Abstract
Background and aims
In studies with small numbers of cases, it has been shown that endoscopic resection of adenomas in ulcerative colitis represents adequate treatment. In a larger study cohort with more prolonged follow up, we assessed the reliability of this finding.
Methods
Between 1988 and 2002, 148 consecutive patients, mainly from private gastroenterologists' practices, with ulcerative colitis were diagnosed as having an adenoma. In 60 patients, histological diagnosis was established in biopsies and in 87 patients in polypectomy specimens; one patient underwent proctocolectomy following diagnosis. The outcome of these patients was analysed after a mean follow up period of 6.0 (3.63) years.
Results
Among 60 patients, surprisingly without endoscopic treatment, 48.3% developed ulcerative colitis associated neoplasia in the same colon segment (23.3% low grade intraepithelial neoplasia; 8.3% high grade intraepithelial neoplasia; 16.7% carcinoma). Among 87 patients undergoing polypectomy of the adenoma, follow up revealed colitis associated neoplasia in other segments of colon in 4.6% of cases.
Conclusion
Development of adenocarcinomas in a total of 6.7% of the overall patient group, and in 2.3% of those undergoing polypectomy, indicates that biopsy based diagnosis of an adenoma in ulcerative colitis must be considered to mandate endoscopic resection of the lesion; 40% of affected patients did not receive any form of endoscopic removal of the lesion. This shows that the most recent guidelines are not followed in a considerable number of patients with ulcerative colitis in private practice in Germany. Although polypectomy of the adenoma represents adequate therapy, further regular follow up examinations are nevertheless necessary.
Keywords: adenoma, ulcerative colitis, follow up, polypectomy
In the 1990s, diagnosis of adenoma in patients with ulcerative colitis was established only when the lesion was located proximal to the ulcerative colitis in an endoscopically and histologically normal mucosa.1,2,3 In all other cases of adenomatous intraepithelial neoplasias located in the colorectal mucosa bearing ulcerative colitis, low grade intraepithelial neoplasia (dysplasia) in ulcerative colitis was assumed, and proctocolectomy recommended.2
Today, in 2006, it is accepted, at least in large specialised centres, that it is possible to distinguish between sporadic adenoma and colitis associated neoplasms in patients with ulcerative colitis, even if no controlled studies of larger patient numbers are available to date.
In 1993, we reported that, in our view, adenomas may also arise within the ulcerative colitis mucosa, and that they can be distinguished from intraepithelial neoplasias in such patients on the basis of gross findings and histological criteria.3 While this view initially met with scepticism on the part of other pathologists,4 it did eventually find acceptance.5,6 Further clinicopathological,7 immunohistochemical,8,9 and molecular‐pathological10 differences between adenomas and intraepithelial neoplasias in ulcerative colitis lent support to the hypothesis that adenomas can indeed be distinguished from colitis associated low grade intraepithelial neoplasias.
The question thus arose as to whether such lesions in ulcerative colitis diagnosed as “adenoma” are adequately treated with endoscopic polypectomy, or whether post‐polypectomy follow up data indicate a need for proctocolectomy. Initial follow up results in patients undergoing polypectomy of an “adenoma”3,5,11,12,13,14 indicated that there is no danger of developing a carcinoma. However, most of these studies investigated only very small numbers of cases. Two studies published in 1999,6,15 involving 30 and 24 cases followed over a mean period of 4.1 and 3.4 years, respectively, also concluded that endoscopic polypectomy represents adequate treatment. In a further study16 involving 34 patients with an adenoma in ulcerative colitis, a carcinoma developed in only 2.9% of cases within a mean follow up period of 5.0 years. However, it must be noted that only very small numbers of cases were involved in all of these studies.
The aim of the present study, involving a relatively large number of 148 patients with such adenomas, was therefore to investigate the question of whether endoscopic resection of an adenoma in ulcerative colitis truly represents adequate therapy or whether proctocolectomy is mandatory.
