Abstract
Background and aims
Ghrelin, the natural ligand of the growth hormone secretagogue receptor 1a, is the most powerful peripherally active orexigenic agent known. In rodents, ghrelin administration stimulates growth hormone release, food intake, and adiposity. Because of these effects, blocking of ghrelin has been widely discussed as a potential treatment for obesity. Spiegelmer NOX‐B11 is a synthetic l‐oligonucleotide, which was previously shown to bind ghrelin. We examined the effects of NOX‐B11 on ghrelin induced neuronal activation and food intake in non‐fasted rats.
Methods
Animals received various doses of NOX‐B11, inactive control Spiegelmer, or vehicle intravenously. Ghrelin or vehicle was administered intraperitoneally 12 hours later and food intake was measured over four hours. Neuronal activation was assessed as c‐Fos‐like immunoreactivity in the arcuate nucleus.
Results
Treatment with NOX‐B11 30 nmol suppressed ghrelin induced c‐Fos‐like immunoreactivity in the arcuate nucleus and blocked the ghrelin induced increase in food intake within the first half hour after ghrelin injection (mean 1.13 (SEM 0.59) g/kg body weight; 4.94 (0.63) g/kg body weight versus 0.58 (0.58) g/kg body weight; p<0.0001). Treatment with NOX‐B11 1 nmol or control Spiegelmer had no effect whereas treatment with NOX‐B11 10 nmol showed an intermediate effect on ghrelin induced food intake.
Conclusions
Spiegelmer NOX‐B11 suppresses ghrelin induced food intake and c‐Fos induction in the arcuate nucleus in rats. The use of an anti‐ghrelin Spiegelmer could be an innovative new approach to inhibit the biological action of circulating ghrelin. This may be of particular relevance to conditions associated with elevated plasma ghrelin, such as the Prader‐Willi syndrome.
Keywords: ghrelin, obesity, antagonism, NOX‐B11, food intake, rat
Ghrelin, a 28 amino acid peptide hormone, was identified in 1999 as the natural ligand of the growth hormone secretagogue receptor 1a (GHS‐R1a).1 Its principal site of synthesis is A‐like endocrine cells of the gastric oxyntic mucosa which contribute approximately 80% of the circulating concentrations of ghrelin.2 Independent from its effect as a growth hormone secretagogue in the pituitary gland,3,4,5 ghrelin has been shown to act as a gut‐brain peptide with potent stimulatory effects on food intake after intracerebroventricular and intraperitoneal administration in rodents.6,7,8 Long term administration was shown to increase weight and adiposity in mice and rats.6,9 In humans, intravenous administration of ghrelin stimulated appetite, leading to a 28% increase in food intake during a self served buffet meal.10 The orexigenic effects of ghrelin are thought to be mediated by stimulation of neurones in the arcuate nucleus (ARC) of the hypothalamus as peripheral administration of ghrelin induces a significant increase in c‐Fos positive neurones in this area in rodents.11,12
Blood ghrelin levels are determined by acute as well as chronic feeding states of the body. Fasting increases plasma ghrelin levels in rats and humans9,13 whereas food consumption results in a decrease in plasma ghrelin.13,14 Gastric bypass surgery was shown to cause unusually low plasma ghrelin levels, a phenomenon that is thought to contribute to the success of the procedure in curbing food intake.15 This observation in combination with others has led to the hypothesis that antagonism of ghrelin and its receptor could represent a therapeutic approach to treat obesity. Recent deletion studies in mice however indicated that loss of the ghrelin gene can be compensated as ghrelin negative animals did not exhibit abnormalities in food intake or weight development.16,17 Nevertheless, studies in rats with [d‐Lys‐3]GHRP‐6, a known GHS receptor antagonist, as well as transgenic expression of antisense GHS‐R1a mRNA, showed a reduction in food intake.18,19
Spiegelmers (German: Spiegel = mirror) are non‐natural nucleic acids with specific binding activity towards a given target molecule.20,21 Because the sugar‐phosphate backbone of Spiegelmers contains d‐ribose in place of the naturally occurring l‐ribose, they are not subject to degradation by nucleases and remain stable for 60 hours or more in biological fluids.20,22 Spiegelmers have been made against small molecules, peptides, and proteins.23 Their generation involves the synthesis of a mirror image of the biological target, which serves as the target in SELEX (systematic evolution of ligands by exponential enrichment), a cyclical process in which binding molecules are identified from a large library of oligonucleotides through alternating steps of selection and enzymatic amplification.24,25 Sequencing of binders identified through SELEX delivers the blueprint for the synthesis of the Spiegelmer. The utility of Spiegelmers as inhibitors of receptor‐peptide interactions has been demonstrated in vitro and in vivo.26,27,28,29
We have previously described the generation of Spiegelmer NOX‐B11, which specifically binds to the bioactive form of ghrelin.29 Interaction with NOX‐B11 interferes with the ability of ghrelin to bind to its receptor and inactivates the hormone's stimulation of the GHS‐R1a in cell culture. Moreover, administration of NOX‐B11 to rats was shown to suppress ghrelin induced release of growth hormone specifically and in a dose dependent manner.29
In the present study, we extended our previous work by examining the effects of NOX‐B11 on two separate ghrelin triggered events: induction of c‐Fos in the ARC of the hypothalamus and the powerful orexigenic effect after systemic ghrelin administration to non‐fasted rats.
