIF expression in gastric epithelial cells and MIF concentration in serum (r = 0.774, p<0.001). Stratified analyses by different gastric pathological changes also showed such a correlation in patients with a normal mucosa (r = 0.763, p = 0.001), gastritis (r = 0.739, p = 0.002), intestinal metaplasia (r = 0.587, p = 0.006), and gastric cancer (r = 0.597, p = 0.005).
Expression of MIF protein and mRNA in non‐malignant and malignant gastric cell lines
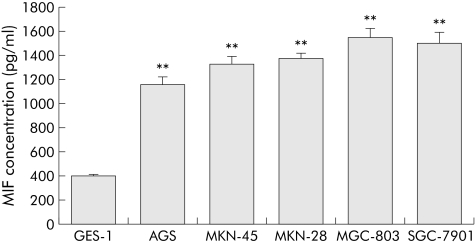
Expression of MIF protein in different gastric cell lines was measured in the supernatant after 24 hours of incubation in the culture medium. MIF expression was significantly higher in malignant cell lines than in the non‐malignant gastric cell line GES‐1 (p<0.01 for all comparisons). MIF expression was not significantly different among the malignant cell lines (fig 4).
Figure 4 Macrophage migration inhibitory factor (MIF) expression in non‐malignant and malignant gastric cell lines. Results are means (SEM) of three independent experiments. **p<0.01 compared with GES‐1.
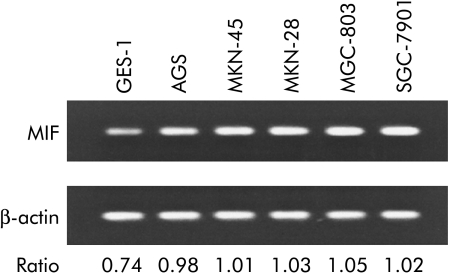
Semi‐quantitative RT‐PCR was used to test if there was a difference in expression of MIF mRNA in the different cell lines. Compared with the non‐malignant cell line (GES‐1), the malignant gastric cell lines expressed higher levels of MIF mRNA. Similarly, there was no apparent difference in MIF mRNA expression among the malignant cell lines (fig 5).
Figure 5 Expression of macrophage migration inhibitory factor (MIF) mRNA in non‐malignant and malignant gastric cell lines. MIF mRNA expression was stronger in cancer cell lines (lanes 2–6) compared with the non‐malignant cell line (GES‐1, lane 1).
Discussion
Previous studies have shown that MIF is secreted by inflammatory cells as a cytokine in response to inflammatory stimuli, or by corticotrophic and thyrotrophic cells of the anterior pituitary, pancreatic β cells, and ovarian and testicular cells as a hormone.25 We have recently demonstrated that MIF expression is significantly increased in activated T cells and macrophages in acetic acid induced ulceration in rats, and in H pylori infected gastritis in humans.9,26 In addition, Maaser et al reported that both MIF protein and mRNA were expressed in human gastric epithelial cells in vivo and gastric cancer cells in vitro.4 However, no study has been conducted evaluating expression of MIF protein and mRNA in association with H pylori infection and gastric pathological changes, leaving the potential role of MIF expression in gastric carcinogenesis elusive. In the present study, MIF expression in gastric epithelial cells was significantly increased in H pylori induced gastric inflammation. Moreover, gastric epithelial MIF expression was associated with progression of gastric pathological changes, which indicates that MIF may play a crucial role in the development and progression of gastric cancer.