Patients and methods
In the period between 1988 and 2002 at the Institute of Pathology, Bayreuth, Germany, 148 ulcerative colitis patients were diagnosed on the basis of biopsy or polypectomy material as having adenomas. A polypectomy specimen was always considered whenever the gastroenterologist indicated a polypectomy or whenever a zone of unaffected mucosa surrounded the neoplasm. Specimens were sent in on a consecutive basis, mostly from private gastroenterologists' practices, and from a few larger hospital centres for gastroenterology. Criteria for diagnosis of a sporadic adenoma within ulcerative colitis were according to Schneider and Stolte's3 endoscopic recognition of a sharply deliminated polypoid or elevated lesion with a smooth regular surface. Histological criteria3,7,21 concerned the morphology of glands, goblet cells, stromal reactions, cell nuclei, proliferation zone, and deliminiation of the lesion. Grading of neoplasia was always based on the worst area of the lesion.
glands: glands in adenoma are, in general, round and oval, and build up regularly. There is an equal configuration referring mainly to size and diameter of the glands. In ulcerative associated neoplasia one would expect varying size and diameter of glands.
goblet cells: in adenomas, goblet cells are configured regularly: size and diameter show equal distribution and are found mainly in the apical part of cells. This is different in colitis associated neoplasms with varying diameters, size, distribution, and localisation within cells.
nuclei: nuclei in adenomas are, in general, elongated, hyperchromatic, and parallel orientated. Configuration, size, chromatin, and orientation do not vary much over a particular lesion.
stromal tissue: in adenomas there is only a little loose stromal tissue in between the cells. In colitis associated neoplasms there is large variation in appearance and width of stromal tissue in between the glands.
proliferation zone: in the luminal part of adenomas, very early zones of the neoplasia can be detected, starting from the apical part of the mucosa and growing downwards (top‐down morphology). Colitis associated neoplasms start from the bottom of the mucosa growing upwards against apical parts of the mucosa.
delimination of the lesion: adenomas show a sharp delimination between neoplastic glands and surrounding mucosa. In colitis associated neoplasms, no such sharp delimination can be seen.
Distinction between sporadic adenoma or colitis associated neoplasia can only be made on all of the above criteria. Use of a single criterion is not diagnostically safe. If unsure of the criteria, lesions were regarded as colitis associated lesions rather than a sporadic adenoma.
Approval by an ethics committee was not necessary as the material was sent in for routine diagnostic work up. Table 1 shows the age and sex distribution of the patients, location of the adenomas within or proximal to ulcerative colitis, extent of colitis, number of adenomas (solitary or multiple (two or more)), and duration of the colitis. Mean follow up of these patients was 6.0 (3.6) years (range 0.25–15). A total of 882.2 patient years were evaluated.
Table 1 Characteristics of the 148 patients with adenomas in ulcerative colitis.
| Age (y) | 61.2 (13.1) |
| Sex (M:F) | 101:47 (2.1:1) |
| Disease duration (y) | 6.9 (8.1) |
| Solitary adenoma (%) | 83.7 |
| Two or more adenomas | 16.3 |
| Location (%) | |
| Proximal to ulcerative colitis | 17.6 |
| Within segment of ulcerative colitis | 82.4 |
| Extent of ulcerative colitis (%) | |
| Total ulcerative colitis (pancolitis) | 29.1 |
| Extensive ulcerative colitis (up to ascending colon) | 12.1 |
| Left sided colitis (up to descending colon) | 43.9 |
| Proctosigmoiditis or ulcerative proctitis | 14.0 |
Location of the adenomas diagnosed in accordance with the criteria published by us in an earlier paper3 is presented in table 2.
Table 2 Location of the 189 adenomas in the 148 patients with ulcerative colitis.
| Rectum | 31.1% |
| Sigmoid | 31.1% |
| Descending colon | 8.8% |
| Transverse colon | 8.9% |
| Ascending colon | 20.1% |
Endoscopic gross appearance of the adenomas was classified as follows:
42.9% pedunculated
25.9% sessile, and
31.2% “polypoid lesion or mass (dysplasia associated lesion or mass)”.
All data were processed using SPSS for Windows software (SPSS, Munich, Germany). Univariate analyses were carried out using the χ2 test or Fisher's exact test. Where applicable, odds ratios were calculated in accordance with the logistic regression model. To compare frequencies of diagnoses at given time points, the Kaplan‐Meier survival rate was used to estimate survival. To compare end points among different patient groups (adenoma and colitis associated neoplasia), log rank testing was used. Results were regarded as significant at p<0.05.