Methods
Animals
Male Sprague‐Dawley rats (Harlan‐Winkelmann Co., Borchen, Germany) were housed under conditions of controlled illumination (12:12 hour light/dark cycle, lights on/off 6:30am/6:30pm), humidity, and temperature (22±2°C) for at least 21 days prior to the experiments. To minimise stress related effects, rats were handled daily beginning at least 14 days before the experiment.
Animals were maintained in colony cages until the start of the experiments and given ad libitum access to standard rat diet (Altromin, Lage, Germany) and tap water. Animal care and experimental procedures followed institutional ethics guidelines and conformed to the requirements of the state authority for animal research conduct.
Peptide and Spiegelmer preparation
Rat ghrelin (GSS (octanoyl) FLS PEH QKA QQR KES KKP PAK LQP R; Bachem AG, Heidelberg, Germany) was dissolved in distilled water (1 mg/ml) and stored at −20°C. Immediately before the experiments, peptide was diluted in sterile 0.1 M phosphate buffered saline (PBS) to reach a final concentration of 6 nmol/ml. The peptide solution was kept on ice for the duration of the experiments.
PEGylated Spiegelmers NOX‐B11 and the inactive control sequence have been described previously.29 For intravenous administration, the PEGylated Spiegelmers were dissolved in sterile 0.1 M PBS at a concentration of 120 nmol/ml.
Experimental design
Feeding studies
On the day before the experiment, animals were briefly anaesthetised (at 09:00pm) with 100 mg/kg body weight ketamine intraperitoneally (Ketanest; Curamed, Karlsruhe, Germany) and 10 mg/kg body weight xylazine intraperitoneally (Rompun 2%; Bayer, Leverkusen, Germany). During anaesthesia, animals received the following substances by intravenous injection of a 250 µl volume in the lateral tail vein: NOX‐B11 in 0.1 M PBS, control Spiegelmer, or 0.1 M PBS. Respective doses of Spiegelmer are indicated in the text and figure legends and were based on previous studies.29 After waking up from the anaesthetic, animals were returned to single home cages and given ad libitum access to food and water.
The feeding experiments were started at 09:00am. After assessment of weight (280–300 g), rats received an intraperitoneal injection of 3 nmol ghrelin (in 0.5 ml 0.1 M PBS) or vehicle solution, and were returned to their single home cages. Preweighed rat chow was made available to the animals and food intake determined by calculating the difference at the indicated time points of the four hour observation period. Food intake was recalculated as cumulative food intake (g)/body weight (kg). Data are expressed as mean (SEM) and were analysed by ANOVA. Differences between groups were evaluated by the least significant difference test; p<0.05 was considered significant.
Effects on c‐Fos‐like immunoreactivity in the arcuate nucleus
Animals received Spiegelmer and ghrelin as described above for the feeding study. At 90 minutes after ghrelin injection,11 animals were anaesthetised and heparinised with 2500 U heparin intraperitoneally (Liquemin; Hoffmann‐La Roche, Grenzach‐Whylen, Germany). Transcardial perfusion was performed as described previously.30 After dissection, brains were kept in a 5% w/v sucrose solution overnight and then cut into 1.0–4.5 mm coronal blocks enclosing the respective hypothalamic regions using a plexiglass brain matrix. For cryoprotection, blocks were moved through a sucrose gradient (15% w/v and 27.3% w/v), shock frozen in hexane at −70°C, and stored at −80°C until further processing.