Furthermore, our in vitro experiments showed that both secretion of MIF protein and expression of MIF mRNA were constitutively expressed in all gastric malignant cell lines studied. Expression of MIF protein and mRNA was significantly higher in malignant cells than in non‐malignant cells, further suggesting a role for MIF in gastric carcinogenesis. However, expression of MIF protein and mRNA was not significantly different among the malignant cell lines with different degrees of differentiation and p53 gene status, indicating that there is no association between differentiation of gastric cancer cells and epithelial MIF expression and that p53 may play no role in regulating MIF production, although MIF appears to inhibit p53 activity.15
A number of studies have indicated that MIF may be centrally involved in carcinogenesis by promoting cell proliferation, tumour angiogenesis, and metastasis.7,10,27,28,29,30 Takahashi et al found that increased MIF expression was associated with enhanced proliferation of murine colon cancer cells in response to growth factors.27 Chesney et al observed that neutralising anti‐MIF antibodies dramatically reduced the initial growth of lymphoma cells in mice but did not significantly affect the growth of established tumours, suggesting an early carcinogenic effect of MIF. They also observed that neutralising anti‐MIF antibodies inhibited endothelial cell growth and reduced the number of tumour capillaries.28 Recently, we observed that H pylori stimulated MIF increased cell proliferation of gastric cancer cells.9 In addition, Yang et al identified MIF as an angiogenic factor released by ectopic human endometrial cells promoting human coronary artery endothelial cell growth.29 Inhibition of tumour angiogenesis by anti‐MIF antibody treatment was also reported in a human melanoma model30 and in murine colon carcinoma cells.10,27 Both increased proliferation of tumour cells and angiogenesis would result in tumour progression and metastasis. How MIF regulates cell proliferation and angiogenesis is presently under intensive investigation. The available evidence suggests that modulation of cell proliferation by MIF may involve a complex regulatory system in which p53, JAB/CSN5/p27Kip1, and ERK1/2, and possibly other signalosome proteins, may play important roles.7,15,31,32 An additional possible mechanism that MIF is involved in tumorigenesis lies in fact that MIF may facilitate tumour cell escape from the host immune response.33,34 Repp et al reported that MIF produced by human uveal melanoma cells inhibited natural killing cell mediated lysis of lymphoma and melanoma cells, and such an effect was inhibited by anti‐MIF antibodies. They also observed that cell lines derived from uveal melanoma‐metastasis produced twice as much MIF as cultures from primary uveal melanoma, suggesting that secretion of MIF by uveal melanoma cells may provide an immune privilege crucial for metastatic growth.33 Furthermore, Abe et al found that splenocytes obtained from mice subcutaneously injected with a tumour cell line (EG.7) secreted high levels of MIF following antigen stimulation, and that neutralising anti‐MIF antibody treatment enhanced the cytotoxic T lymphocyte response in vivo and in vitro, indicating that MIF may play an important role in the regulation and trafficking of anti‐tumour T lymphocytes in vivo.34
H pylori infection is the primary cause of chronic gastritis and gastric cancer. Our previous studies have demonstrated that gastric MIF expression is associated with H pylori infection in vivo, H pylori infection induces MIF expression in vitro, and H pylori induced MIF increases proliferation of gastric epithelial cells.8,9 In this study, we further observed that MIF expression in gastric epithelial cells and serum was progressively associated with gastric pathological changes, indicating that MIF is involved in gastric carcinogenesis. It is likely that colonisation of H pylori in the stomach directly stimulates and/or indirectly facilitates gastric epithelial cells to release MIF, which then promotes local infiltration of inflammatory cells, including polymorphic neutrophils, T and B lymphocytes, and macrophages, resulting in the production of inducible nitrite oxide synthase and cytotoxic cytokines such as tumour necrosis factor α, interferon γ, and intracellular adhesion molecular 1.26 Consequently, infiltrated activated T cells and macrophages per se release a large amount of MIF. In the presence of chronic H pylori infection, this cycle continues, which contributes to more severe gastric inflammation and injury such as gastric erosion and ulcerations. More importantly, MIF released by infiltrated inflammatory cells, in particular by gastric epithelial cells, may be the initiating factor that “switches” chronic inflammation to carcinogenesis in the stomach, although the underlying mechanisms need further investigation.4,7,26
Finally, the observation of high serum MIF concentrations in patients with intestinal metaplasia and gastric cancer may have significant clinical implications. Serum MIF concentration was approximately 10‐fold (4497.7 (252.8) pg/ml) and 20‐fold (9737.1 (1249.0) pg/ml) higher in patients with gastric intestinal metaplasia and gastric cancer, respectively, than that (576.4 (82.2) pg/ml) in patients with a normal gastric mucosa. Moreover, the serum MIF concentration was highly correlated with gastric epithelial MIF expression. Therefore, it may be that measurement of serum MIF may prove to be a useful biomarker of patients with gastric cancer, with serum MIF concentrations higher than 5000 pg/ml being suggestive of malignancy. This remains to be fully tested, as does the further question of whether serum MIF concentrations may be of prognostic value in gastric cancer.