Results
In one (0.7%) of our 148 patients diagnosed as having an adenoma in ulcerative colitis, a proctocolectomy was nevertheless carried out. An incidental pT2 carcinoma was found in the surgical specimen that was not diagnosed prior to surgery.
Following histological diagnosis of adenoma in forceps biopsy material, 60 patients (40.5%) received only medical treatment for their ulcerative colitis but no endoscopic resection of the histologically diagnosed adenoma.
In 87 patients (58.9%) the adenoma was removed completely via the endoscope.
Course of disease in patients with histologically diagnosed adenoma not receiving endoscopic treatment
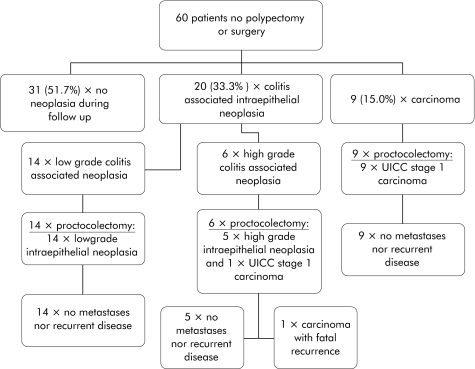
Figure 1 shows the results of the follow up investigations in the 60 patients who did not undergo endoscopic treatment. In 31 patients (51.7%) the endoscopy/biopsy follow up examinations within the period under observation, covering a mean of 87 (41.7) months (range 3–180), revealed no further neoplasia. Twenty nine patients (48.3%) developed an ulcerative colitis associated neoplasia in the same segment of the colon previously containing the adenoma. In 20 of these patients (33.3%) colitis associated intraepithelial neoplasia—in 14 patients (23.3%) low grade and in six patients (10%) high grade—was diagnosed on average after 20.6 (24.4) months (range 1–95). All 20 patients then underwent proctocolectomy. In one of the six patients with high grade intraepithelial neoplasia, a UICC stage I (pT2, pN0, pM0) colorectal carcinoma was detected in the surgical specimen. This patient died 46 months after surgery of the sequelae of a locoregional recurrence with metastases. The other 19 patients (14 with low grade and five with high grade intraepithelial neoplasia) remained tumour free after proctocolectomy for the rest of the observation period of, on average, 97 (35) months (range 23–155).
Figure 1 Overview of the clinical data of 60 patients with ulcerative colitis and sporadic adenoma receiving no further therapy.
The nine patients with colitis associated carcinoma (15%) diagnosed by endoscopy/biopsy also underwent proctocolectomy. All of these patients had a colitis associated UICC stage I carcinoma. During the further follow up period of 97 (11.3) months (range 65–154), patients remained recurrence and metastasis free.
Course of disease in patients undergoing polypectomy of histologically diagnosed adenoma
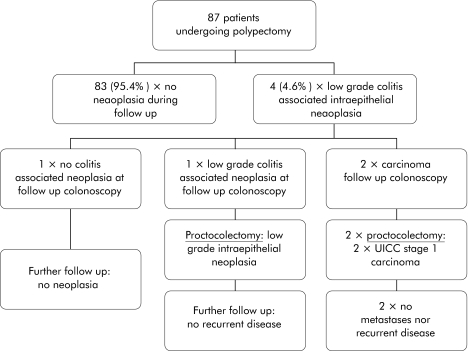
Figure 2 shows the course of disease in the 87 patients with ulcerative colitis who underwent polypectomy of the histologically diagnosed adenoma.
Figure 2 Overview of follow up of patients with ulcerative colitis and polypectomy of sporadic adenoma.
Within the mean follow up period of 53 (8.9) months (range 3–158), 83 patients (95.4%) developed no further neoplasia. In four patients (4.6%), the endoscopy/biopsy follow up examinations revealed a low grade intraepithelial neoplasia. This was not located at the site of the endoscopically removed adenoma but in another segment of the colon. Initially, all four patients were closely followed up with endoscopy/biopsy examinations performed at intervals of 3–6 months. In two of these four patients, a colitis associated adenocarcinoma was diagnosed after 11 and 57 months, respectively. Both patients underwent proctocolectomy, and the surgical specimen contained a colitis associated UICC stage I carcinoma. After 17 and 83 months, respectively, patients were both recurrence and metastasis free.