Staining for c‐Fos‐like immunoreactivity (c‐FLI) and analysis
Free floating 25 µm sections were pretreated with 1% w/v sodium borohydride in PBS for 15 minutes. Sections were blocked for 60 minutes in blocking buffer (10% w/v bovine serum albumin and 0.3% v/v Triton X‐100 in PBS) and incubated for 24 hours at room temperature with anti‐c‐Fos antibody (Oncogene Research Products, Boston, Massachusetts, USA) diluted 1:10 000 in blocking buffer containing 0.1% w/v sodium azide.
Sections were rinsed three times in PBS and incubated in blocking buffer for one hour. FITC labelled goat‐anti‐rabbit IgG (Sigma, St Louis, Missouri, USA), diluted 1:800 in 10% w/v bovine serum albumin in PBS, was applied and sections incubated for 12 hours at room temperature. Sections were rinsed three times in PBS and stained with propidium iodide (2.5 µg/ml in PBS) for 15 minutes to counterstain cell chromatin. Tissue sections were embedded in 15 µl anti‐fading solution (100 mg/ml 1,4‐diazabicyclo[2.2.2]octane (Sigma) in 90% v/v glycerin, 10% v/v PBS, pH 7.4) and analysed using a confocal laser scanning microscope (cLSM 510; Carl Zeiss, Germany).30 Semiquantitative assessment of c‐FLI was achieved by counting the number of cells with bright green nuclear staining. For the entire collection of consecutive coronal 25 µm sections, every other section was counted for c‐FLI positive staining bilaterally in the ARC throughout their rostrocaudal extent. c‐FLI positive neurones were counted in 15 sections per rat of the ARC. Anatomical correlation was made according to landmarks given in a stereotaxic atlas.31 Identical numbers of brains (n = 5) were processed for all groups investigated to achieve maximal consistency of the results. To avoid bias, counting was carried out without knowledge of the treatments received by the animals. The average number of c‐FLI positive cells per section for the brain nuclei mentioned above was calculated for each rat. Data are expressed as median (interquartile range) of the average number of cells/section. Differences between groups were evaluated by the non‐parametric Kruskal‐Wallis and Mann‐Whitney U‐tests; p<0.05 was considered significant.
Results
Effects of ghrelin and Spiegelmer plus ghrelin on food intake
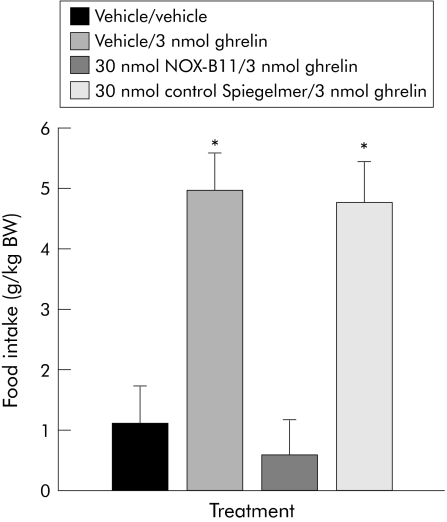
Our aim was to investigate the effects of Spiegelmer NOX‐B11 on short term food intake induced by peripheral ghrelin. Stimulation of food intake was elicited with an intraperitoneal injection of ghrelin, as previously described.9 In order to keep the animals' stress levels to a minimum, we chose to administer 30 nmol of Spiegelmer or PBS 12 hours before stimulation with ghrelin. Based on the half life of Spiegelmer NOX‐B11 of 12 hours in plasma (data not shown), this dose and timing was calculated to provide sufficiently high plasma levels of NOX‐B11 during the feeding experiment while at the same time allowing animals to recover from the intravenous injection. The results are shown in fig 1. In the positive control group, treatment with PBS and 3 nmol ghrelin (vehicle/ghrelin group) significantly increased food intake within the first half hour following intraperitoneal injection (4.94 (0.63) g/kg body weight; fig 1) compared with the vehicle/vehicle group (1.13 (0.59) g/kg body weight, p<0.0002) (fig 1). Pretreatment with 30 nmol NOX‐B11 blocked the stimulatory effect of ghrelin on food intake (0.58 (0.58) g/kg body weight; p<0.0001) (fig 1). In contrast, administration of a control Spiegelmer consisting of a random sequence had no such inhibitory effect, leaving ghrelin induced stimulation of food intake intact (4.77 (0.66) g/kg body weight; p>0.864) (fig 1).