In conclusion, epithelial and serum MIF expression is progressively increased in H pylori induced gastritis, intestinal metaplasia, and gastric cancer, suggesting that MIF is involved in gastric carcinogenesis, and may be a valuable biomarker for the early detection of gastric cancer.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (NSFC) (30470435), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China (SEM (2003) No 406), and the Provincial Natural Science Foundation of Guangdong (031766) to Dr XX He, and competitive earmarked research grants from the Research Grants Council of Hong Kong SAR, China (HKU7493/03M and HKU7472/04M) to Dr HHX Xia. We thank Mr Yingjie Liang, Department of Pathology, Sun Yat‐Sen University, Guangzhou, China, for technical assistance.
Abbreviations
MIF - macrophage migration inhibitory factor
ELISA - enzyme linked immunosorbent assay
RT‐PCR - reverse transcription‐polymerase chain reaction
LI - labelling index
Footnotes
Conflict of interest: None declared.
References
- 1.Shibuya K, Mathers C D, Boschi‐Pinto C.et al Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer 2002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernst P B, Gold B D. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol 200054615–640. [DOI] [PubMed] [Google Scholar]
- 3.Macarthur M, Hold G L, El‐Omar E M. Inflammation and cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol 2004286G515–G520. [DOI] [PubMed] [Google Scholar]
- 4.Maaser C, Eckmann L, Paesold G.et al Ubiquitous production of macrophage migration inhibitory factor by human gastric and intestinal epithelium. Gastroenterology 2002122667–680. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z W, Farthing M J. Molecular mechanisms of H. pylori associated gastric carcinogenesis. World J Gastroenterol 19995369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernhagen J, Calandra T, Bucala R. Regulation of the immune response by macrophage migration inhibitory factor: biological and structural features. J Mol Med 199876151–161. [DOI] [PubMed] [Google Scholar]
- 7.Lue H, Kleemann R, Calandra T.et al Macrophage migration inhibitory factor (MIF): mechanisms of action and role in disease. Microbes Infect 20024449–460. [DOI] [PubMed] [Google Scholar]
- 8.Xia H H, Lam S K, Huang X R.et al Helicobacter pylori infection is associated with increased expression of macrophage migratory inhibitory factor—by epithelial cells, T cells, and macrophages—in gastric mucosa. J Infect Dis 2004190293–302. [DOI] [PubMed] [Google Scholar]
- 9.Xia H H, Lam S K, Chan A O.et al Macrophage migration inhibitory factor stimulated by Helicobacter pylori increases proliferation of gastric epithelial cells. World J Gastroenterol 2005111946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White E S, Flaherty K R, Carskadon S.et al Macrophage migration inhibitory factor and CXC chemokine expression in non‐small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res 20039853–860. [PubMed] [Google Scholar]
- 11.Akbar S M, Abe M, Murakami H.et al Macrophage migration inhibitory factor in hepatocellular carcinoma and liver cirrhosis; relevance to pathogenesis. Cancer Lett 2001171125–132. [DOI] [PubMed] [Google Scholar]
- 12.Bando H, Matsumoto G, Bando M.et al Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res 200293389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legendre H, Decaestecker C, Nagy N.et al Prognostic values of galectin‐3 and the macrophage migration inhibitory factor (MIF) in human colorectal cancers. Mod Pathol 200316491–504. [DOI] [PubMed] [Google Scholar]
- 14.del Vecchio M T, Tripodi S A, Arcuri F.et al Macrophage migration inhibitory factor in prostatic adenocarcinoma: correlation with tumor grading and combination endocrine treatment‐related changes. Prostate 20004551–57. [DOI] [PubMed] [Google Scholar]