In one of the two remaining patients, the endoscopy/biopsy follow up again revealed a low grade intraepithelial neoplasia, and a proctocolectomy was performed. The low grade intraepithelial neoplasia was confirmed in the surgical specimen. In the remaining patient, follow up colonoscopy revealed no further signs of intraepithelial neoplasia, and this patient did not undergo surgery. Within the further follow up period of 76 and 90 months, respectively, neither patient developed a further neoplasia.
Comparison of outcomes in relation to treatment
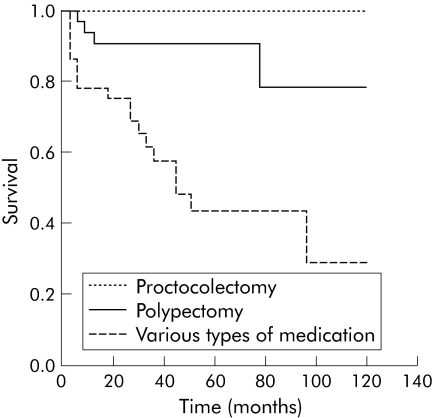
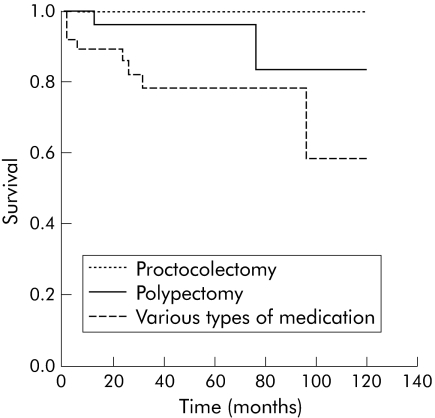
Comparison of the results achieved with different treatments (medical treatment versus polypectomy or proctocolectomy) in terms of development of intraepithelial neoplasia or carcinoma (figs 3, 4) revealed significant differences (p<0.01 and p<0.05). Differences between polypectomy and proctocolectomy were not statistically significant.
Figure 3 Comparison of the types of treatment (medical treatment versus polypectomy and proctocolectomy) over time (months) and the end point colitis associated intraepithelial neoplasia, using the Kaplan‐Meier survival function with log rank (p = 0.0019).
Figure 4 Comparison of the types of treatment (medical treatment versus polypectomy and proctocolectomy, and polypectomy versus proctocolectomy) over time (months) and the end point carcinoma, using the Kaplan‐Meier survival function with log rank (p = 0.0387) and log rank (p = 0.6729), respectively.
Discussion
Our results, established in the largest number of patients with adenoma in ulcerative colitis to date, and over a relatively long follow up period (six years on average) with endoscopy/biopsy examinations, showed that endoscopic resection of these adenomas represents adequate treatment, and that proctocolectomy, often previously applied in such patients, must therefore be considered over treatment.
However, our retrospective study also showed that an adenoma diagnosed in biopsy material from a patient with ulcerative colitis must be subjected to endoscopic resection, both to confirm the biopsy based adenoma diagnosis and to exclude colitis associated intraepithelial neoplasia.
Furthermore, our results showed that endoscopic resection of an adenoma must be followed by regular colonoscopy/biopsy follow up examinations with the aim of ensuring the timely diagnosis and treatment of neoplasia developing at other locations.
Over an average follow up of six years, 10 (6.7%) of our 148 patients with adenomas developed a colitis associated carcinoma. In the group of 87 patients undergoing endoscopic resection of the adenoma however, only two patients (2.3%) developed a colitis associated carcinoma, which was located in a segment of the colon other than that bearing the adenoma. These results confirm data from the literature. In seven studies published on this topic (most of which had small numbers of cases and different follow up periods), no carcinoma was diagnosed following polypectomy of the adenoma. Only in the study by Odze and colleagues16 did one of the 34 patients (2.9%) develop a carcinoma post‐polypectomy.
