Abstract
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are frequently used in the treatment of irritable bowel syndrome (IBS) although evidence of their efficacy is scarce.
Aim
Twenty three non‐depressed IBS patients were recruited from a tertiary care centre and included in a crossover trial comparing six weeks of treatment with the SSRI citalopram (20 mg for three weeks, 40 mg for three weeks) with placebo. IBS symptom severity was the primary outcome measure, and depression and anxiety scores were also measured. The effect of acute administration of citalopram on colonic sensitivity and on colonic response to feeding was investigated as a putative predictor of symptomatic response to the drug.
Results
After three and six weeks of treatment, citalopram significantly improved abdominal pain, bloating, impact of symptoms on daily life, and overall well being compared with placebo. There was only a modest effect on stool pattern. Changes in depression or anxiety scores were not related to symptom improvement. The effect of acute administration of citalopram during a colonic barostat study did not predict clinical outcome. Analysis of the first treatment period as a double blind parallel arm study confirmed the benefit of citalopram over placebo.
Conclusions
The SSRI citalopram significantly improves IBS symptoms, including abdominal pain, compared with placebo. The therapeutic effect is independent of effects on anxiety, depression, and colonic sensorimotor function.
Keywords: selective serotonin reuptake inhibitor, citalopram, irritable bowel syndrome
The irritable bowel syndrome (IBS) is probably the most commonly encountered disorder by gastroenterologists in the industrialised world. It is defined by the presence of abdominal pain, associated with altered bowel habits, in the absence of organic disease.1 IBS is characterised by a symptom cluster which includes abdominal pain, often relieved by defecation, distension of the abdomen, disordered bowel habit, frequent feeling of incomplete evacuation, mucus in stool, looser stools with pain onset, and more frequent stools with pain onset.1
Several pharmacological treatments have been proposed for the treatment of IBS but the therapeutic effects in the IBS population as a whole are limited, especially concerning key symptoms such as abdominal pain and bloating.2,3 Recent developments have focused on serotonin mediated alterations in colonic transit to achieve symptomatic benefit in subgroups of IBS patients.2,3,4 Moreover, serotonin (5‐HT) modulates processing, sensation, and perception of visceral afferent information at the level of the central nervous system (CNS) (spinal chord and/or brain), a mechanism by which 5‐HT may influence visceral sensitivity.2,3,4 Serotonin3 (5‐HT3) receptor antagonists inhibit colonic motor activity in humans via a neural pathway2,3,4 and 5‐HT3 receptors are involved in the processing of visceral afferent information at the CNS level.4,5,6,7 The efficacy of alosetron, a 5‐HT3 receptor antagonist, in alleviating symptoms in female IBS patients has been demonstrated but the use of the drug was limited because of an unfavourable side effect profile.2,3,4,8,9 5‐HT4 receptor agonists enhance colonic transit, and the 5‐HT4 receptor agonist tegaserod was shown to be beneficial in the treatment of constipation predominant IBS.2,3,4,10 Finally, 5‐HT is a key neurotransmitter in the pathophysiology of mood and anxiety disorders, which frequently share comorbidity with IBS.11,12,13
Tricyclic antidepressants (TCA) are frequently used to treat patients with IBS, especially those with more severe or more refractory symptoms.3,14 Initially they were used because of the high prevalence of depression and anxiety disorders in patients with IBS although the doses that are used in IBS are generally lower than those needed to treat mood and anxiety disorders.3,14 TCAs not only have antidepressant but also neuromodulatory and analgesic properties. They are non‐selective serotonin reuptake inhibitors and they also have anticholinergic properties.15 Several placebo controlled studies have confirmed the efficacy of low doses of TCAs in IBS.3,14,15,16,17 However, in spite of these studies, it is unclear whether they act by influencing mood or anxiety, through a central or peripheral neuromodulatory effect, or through an analgesic effect. It is also unclear whether their effect in IBS is constituted by their anticholinergic or by their serotonin reuptake inhibitor effects.14 There is recent evidence that the TCA amitriptyline alters CNS processing of visceral afferent information, specifically during stressful conditions.18
The potential role of selective 5‐HT reuptake inhibitors (SSRIs) in the treatment of IBS has only been studied in a few trials and results have been inconsistent.19,20,21,22 Patients with IBS show increased sensitivity to distension of the colon compared with controls.15 In addition, an exaggerated colonic response to feeding is present in a subset of patients.15 Recently, we demonstrated that administration of the SSRI citalopram in healthy subjects decreases the sensitivity of the colon to distension and inhibits the colonic response to feeding.23 These observations may provide a rationale for use of citalopram in the treatment of IBS.
In the present study, we investigated the effect of citalopram on symptom severity in patients with IBS. We hypothesised that acute intravenous administration would affect colonic sensorimotor function in IBS, similar to the observations in healthy controls,23 and that the magnitude of this response might be a predictor of responsiveness to longer term oral treatment. Therefore, we also investigated whether the effect of acute administration of citalopram on colonic sensitivity and on colonic response to a meal would be able to predict the symptomatic response to the drug.