Figure 1 Thirty minutes after intraperitoneal ghrelin administration, NOX‐B11 inhibited ghrelin induced food intake. Following intraperitoneal injection of 3 nmol ghrelin, animals who had been treated with 0.1 M phosphate buffered saline (n = 11) or 30 nmol control Spiegelmer (n = 8) exhibited a significant increase in food intake. The stimulatory effect of ghrelin on food intake was abolished in animals pretreated with 30 nmol NOX‐B11 (n = 8). Data are expressed as mean (SEM). *p<0.05 versus vehicle/vehicle and NOX‐B11.
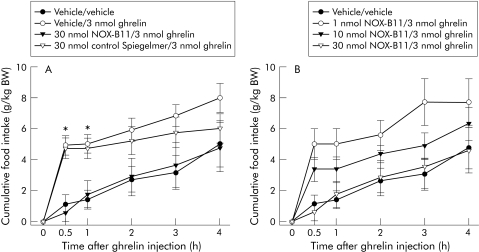
In the ghrelin stimulated groups, food intake followed promptly on administration and lasted for 30 minutes. Virtually no feeding was observed for the next 30 minutes (fig 2A). Nevertheless, one hour after ghrelin treatment the increases in cumulative food intake in the vehicle/ghrelin group and the control Spiegelmer/ghrelin group (5.04 (0.59) g/kg body weight and 4.77 (0.66) g/kg body weight, respectively) were still statistically significant compared with the vehicle/vehicle group (1.44 (0.59) g/kg body weight; p<0.0005 and 0.003) (fig 2A) and the 30 nmol NOX‐B11/ghrelin group (1.78 (0.87) g/kg body weight; p<0.004) (fig 2A). For the remaining three hours of the four hour observation period, food intake continued to be lower in animals that had exhibited ghrelin induced stimulation in the first half hour. Rats treated with vehicle and ghrelin or control Spiegelmer and ghrelin ate less in this time span compared with animals who had received NOX‐B11 and ghrelin or vehicle alone (fig 2A). Despite this catching up of the vehicle/vehicle and NOX‐B11/ghrelin groups over time, a trend towards increased cumulative food consumption in the vehicle/ghrelin group and the control Spiegelmer/ghrelin group persisted at the end of the four hour observation period. Statistical significance however was limited to the first hour after ghrelin injection.
Figure 2 Four hour time course of ghrelin induced food intake after treatment with different Spiegelmers (A) or various doses of Spiegelmer NOX‐B11 (B). (A) Animals given vehicle and ghrelin (0.1 M phosphate buffered saline intravenously, 3 nmol ghrelin intraperitoneally, n = 11) or control Spiegelmer and ghrelin (30 nmol control Spiegelmer intravenously, 3 nmol ghrelin intraperitoneally, n = 8) displayed an increase in cumulative food intake compared with rats given vehicle alone (0.1 M phosphate buffered saline intravenously, 0.1 M PBS intraperitoneally, n = 9) or NOX‐B11 and ghrelin (30 nmol Spiegelmer NOX‐B11 intravenously, 3 nmol ghrelin intraperitoneally; n = 8). The increase in food intake was most pronounced 30 minutes after ghrelin administration and remained statistically significant for the first hour. (B) Doses of 1 nmol (n = 8), 10 nmol (n = 8), and 30 nmol (n = 8) NOX‐B11 exhibited varying degrees of inhibition of ghrelin induced food intake. Complete neutralisation of stimulation of food intake with 3 nmol ghrelin was observed at 30 nmol NOX‐B11. All data are expressed as mean (SEM). *p<0.05 versus vehicle/vehicle and NOX‐B11.
The inhibitory effect of NOX‐B11 on food intake proved to be strictly dose dependent. A dose of 1 nmol Spiegelmer NOX‐B11 had no effect on ghrelin's stimulation of food intake. An intermediate effect was observed for a dose of 10 nmol NOX‐B11; at this dose level, the stimulatory effect of 3 nmol ghrelin during the first 30 minutes was moderated (3.51 (0.66) g/kg body weight v 4.94 (0.63) g/kg body weight; p>0.159 v ghrelin alone). Taken together, these results demonstrate that NOX‐B11 is capable of neutralising the transient orexigenic effect of exogenous ghrelin. This inhibitory effect is specific to the ghrelin binding Spiegelmer and dose dependent. Interestingly, animals that received NOX‐B11and ghrelin displayed the same basal rate of feeding as the vehicle/vehicle group throughout the entire four hour observation period after ghrelin injection.