In addition, our study showed for the first time results of follow up examinations in patients with ulcerative colitis in whom an adenoma was diagnosed only in biopsy material and who did not subsequently undergo endoscopic resection of the adenoma. Of these 60 patients, 43.8% developed neoplastic lesions during the course of further follow up, most of which were intraepithelial neoplasia (33.3%) but some were carcinomas (15.0%). All of these neoplasms were located in the same segment of the colon as the previously biopsy diagnosed adenoma.
All of these patients underwent proctocolectomy. The nine carcinomas were all UICC stage I lesions and none of the patients developed recurrent carcinoma or metastases during the follow up period. Among the 20 patients with intraepithelial neoplasia, follow up biopsy material revealed high grade intraepithelial neoplasia in six cases and a UICC stage I carcinoma in the surgical specimen of one of these. Despite the fact that his carcinoma was in a favourable stage, this patient subsequently died of locoregional recurrence with metastases.
The outcome of these patients diagnosed as having an adenoma in biopsy material was, for us, most surprising. We had proceeded on the assumption that a diagnosis of adenoma in forceps biopsy material would always prompt a polypectomy. In our pathology reports containing a diagnosis of biopsy based adenoma, we always point out that the differential diagnosis between adenoma and low grade intraepithelial neoplasia in biopsy material is not reliably, and therefore follow up colonoscopy involving a subtle search for endoscopically visible dysplasia associated lesions or mass, wherever possible with magnification chromoendoscopy,17,18,19,20 and endoscopic resection of the lesion previously diagnosed as adenoma, is indicated.
Although statistically significant differences are found between adenomas and low grade intraepithelial neoplasia in terms of age distribution, duration of ulcerative colitis, endoscopic gross appearance, number of lesions,3,21 immunohistochemical expression of p53, Ki67, and Bcl‐2,22 DNA cytometry,23 and loss of heterozygosity of the Hippel Lindau gene,24 all of these differences overlap between adenoma and low grade intraepithelial neoplasia. This underscores the perception that the biopsy based diagnosis of adenoma is uncertain and that there continues to be a need for endoscopic resection of the lesion for further diagnostic work up to obtain a basis for deciding the necessary treatment. This point needs to be given greater emphasis than has previously been the case in the pathologist's comments on a report indicating a diagnosis or suspected diagnosis of adenoma in forceps biopsy material. When confronted by such biopsy material, it might be better to follow the proposal of Odze5 and establish a diagnosis of adenoma‐like dysplasia so as to underscore the uncertainty of the biopsy based differential diagnosis between adenoma and low grade intraepithelial neoplasia, and thus add more urgency to the need to send the patient for further follow up colonoscopy.
In conclusion, our retrospective study showed that endoscopic resection of an adenoma in ulcerative colitis represents adequate treatment. If, however, an adenoma has been diagnosed only on the basis of biopsy material, it should be removed in toto via the endoscope at the next follow up endoscopy, which should not be delayed unduly. Furthermore, all patients with ulcerative colitis undergoing polypectomy of an adenoma should undergo regular surveillance colonoscopy with the aim of detecting and treating subsequently developing neoplasia in good time.
Footnotes
Conflict of interest: None declared.