Materials and methods
Patient selection
Patients aged 20–70 years were eligible for the study if they fulfilled the Rome II criteria for IBS.1 Organic or metabolic disease had to be adequately excluded by routine biochemistry and colonoscopy in the past five years. In patients with diarrhoea, stool cultures and microscopic examination had to be negative and a lactose tolerance test had to be normal. Patients with a history of colonic or major abdominal surgery were excluded. All patients were seen by a psychiatrist for a structured psychiatric interview (structured clinical interview for DSM‐IV axis I disorders—patient edition, SCID‐P).24 Patients with current depression and those receiving therapy with neuroleptics, SSRIs, or TCAs during the past six weeks were not eligible. Pregnant female patients or patients of childbearing potential without effective contraception were excluded from the trial. All analgesic drugs, except for paracetamol, all prokinetic, and all spasmolytic drugs were forbidden for the entire course of the study; drugs that inhibit gastric acid secretion were allowed.
Study design
After a two week run in period, the study consisted of two treatment periods of six weeks each, separated by a three week washout period. Treatment comprised citalopram 20 mg during the first three weeks and 40 mg during the second three weeks, or matching placebo intake. A visit to the outpatient clinic was scheduled at the start of the run in period and at the beginning and end of each six week treatment period. After three weeks of treatment, a telephone call was scheduled to assess symptoms, adverse events, and to instruct the patient to double the dose of drug.
IBS symptom severity was assessed by a symptom questionnaire at the beginning and end of the run in, at the end of the washout period, and after three and six weeks treatment. This questionnaire assessed the same items as the daily diaries (see below) but provided more details regarding frequency, average severity, and severity of the worst episodes of symptoms. Using a 10 cm visual analogue scale (VAS), the severity of abdominal pain, severity of the worst episode of abdominal pain, severity of bloating, severity of the worst episode of bloating, severity of stool problems, interference of abdominal symptoms with daily life, and global assessment over the past two weeks were scored by the patient. Questions were asked to assess the number of days with abdominal pain and the number of days with bloating over the past two weeks. Similarly, stool pattern was assessed in more detail, and the patient registered the number of days with loose or watery stools, number of days with hard pellety stools, number of days with urgency, number of days with straining, and number of days with a sense of incomplete rectal evacuation.
Throughout the study, patients completed a daily diary to assess number of stools and indicated on a 10 cm VAS duration of abdominal pain, severity of abdominal pain, severity of bloating, stool consistency, and interference of abdominal symptoms with daily life. The diary indicated that the VAS scale ranged from absent to presence for symptoms, from watery to extremely hard for stool consistency, and from no influence to completely for interference with daily life.
At the beginning of the study, an extensive clinical psychiatric assessment was performed, and the symptom checklist‐90‐revised (SCL‐90R)25 and hospital anxiety and depression scale (HADS)26 were completed. At the end of each treatment period, the same questionnaires were repeatedly filled out. Both questionnaires have been extensively validated and used in medical and consultation‐liaison psychiatric settings.27,28
Colonic barostat study
A separate mechanistic barostat study, assessing the influence of citalopram or placebo on colonic sensitivity to distension and on the colonic response to feeding, was performed at least one week before the run in period of the clinical trial. Patients were offered the possibility of undergoing this examination again at the end of the first treatment period.
After an overnight fast, patients presented to the endoscopy department in the morning. After tap water enema cleansing and sedation with midazolam up to 5 mg intravenously, a left sided colonoscopy was performed and a colonic barostat/manometry probe was placed into the descending colon. Stepwise distensions by 2 mm Hg increments at two minute intervals were performed. Subjects were instructed to score the intensity of their perception of colonic distension at the end of every distending step using a graphic rating scale that combined verbal descriptors on a scale graded 0–6 (0 = absent, 1 = vague, 2 = mild, 3 = moderate, 4 = prominent, 5 = discomfort, and 6 = pain). The end point of each sequence of distensions was established at an intraballoon volume of 300 ml or when subjects reported discomfort or pain (score 5 or 6). Subsequently, placebo or 20 mg citalopram were administered intravenously over 20 minutes and the distensions were repeated. Afterwards, isobaric tone measurements were performed 30 minutes before and 90 minutes after ingestion of a mixed liquid meal (200 ml, 300 kcal).
Data analysis
The primary outcome variable was number of days with abdominal pain, assessed on the IBS symptom severity questionnaires. In a post hoc analysis, responders were defined as patients who had ⩾50% reduction in number of days with abdominal pain assessed at the end of the six week treatment. Secondary outcome variables were other symptoms on the IBS symptom severity questionnaires, daily diaries, and psychosocial questionnaires.