Effects of peripheral ghrelin and Spiegelmer on c‐Fos‐like immunoreactivity (c‐FLI) in the arcuate nucleus
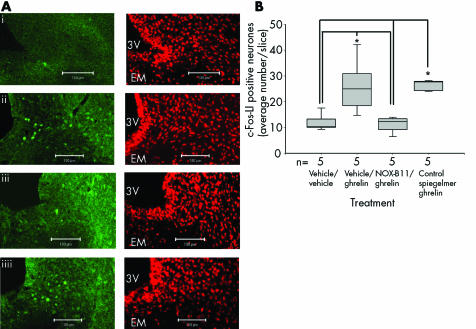
We and others have previously shown that the orexigenic effect of ghrelin after peripheral administration correlates with neuronal activation, as assessed by c‐FLI, in the ARC of the hypothalamus. We therefore wished to examine whether NOX‐B11 is capable of suppressing this change in neuronal activity of ARC neurones. Intraperitoneal administration of 3 nmol ghrelin per rat (vehicle/ghrelin group) induced an increase in the density of c‐FLI positive neurones, predominantly in the ventromedial part of the ARC, compared with animals receiving vehicle (fig 3A and 3B, compare (i) and (ii); median number of c‐FLI positive neurones/section 25 v 10.3; p<0.016). This effect of peripheral ghrelin on neuronal activity in the ARC was significantly diminished by pretreatment with 30 nmol NOX‐B11 (fig 3A (iii) and 3B; median number of c‐FLI positive neurones/section 12.23; p<0.009) whereas the control Spiegelmer had no effect (27.66; p>0.46). The result demonstrates that intravenous administration of NOX‐B11 prevents ghrelin's activation of neurones in the ARC.
Figure 3 NOX‐B11 inhibited ghrelin induced c‐Fos‐like immunoreactivity (c‐Fos‐LI) in the arcuate nucleus (ARC) of the hypothalamus. (A) Intraperitoneal injection of 3 nmol ghrelin per rat (vehicle/ghrelin group) induced an increase in the density of c‐FLI positive neurones in the ventromedial part of the ARC (ii) compared with injection of vehicle alone (i). This effect of peripheral ghrelin on neuronal activity in the ARC was diminished by pretreatment with NOX‐B11 (iii) but not with control Spiegelmer (iiii). To visualise cell nuclei, the same sections were counterstained with propidium iodide. The scale bar represents 100 µm. 3V, third ventricle, EM, median eminence. (B) Statistical analysis of c‐Fos‐LI in the ARC of the hypothalamus. Results are shown as box and whiskers plots. The upper hinge of the box represents the 75th and the lower the 25th percentile. The median is shown as a line across the box. The whiskers above and below the boxes represent the largest and smallest observed scores that were less than 1.5 box lengths from that of the box. *p<0.05 versus vehicle/vehicle and NOX‐B11/ghrelin.
Discussion
We have previously shown that Spiegelmer NOX‐B11 binds ghrelin and thereby prevents the interaction of the peptide with its target receptor, GHS‐R1a.29 Moreover, systemic administration of NOX‐B11 suppresses ghrelin's stimulation of GH release in rats.29 However, the primary function of ghrelin is thought to be in the regulation of energy balance.6,8,32 The goal of the present study was therefore to investigate the effects ofNOX‐B11 on ghrelin induced food intake and the concomitant neuronal activation in the ARC.
Our results showed that the increase in food intake during the first 60 minutes after intraperitoneal injection of ghrelin can be abolished by intravenous pretreatment with 30 nmol NOX‐B11. This inhibitory effect was specific as the same treatment with a control Spiegelmer consisting of a random sequence has no influence on food intake. Moreover, administration of lower doses of NOX‐B11 resulted in moderation of ghrelin inhibition (10 nmol) or lack of an inhibitory effect (1 nmol). These findings support the target specificity previously described for NOX‐B11. Our examination of the ARC for ghrelin stimulated c‐FLI indicated that the presence of NOX‐B11 curbed neuronal activation whereas the control Spiegelmer had no effect. Taken together, our results demonstrated that NOX‐B11 suppressed ghrelin mediated effects directly linked to energy regulation.