- 15.Hudson J D, Shoaibi M A, Maestro R.et al A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 19991901375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shun C T, Lin J T, Huang S P.et al Expression of macrophage migration inhibitory factor is associated with enhanced angiogenesis and advanced stage in gastric carcinomas. World J Gastroenterol 2005113767–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun B, Nishihira J, Suzuki M.et al Induction of macrophage migration inhibitory factor by lysophosphatidic acid: relevance to tumor growth and angiogenesis. Int J Mol Med 200312633–641. [PubMed] [Google Scholar]
- 18.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992526735–6740. [PubMed] [Google Scholar]
- 19.Wong B C, Wong W M, Wang W H.et al An evaluation of invasive and non‐invasive tests for the diagnosis of Helicobacter pylori infection in Chinese. Aliment Pharmacol Ther 200115505–511. [DOI] [PubMed] [Google Scholar]
- 20.Xia H H, Wong B C, Wong W M.et al Optimal serological tests for the detection of Helicobacter pylori infection in the Chinese population. Aliment Pharmacol Ther 200216521–526. [DOI] [PubMed] [Google Scholar]
- 21.Dixon M F, Genta R M, Yardley J H.et al Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996201161–1181. [DOI] [PubMed] [Google Scholar]
- 22.Ke Y, Ning T, Wang B. Establishment and characterization of a SV40 transformed human fetal gastric epithelial cell line‐GES‐1. Zhonghua Zhong Liu Za Zhi 1994167–10. [PubMed] [Google Scholar]
- 23.Jiang X H, Wong B C, Lin M C.et al Functional p53 is required for triptolide‐induced apoptosis and AP‐1 and nuclear factor‐kappaB activation in gastric cancer cells. Oncogene 2001208009–8018. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Xia H H, Tu S P.et al 15‐Lipoxygenase‐1 mediates cyclooxygenase‐2 inhibitor‐induced apoptosis in gastric cancer. Carcinogenesis 200324243–247. [DOI] [PubMed] [Google Scholar]
- 25.Swope M D, Lolis E. Macrophage migration inhibitory factor: cytokine, hormone, or enzyme? Rev Physiol Biochem Pharmacol 19991391–32. [DOI] [PubMed] [Google Scholar]
- 26.Huang X R, Chun Hui C W, Chen Y X.et al Macrophage migration inhibitory factor is an important mediator in the pathogenesis of gastric inflammation in rats. Gastroenterology 2001121619–630. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Nishihira J, Sato Y.et al Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol Med 19984707–714. [PMC free article] [PubMed] [Google Scholar]
- 28.Chesney J, Metz C, Bacher M.et al An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med 19995181–191. [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Degranpre P, Kharfi A.et al Identification of macrophage migration inhibitory factor as a potent endothelial cell growth‐promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab 2000854721–4727. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa H, Nishihira J, Sato Y.et al An antibody for macrophage migration inhibitory factor suppresses tumour growth and inhibits tumour‐associated angiogenesis. Cytokine 200012309–314. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell R A, Liao H, Chesney J.et al Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A 200299345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell R A, Metz C N, Peng T.et al Sustained mitogen‐activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J Biol Chem 199927418100–18106. [DOI] [PubMed] [Google Scholar]
- 33.Repp A C, Mayhew E S, Apte S.et al Human uveal melanoma cells produce macrophage migration‐inhibitory factor to prevent lysis by NK cells. J Immunol 2000165710–715. [DOI] [PubMed] [Google Scholar]
- 34.Abe R, Peng T, Sailors J.et al Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol 2001166747–753. [DOI] [PubMed] [Google Scholar]