Short abstract
Early experience of fontolizumab, a humanised anti‐interferon γ antibody, in active Crohn's disease has shown that the drug caused a significant decrease in endoscopic severity scores and CRP and was reasonably well tolerated. Fontolizumab may be worthy of further clinical trials
Keywords: interferon γ, monoclonal antibody, fontolizumab, immunogenicity, Crohn's disease
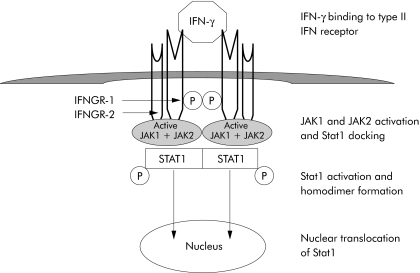
Interferons (IFN) are cytokines produced by immune cells in response to virus, bacteria, parasites, and tumour cells. Interferons exhibit immunomodulatory and antitumour activity in addition to the antiviral activity which defined the interferons. Three types of interferons are recognised in humans. Type I interferons consist of 14 alpha isoforms and beta, epsilon, omega, and kappa isoforms. Type I interferon receptor is composed of two chains, IFNAR‐1 and IFNAR‐2, and the signal transduction pathway involves constitutively expressed Janus family kinases Jak 1 and Tyk 2 with activation of Stat1, Stat2, Stat3, and Stat5. Interferon γ (IFN‐γ) is classified as a type II interferon. Type II interferon receptor is composed of IFNGR‐1 and IFNGR‐2 subunits and the signal transduction pathway involves Jak 1 and Jak 2 kinases with phosphorylation and activation of Stat1 (fig 1). The third type consists of interferon lambda with three isoforms.
Figure 1 Binding of interferon γ to type II interferon receptor composed of IFNGR‐1 and IFNGR‐2 subunits. This leads to activation of Janus family kinases Jak1 and Jak2 and phosphorylation of Stat1. Stat1 homodimers are translocated into the nucleus with initiation of gene transcription.
IFN‐γ drives expression of MHC class II on antigen presenting cells, increases chemokine secretion, and activates macrophages, lymphocytes, and endothelial cells. In addition to activation of Stat1, IFN‐γ also activates Raf‐1 and β‐Raf kinases with resulting activation of p42 mitogen activated protein kinase. A further signalling pathway activated by interferons is the ubiquitously expressed phosphatidylinositol 3′ kinase enzymes. In inflammatory bowel disease, both Crohn's disease (CD) and ulcerative colitis (UC), plasma and tissue concentrations of proinflammatory cytokines such as tumour necrosis factor TNF, IFN‐ γ, interleukin (IL)‐1β, IL‐6, and IL‐8 are increased. It is customary to consider CD as a TH1 lymphocyte mediated disease with lamina propria T lymphocytes producing large amounts of IFN‐γ and small amounts of IL‐13, while UC resembles an atypical TH2 lymphocyte mediated disease with little IFN‐γ but marked IL‐13 secretion from natural killer T cells.1 However, other researchers have described high expression of IFN‐γ and TNF in the mucosa of at least a proportion of patients with active UC.2 Immunoregulatory therapy with type I interferons such as IFN‐α or IFN‐β can inhibit production of TNF and IFN‐γ, antagonise IFN‐γ signalling pathway, and increase production of anti‐inflammatory cytokine IL‐10, although most studies have been in multiple sclerosis.3,4 IFN‐β has also been shown to be immunoregulatory by enhancing regulatory T lymphocyte and natural killer cell activity.5,6 Pilot studies with natural or recombinant IFN‐β in 25 refractory UC patients showed high response rates in an open study.7 Subcutaneous recombinant IFN‐β‐1a administered three times per week in a randomised dose escalation study in moderately active UC showed some beneficial anti‐inflammatory effect with remission induced in three of 10 IFN‐β‐1a treated patients compared with 0/7 placebo treated patients (p = 0.02).8 However, a further and larger randomised controlled trial (n = 91) of recombinant IFN‐β‐1a in active steroid refractory UC was negative for clinical efficacy end points.9 Subcutaneous IFN‐α‐2A significantly improved Powell‐Tuck disease activity index, inflammatory bowel disease questionnaire (IBDQ) score, and histology score compared with baseline in 16 patients with active left sided UC.10 However, in this randomised open label study, the 16 patients who received prednisolone enemas were not different in terms of clinical disease activity index, IBDQ, and histology score from IFN‐α‐2A treated patients. The largest randomized controlled trial of pegylated IFN‐α in 60 patients with active UC however failed to show clinical efficacy, although the higher dose of 1 μg/kg subcutaneously showed biological efficacy with a significant decrease in C reactive protein (CRP) concentrations.11 Overall, the evidence for the efficacy of type I interferons in IBD is therefore unconvincing.