Because PEGylated Spiegelmer does not cross the blood‐brain barrier due to its charge and size, neutralisation of ghrelin most likely occurs in the periphery. However, the proximity of the basal parts of the ARC to the circumventricular area of the median eminence implies that the hypothalamic targets within this area may be only weakly protected by the blood‐brain barrier. Therefore, we cannot rule out the fact that part of the inhibition takes place in the ARC itself. In any case, complete suppression of ghrelin induced effects with a dose of 30 nmol NOX‐B11 argues that the amount of exogenous peptide is quantitatively bound and inactivated under these conditions.
Interestingly, NOX‐B11 did not influence the basal rate of food ingestion, as shown by the fact that the amount of ingested food did not fall below that of the vehicle/vehicle group. While this indicates that the effect of NOX‐B11 on feeding is ghrelin specific, it also implies that neutralisation of endogenous circulating ghrelin does not affect the basic level of feeding under our experimental ad libitum conditions. There are three possible explanations for this observation: firstly, the unstimulated rate of feeding under non‐fasting conditions is under the control of hypothalamic ghrelin which cannot be accessed by NOX‐B11 due to protection by the blood‐brain barrier; secondly, the role of ghrelin in the regulation of feeding is redundant and its neutralisation is compensated for by other factors; or thirdly, endogenous ghrelin is not involved in the regulation of non‐fasted food intake. Absence of a distinct phenotype in studies with mice carrying disruption of the ghrelin gene locus argues that other factors can counterbalance loss of the hormone,16,17,33 at least under laboratory living conditions. Our results confirm previous studies showing that high peripheral ghrelin levels trigger powerful stimulation of feeding9 and neuronal activity in the ARC.12 The response to the ghrelin stimulus, but not unstimulated food intake, can be offset by the ghrelin antagonist. Our result is therefore suggestive of a role for ghrelin in the adaptive behavioural response to conditions of low energy intake (that is, “hunger”) rather than in driving basal food intake (that is, “appetite”). The observation that ghrelin's stimulation of feeding is followed by a period of reduced food intake is in agreement with such a view.
Taken together, our results indicate that treatment with the ghrelin binding Spiegelmer NOX‐B11 provides a feasible approach to blocking the effect of circulating ghrelin on food intake and induction of c‐Fos in ARC neurones. Although many questions about the role of ghrelin in the regulation of feeding and energy balance remain, NOX‐B11 may lead the way in a new approach to the treatment of obesity and obesity related diseases. This could be especially relevant in diseases that are associated with high levels of circulating ghrelin, such as the Prader‐Willi syndrome.34,35
Acknowledgements
We would like to thank all members of NOXXON involved in the supply of Spiegelmers used in this study. This work was supported by a grant from the German Research Foundation (DFG) to HM (DFG: Mö 458/4‐1) and the German Ministry for Education and Research (BMBF) to NOXXON (BMBF 0313103).
Abbreviations
GHS‐R1a - growth hormone secretagogue receptor 1a
ARC - arcuate nucleus
SELEX - systematic evolution of ligands by exponential enrichment
PBS - phosphate buffered saline
c‐FLI - c‐Fos‐like immunoreactivity
Footnotes
Conflict of interest: None declared.
