Abstract
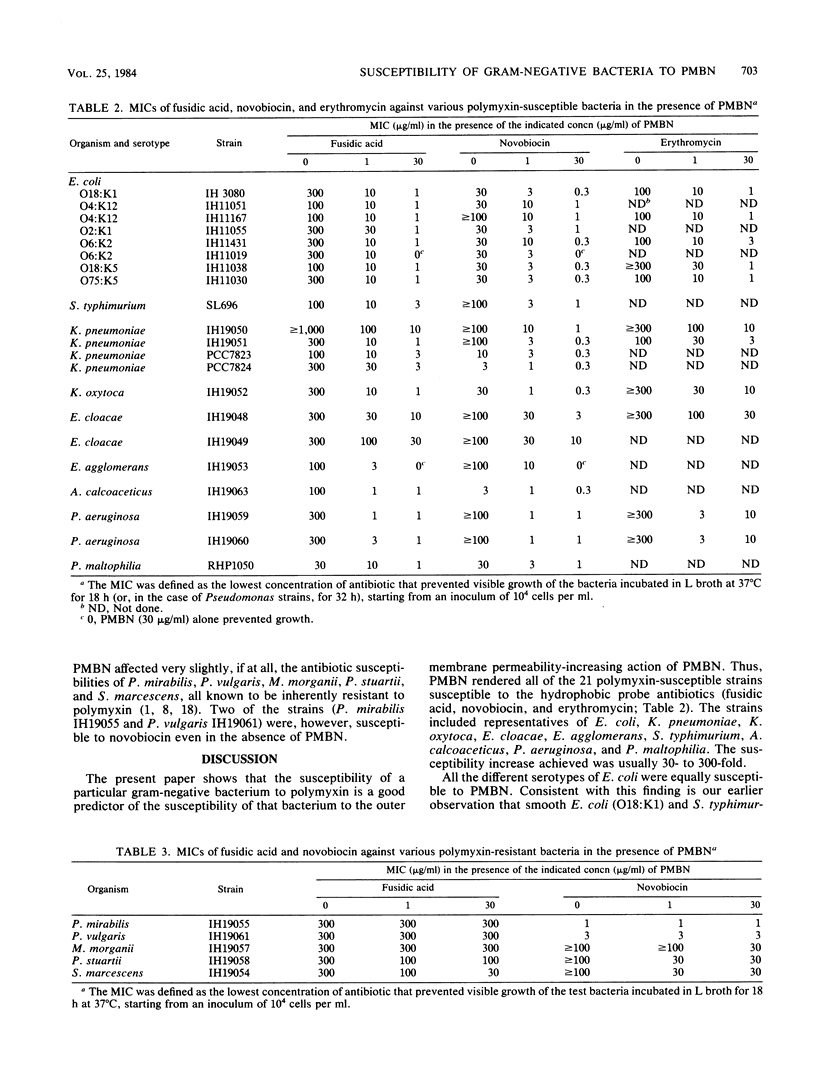
Subinhibitory concentrations of polymyxin B nonapeptide sensitized all 21 polymyxin-susceptible gram-negative bacterial strains studied to hydrophobic antibiotics such as fusidic acid, novobiocin, and erythromycin. The susceptibility increases were usually 30- to 300-fold. The strains included representatives of Escherichia coli with different O- and K-antigens, Klebsiella pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, Enterobacter agglomerans, Salmonella typhimurium, Acinetobacter calcoaceticus, Pseudomonas aeruginosa, and Pseudomonas maltophilia. In contrast, polymyxin-resistant strains (Proteus mirabilis, Proteus vulgaris, Morganella morganii, Providencia stuartii, and Serratia marcescens) were resistant to the action of polymyxin B nonapeptide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chedid L., Parant M., Parant F., Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968 Feb;100(2):292–306. [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- Galelli A., le Garrec Y., Chedid L. Transfer by bone marrow cells of increased natural resistance to Klebsiella pneumoniae induced by lipopolysaccharide in genetically deficient C3H/He mice. Infect Immun. 1979 Feb;23(2):232–238. doi: 10.1128/iai.23.2.232-238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Takeda H., Ito H., Sato H., Shimizu M. [Studies on the compounds related to colistin. IX. On the chemical deacylation of colistin and colistin derivatives (author's transl)]. Yakugaku Zasshi. 1974 Nov;94(11):1491–1494. doi: 10.1248/yakushi1947.94.11_1491. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Neisseria gonorrhoeae cell envelope: permeability to hydrophobic molecules. J Bacteriol. 1981 Feb;145(2):946–952. doi: 10.1128/jb.145.2.946-952.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979 Oct 2;18(20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Franson R. C., Beckerdite S., Schmeidler K., Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Invest. 1975 Jan;55(1):33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Stendhal O., Elsbach P. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J Clin Invest. 1982 Apr;69(4):959–970. doi: 10.1172/JCI110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]