References
- 1.Fenoglio C M, Haggitt R C, Hamilton S R.et al Colonic dysplasia. Pathol Annu 19811181–213. [PubMed] [Google Scholar]
- 2.Kornbluth A, Sachar D B. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, practice parameters committee. Am J Gastroenterol 2004991371–1385. [DOI] [PubMed] [Google Scholar]
- 3.Schneider A, Stolte M. Differential diagnosis of adenomas and dysplastic lesions in patients with ulcerative colitis. Z Gastroenterol 199331653–656. [PubMed] [Google Scholar]
- 4.Torres C, Antonioli D, Odze R D. Polypoid dysplasia and adenomas in inflammatory bowel disease: a clinical, pathologic, and follow‐up study of 89 polyps from 59 patients. Am J Surg Pathol 199822275–284. [DOI] [PubMed] [Google Scholar]
- 5.Odze R D. Adenomas and adenoma‐like DALMs in chronic ulcerative colitis: a clinical, pathological, and molecular review. Am J Gastroenterol 1999941746–1750. [DOI] [PubMed] [Google Scholar]
- 6.Engelsgjerd M, Farraye F A, Odze R D. Polypectomy may be adequate treatment for adenoma‐like dysplastic lesions in chronic ulcerative colitis. Gastroenterology 19991171288–1294. [DOI] [PubMed] [Google Scholar]
- 7.Vieth M, Stolte M, Mueller E.et al Bioptical differential diagnosis of adenomas, dysplasias and carcinomas in patients with ulcerative colitis. Leber Magen Darm 200030125–132. [Google Scholar]
- 8.Walsh S V, Loda M, Torres C M.et al P53 and beta catenin expression in chronic ulcerative colitis‐associated polypoid dysplasia and sporadic adenomas: an immunohistochemical study. Am J Surg Pathol 199923963–969. [DOI] [PubMed] [Google Scholar]
- 9.Mueller E, Vieth M, Stolte M.et al The differentiation of true adenomas from colitis‐associated dysplasia in ulcerative colitis: a comparative immunohistochemical study. Hum Pathol 199930898–905. [DOI] [PubMed] [Google Scholar]
- 10.Fogt F, Urbanski S J, Sanders M E.et al Distinction between dysplasia‐associated lesion or mass (DALM) and adenoma in patients with ulcerative colitis. Hum Pathol 200031288–291. [DOI] [PubMed] [Google Scholar]
- 11.Connell W R, Lennard‐Jones J E, Williams C B.et al Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994107934–944. [DOI] [PubMed] [Google Scholar]
- 12.Rozen P, Baratz M, Fefer F.et al Low incidence of significant dysplasia in a successful endoscopic surveillance program of patients with ulcerative colitis. Gastroenterology 19951081361–1370. [DOI] [PubMed] [Google Scholar]
- 13.Medlicott S A, Jewell L D, Price L.et al Conservative management of small adenomata in ulcerative colitis. Am J Gastroenterol 1997922094–2098. [PubMed] [Google Scholar]
- 14.Rubin P H. Adenomas in ulcerative colitis: endoscopic polypectomy or colectomy? Inflamm Bowel Dis 19995304–305. [DOI] [PubMed] [Google Scholar]
- 15.Rubin P H, Friedman S, Harpaz N.et al Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology 19991171295–1300. [DOI] [PubMed] [Google Scholar]
- 16.Odze R D, Farraye F A, Hecht J L.et al Long‐term follow‐up after polypectomy treatment for adenoma‐like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 20042534–541. [DOI] [PubMed] [Google Scholar]
- 17.Kiesslich R, Fritsch J, Holtmann M.et al Methylene blue‐aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003124880–888. [DOI] [PubMed] [Google Scholar]
- 18.Kiesslich R, Burg J, Vieth M.et al Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 2004127706–713. [DOI] [PubMed] [Google Scholar]
- 19.Rutter M, Bernstein C, Matsumoto T.et al Endoscopic appearance of dysplasia in ulcerative colitis and the role of staining. Endoscopy 2004361109–1114. [DOI] [PubMed] [Google Scholar]
- 20.Hurlstone D P, McAlindon M E, Sanders D S.et al Further validation of high‐magnification chromoscopic‐colonoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2004126376–378. [DOI] [PubMed] [Google Scholar]
- 21.Vieth M, Behrens H, Stolte M. Sporadic adenoma and colitis‐associated intraepithelial neoplasia: a difficult differential diagnosis. Pathologe 20032436–43. [DOI] [PubMed] [Google Scholar]
- 22.Mueller E, Vieth M, Stolte M.et al The differentiation of true adenomas from colitis‐associated dysplasia in ulcerative colitis: a comparative immunohistochemical study. Hum Pathol 199930898–905. [DOI] [PubMed] [Google Scholar]
- 23.Porschen R, Robin U, Schumacher A.et al DNA aneuploidy in Crohn's disease and ulcerative colitis: results of a comparative flow cytometric study. Gut 199233663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogt F, Vortmeyer A O, Stolte M.et al Loss of heterozygosity of the von Hippel Lindau gene locus in polypoid dysplasia but not flat dysplasia in ulcerative colitis or sporadic adenomas. Hum Pathol 199829961–964. [DOI] [PubMed] [Google Scholar]