References
- 1.Kojima M, Hosoda H, Date Y.et al Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature 1999402656–660. [DOI] [PubMed] [Google Scholar]
- 2.Date Y, Kojima M, Hosoda H.et al Ghrelin, a novel growth hormone‐releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 20001414255–4261. [DOI] [PubMed] [Google Scholar]
- 3.Date Y, Murakami N, Kojima M.et al Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem Biophys Res Commun 2000275477–480. [DOI] [PubMed] [Google Scholar]
- 4.Seoane L M, Tovar S, Baldelli R.et al Ghrelin elicits a marked stimulatory effect on GH secretion in freely‐moving rats. Eur J Endocrinol 2000143R7–R9. [DOI] [PubMed] [Google Scholar]
- 5.Tolle V, Zizzari P, Tomasetto C.et al In vivo and in vitro effects of ghrelin/motilin‐related peptide on growth hormone secretion in the rat. Neuroendocrinology 20017354–61. [DOI] [PubMed] [Google Scholar]
- 6.Tschop M, Smiley D L, Heiman M L. Ghrelin induces adiposity in rodents. Nature 2000407908–913. [DOI] [PubMed] [Google Scholar]
- 7.Wren A M, Small C J, Ward H L.et al The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 20001414325–4328. [DOI] [PubMed] [Google Scholar]
- 8.Asakawa A, Inui A, Kaga T.et al Ghrelin is an appetite‐stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001120337–345. [DOI] [PubMed] [Google Scholar]
- 9.Wren A M, Small C J, Abbott C R.et al Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001502540–2547. [DOI] [PubMed] [Google Scholar]
- 10.Wren A M, Seal L J, Cohen M A.et al Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001865992. [DOI] [PubMed] [Google Scholar]
- 11.Hewson A K, Dickson S L. Systemic administration of ghrelin induces Fos and Egr‐1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol 2000121047–1049. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Saint‐Pierre D H, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y‐synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 200232547–51. [DOI] [PubMed] [Google Scholar]
- 13.Tschop M, Weyer C, Tataranni P A.et al Circulating ghrelin levels are decreased in human obesity. Diabetes 200150707–709. [DOI] [PubMed] [Google Scholar]
- 14.Cummings D E, Purnell J Q, Frayo R S.et al A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001501714–1719. [DOI] [PubMed] [Google Scholar]
- 15.Cummings D E, Weigle D S, Frayo R S.et al Plasma ghrelin levels after diet‐induced weight loss or gastric bypass surgery. N Engl J Med 20023461623–1630. [DOI] [PubMed] [Google Scholar]
- 16.Wortley K E, Anderson K D, Garcia K.et al Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A 20041018227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Ahmed S, Smith R G. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 2003237973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuto Y, Shibasaki T, Otagiri A.et al Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 20021091429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asakawa A, Inui A, Kaga T.et al Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 200352947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klussmann S, Nolte A, Bald R.et al Mirror‐image RNA that binds D‐adenosine. Nat Biotechnol 1996141112–1115. [DOI] [PubMed] [Google Scholar]
- 21.Nolte A, Klussmann S, Bald R.et al Mirror‐design of L‐oligonucleotide ligands binding to L‐arginine. Nat Biotechnol 1996141116–1119. [DOI] [PubMed] [Google Scholar]
- 22.Eulberg D, Klussmann S. Spiegelmers: biostable aptamers. Chembiochem 20034979–983. [DOI] [PubMed] [Google Scholar]
- 23.Vater A, Klussmann S. Toward third‐generation aptamers: Spiegelmers and their therapeutic prospects. Curr Opin Drug Discov Dev 20036253–261. [PubMed] [Google Scholar]
- 24.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990249505–510. [DOI] [PubMed] [Google Scholar]
- 25.Ellington A D, Szostak J W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990346818–822. [DOI] [PubMed] [Google Scholar]
- 26.Leva S, Lichte A, Burmeister J.et al GnRH binding RNA and DNA Spiegelmers: a novel approach toward GnRH antagonism. Chem Biol 20029351–359. [DOI] [PubMed] [Google Scholar]
- 27.Purschke W G, Radtke F, Kleinjung F.et al A DNA Spiegelmer to staphylococcal enterotoxin B. Nucleic Acids Res 2003313027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wlotzka B, Leva S, Eschgfaller B.et al In vivo properties of an anti‐GnRH Spiegelmer: an example of an oligonucleotide‐based therapeutic substance class. Proc Natl Acad Sci U S A 2002998898–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helmling S, Maasch C, Eulberg D.et al Inhibition of ghrelin action in vitro and in vivo by an RNA‐Spiegelmer. Proc Natl Acad Sci U S A 200410113174–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobelt P, Tebbe J J, Tjandra I.et al Two immunocytochemical protocols for immunofluorescent detection of c‐Fos positive neurons in the rat brain. Brain Res Brain Res Protoc 20041345–52. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G W C.The rat brain in stereotaxic coordinates, 3rd edn. San Diego: Academic Press, 1997
- 32.Nakazato M, Murakami N, Date Y.et al A role for ghrelin in the central regulation of feeding. Nature 2001409194–198. [DOI] [PubMed] [Google Scholar]
- 33.Wortley K E, Anderson K, Garcia K.et al Deletion of ghrelin reveals no effect on food intake, but a primary role in energy balance. Obes Res 200412170 [Google Scholar]
- 34.Cummings D E, Clement K, Purnell J Q.et al Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 20028643–644. [DOI] [PubMed] [Google Scholar]
- 35.DelParigi A, Tschop M, Heiman M L.et al High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader‐Willi syndrome. J Clin Endocrinol Metab 2002875461–5464. [DOI] [PubMed] [Google Scholar]