In the era of biological therapies with monoclonal antibodies, IFN‐γ predictably has been identified as a target cytokine in chronic inflammatory diseases. In two studies published in this issue of the Gut,12,13 the early experience of anti‐IFN‐γ antibody in active CD has been reported (see pages 1131 and 1138). In a phase I/II dose ranging (0.1, 1.0, and 4.0 mg/kg) evaluation in active CD, fontolizumab, a humanised anti‐IFN‐γ antibody, was reasonably well tolerated with minimal immunogenicity. Flu‐like symptoms, chills, and fever were noted in the active treatment groups but not in the placebo group.12 Although in this small study (n = 45) with a high placebo response rate clinical efficacy end points did not reach statistical significance, a significant decrease in median CD endoscopic index of severity score was noted in the highest dose (4.0 mg/kg) subgroup compared with placebo. At day 29, evidence of biological efficacy was also noted with a significant decrease in CRP from baseline in the 1.0 and 4.0 mg/kg intravenous cohort. In addition to mucosal healing, aberrant HLA‐DR, CD3, Stat‐1, CXCR3, and Ki‐67 expression decreased in mucosal biopsies in the higher dose (especially 4 mg/kg) group.12 This would suggest that the 4.0 mg/kg dose of fontolizumab would be worthy of further clinical trials, although even higher doses may need to be explored.
Therefore, in a larger phase II study involving 133 patients with active CD, fontolizumab was administered in a single dose of either 4 or 10 mg/kg compared with placebo.13 The study did not reach its primary efficacy end point, either clinical response (decrease in Crohn's disease activity index (CDAI) ⩽100) or remission (CDAI <150). However, the 91 patients who received two doses at 0 and 28 days showed a significant increase in clinical response rates at day 56 compared with the placebo group. There was no indication of any additional advantage of the 10 mg/kg dose compared with the 4 mg/kg dose.
A high placebo response was noted in both fontolizumab trials which could be diminished by stratification into elevated CRP at baseline. This has been noted in trials of other biological therapies such as certolizumab pegol phase II14 and natalizumab phase II and III.15,16 In these trials it is likely that elevated CRP identified a subgroup of recruited patients with genuine inflammatory activity, and therefore served as a quality control for the subjective nature of CDAI, which may be elevated with irritable bowel syndrome‐like symptoms associated with IBD.17
Fontolizumab was generally well tolerated. Flu‐like symptoms12 are probably a consequence of action of type I interferons as a result of inhibition of IFN‐γ. Type I interferons may induce transient chemokine release (such as CXCL10) which coincides with occurrence of flu‐like symptoms.18 Immunogenicity was modest, but neutralising antibodies were detected in a proportion of patients.13
Interferons were the first recombinant DNA technology product to be widely used in cancer therapeutics and are still very valuable therapeutic agents in the treatment of chronic leukaemia, hairy cell leukaemia, lymphoma, renal carcinoma, bladder carcinoma, and melanoma.19 The antiviral efficacy of interferons have found established clinical use in chronic hepatitis B and hepatitis C (table 1). However, the use of interferons in chronic immune mediated inflammatory diseases is still limited, apart from relapsing and remitting multiple sclerosis. IFN‐γ is the signature cytokine of TH1 lymphocytes which characterise CD, suggesting that interfering with IFN‐γ signalling may be an attractive therapeutic strategy in CD. Dogmatic classification of CD as a TH1 mediated disease and UC as a TH2 like disease has however hampered the progress of targeted therapy in both diseases. For example, recent evidence suggests that anti‐TNF therapy is equally effective in both CD and UC, despite the initial reluctance to use anti‐TNF therapy in a TH2‐like disease.20 Classifying chronic inflammatory diseases into TH1 (IFN‐γ, IL‐2) or TH2 (IL‐4, IL‐5, IL‐13) mediated diseases is an over simplification. IL‐12 is crucial in the development of IFN‐γ producing TH1 lymphocytes and anti‐IL‐12p40 antibody has been shown to be effective in CD.21 However, the IL‐12p40 chain is also a component of IL‐23, a key cytokine for driving IL‐17 production, which is a recently described potent proinflammatory cytokine which upregulates chemokine production in target cells.22 Both IL‐12 and IL‐23 interact with receptors that share the IL‐12 receptor β1 (IL12Rβ1) subunit. IL‐23 promotes the development of a pathogenic T lymphocyte population described as TH‐17, distinct from TH1 and TH2, which is characterized by IL‐6, IL‐17, and TNF production.23 Therefore, it is possible that antagonism of IFN‐γ signalling may have a more restricted effect compared with antagonism of IL‐12p40 (antagonising both IL‐12 and IL‐23) and antagonism of TNF (fig 2). In addition, blockade of IFN‐γ may enhance the development of TH17 effector cells. Chronic inflammation is a nexus of pathways, and multipoint blockade may be necessary to increase clinical efficacy. Further clinical trial experience with anti‐IL‐12p40 and anti‐IFN‐γ is necessary to determine which one of these monoclonal antibodies prove to be more effective in the treatment of inflammatory bowel disease, including CD and UC.
Table 1 Clinical uses of interferon modulation.
| Type of interferon | Established or potential clinical use |
|---|---|
| Interferon α | Chronic myelogenous leukaemia |
| Hairy cell leukaemia | |
| Follicular lymphoma | |
| Renal cell carcinoma | |
| Bladder carcinoma | |
| Liver metastasis of carcinoid tumour | |
| Malignant melanoma | |
| AIDS related Kaposi's sarcoma | |
| Chronic hepatitis B | |
| Chronic hepatitis C | |
| Interferon β | Multiple sclerosis |
| ?Ulcerative colitis | |
| Interferon γ | Chronic granulomatous disease |
| Severe malignant osteopetrosis | |
| Anti‐interferon γ (Fontolizumab) | ? Crohn's disease |
| ? Rheumatoid arthritis |
Figure 2 Schematic diagram showing the development of naïve CD4+ T lymphocytes into TH1, TH2, and TH17 effector cells. Interferon γ (IFN‐γ) characterises the TH1 lymphocytes. Interleukin (IL)‐12 and IL‐23 bind to receptors that share the IL‐12Rβ1 subunit. The putative blocking effects (⊥) of anti‐IFN‐γ, anti‐tumour necrosis factor (TNF), and anti‐IL12p40 are shown.
Footnotes
Conflict of interest: None declared.
References
- 1.Fuss I J, Heller F, Boirivant M.et al Nonclassical CD1d‐restricted NK T cells that produce IL‐13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 20041131490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukada T, Nakamura T, Limura M.et al Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytopheresis. Am J Gastroenterol 2002972820–2828. [DOI] [PubMed] [Google Scholar]
- 3.Panitch H S, Folus J S, Johnson K P. Recombinant IFN‐β inhibits IFN‐gamma production in MS. Ann Neurol 198722137 [Google Scholar]
- 4.Brod S A, Marshall G D, Henninger E M.et al Interferon‐β 1b treatment decreases tumour necrosis factor‐alpha and increases interleukin‐6 production in multiple sclerosis. Neurology 1996461633–1638. [DOI] [PubMed] [Google Scholar]
- 5.Rudick R A, Ransohoff R M, Peppler R.et al Interferon beta induces interleukin‐10 expression: relevance to multiple sclerosis. Ann Neurol 199640618–627. [DOI] [PubMed] [Google Scholar]
- 6.Schnaper H W, Aune T M, Pierce C W. Suppressor T cell activation by human leukocyte IFN. J Immunol 19831312301–2306. [PubMed] [Google Scholar]
- 7.Musch E, Andus T, Malek M. Induction and maintenance of clinical remission by interferon‐beta in patients with steroid‐refractory active ulcerative colitis—an open long‐term pilot trial. Aliment Pharmacol Ther 2002161233–1239. [DOI] [PubMed] [Google Scholar]
- 8.Nikolaus S, Rutgeerts P, Fedorak R.et al Interferon β‐1a in ulcerative colitis: a placebo‐controlled, randomized, dose escalating study. Gut 2003521286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musch E, Andus T, Kruis W.et al Interleukin‐beta‐1a for the treatment of steroid‐refractory ulcerative colitis: a randomized, double‐blind, placebo‐controlled trial. Clin Gastroenterol Hepatol 20053581–586. [DOI] [PubMed] [Google Scholar]
- 10.Madsen S M, Schlichting P, Davidsen B.et al An open‐labeled, randomized study comparing systemic interferon‐α‐2A and prednisolone enemas in the treatment of left‐sided ulcerative colitis. Am J Gastroenterol 2001961807–1815. [DOI] [PubMed] [Google Scholar]
- 11.Tilg H, Vogelsang H, Ludwiczek O.et al A randomized placebo controlled trial of pegylated interferon α in active ulcerative colitis. Gut 2003521728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinisch W, Hommes D W, Van Assche G.et al A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti‐interferon γ antibody, in patients with moderate to severe Crohn's disease. Gut 2006551138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hommes D W, Mikhajlova T L, Stoinov S.et al Fontolizumab, a humanised anti‐interferon γ antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease. Gut 2006551131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber S, Rutgeerts P, Fedorak R.et al A randomized, placebo controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology 2005129807–818. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Goldin E, Gordon F.et al Natalizumab for active Crohn's disease. N Engl J Med 200334824–32. [DOI] [PubMed] [Google Scholar]
- 16.Sandborn W J, Colombel J F, Enns R.et al Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med 20053531912–1925. [DOI] [PubMed] [Google Scholar]
- 17.Acciuffi S, Ghosh S, Ferguson A. Strengths and limitations of the Crohn's disease activity index, revealed by an objective gut lavage test of gastrointestinal protein loss. Aliment Pharmacol Ther 199610321–326. [DOI] [PubMed] [Google Scholar]
- 18.Buttmann M, Merzyn C, Rieckmann P. Interferon‐beta induces transient systemic IP‐10/CXCL10 chemokine release in patients with multiple sclerosis. J Neuroimmunol 2004156195–203. [DOI] [PubMed] [Google Scholar]
- 19.Borden E C. Interferons and cancer: where from here? J Interferon Cytokine Res 200525511–527. [DOI] [PubMed] [Google Scholar]
- 20.Rutgeerts P, Sandborn W J, Feagan B G.et al Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 20053532462–2476. [DOI] [PubMed] [Google Scholar]
- 21.Mannon P J, Fuss I J, Mayer L.et al Anti‐interleukin‐12 antibody for active Crohn's disease. N Engl J Med 20043512069–2079. [DOI] [PubMed] [Google Scholar]
- 22.Kolls J K, Linden A. Interleukin‐17 family members and inflammation. Immunity 200421467–476. [DOI] [PubMed] [Google Scholar]
- 23.Langrish C L, Chen Y, Blumenschein W M.et al IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005201233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]