Daily diary severity scores were averaged into weekly scores. IBS symptom severity on the questionnaires was assessed by measuring VAS scores. All placebo and all citalopram treatment periods of the crossover study were analysed separately. Evolution of symptom severity during placebo or citalopram was assessed by comparing symptom severity at three and at six weeks to symptom severity at the start of treatment. In addition, to avoid any carryover effects, the first part of the study prior to crossover was analysed as a placebo controlled parallel group study. Mean weekly scores on the diaries were analysed using the same approach.
During the barostat studies, for each two minute isobaric distending period, the corresponding intraballoon volume was calculated by averaging the recording. The thresholds for perception and discomfort were computed after the experiments by analysing the perception score corresponding to each distension step. Perception threshold was defined as the first level of pressure and the corresponding volume that had evoked a perception score of 1 or more. Discomfort threshold was defined as the first level of pressure and the corresponding volume that had provoked a perception score of 5 or more. Colonic compliance was calculated as the slope of the pressure‐volume curve obtained during stepwise isobaric distensions.
To evaluate colonic tone before and after administration of citalopram or placebo, mean intraballoon volume was calculated over consecutive five minute intervals. The change in colonic tone was quantified by calculating the difference between the average intraballoon volume during the 30 minutes before and 30 minutes after drug administration.
To evaluate colonic tone before and after administration of the meal, mean intraballoon volume was calculated over consecutive five minute intervals. The colonic response to feeding was quantified by calculating the difference between the average intraballoon volume during the 30 minutes before and the first 60 minutes after administration of the meal. The maximum postprandial volume decrease and the time needed to reach the lowest postprandial volume were calculated.
Statistical analysis
Symptom scores and changes in scores on the IBS questionnaires and in the IBS diaries, as well as HADS and SCL‐90R questionnaire scores during treatment with citalopram or placebo, were compared by paired t test and by two way ANOVA for repeated measures. The correlation between changes in symptom severity and changes in the HADS and SCL‐90R scores were assessed by Pearson correlation analysis.
The threshold for discomfort during colonic barostat distension and mean changes in intraballoon volume after drug administration or after the meal were calculated. Barostat data (mean (SEM)) were compared by t test and by two way ANOVA. Symptom assessments during the barostat studies were analysed with a paired samples t test (and controlled with the non‐parametric Wilcoxon signed rank test).
Results
Conduct of the study
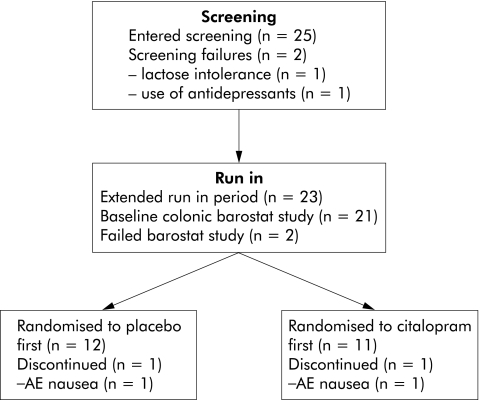
Of our 25 screened patients, one was excluded because of a positive lactose tolerance test and one patient was excluded because his general practitioner started an antidepressant during the run in period (fig 1). Twenty three IBS patients (18 women, mean age 39 (3) years) participated in the study. According to the Rome II definitions, four patients were constipation predominant, five were diarrhoea predominant, and all of the remaining patients had alternating bowel habits. Table 1 summarises the symptoms reported by the patients at the end of the run in period. Eleven patients were randomised to receive citalopram first, and twelve patients received placebo first. The groups did not differ in mean age (40.7 (4.6) v 38.1 (3.0); NS) or sex distribution (2/11 v 3/12 males; NS).
Figure 1 Flow chart depicting the selection and evolution of patients during the different phases of the study. AE, adverse event.
Table 1 Symptom severity before the run in period and before each treatment period.
| Visit 0 (before run in) | Visit 1 (before 1st treatment period) | Visit 4 (before 2nd treatment period) | |
|---|---|---|---|
| Overall severity assessment | 7.5 (0.3) | 7.5 (0.3) | 6.2 (0.6)* |
| No of days with impact on daily life | 5.0 (0.2) | 5.0 (0.2) | 4.3 (0.3)* |
| Severity of impact on daily life | 6.9 (0.3) | 6.7 (0.4) | 6.3 (0.5) |
| No of days abdominal pain | 5.4 (0.2) | 5.4 (0.2) | 4.7 (0.3)* |
| Severity of abdominal pain | 7.5 (0.3) | 7.5 (0.3) | 6.4 (0.6) |
| Worst episode of abdominal pain | 8.5 (0.3) | 8.4 (0.3) | 6.7 (0.7)* |
| No of days bloating | 4.9 (0.3) | 4.8 (0.3) | 4.2 (0.4)* |
| Severity of bloating | 6.2 (0.5) | 6.2 (0.5) | 5.1 (0.6)* |
| Worst episode of bloating | 7.3 (0.6) | 7.1 (0.6) | 6.3 (0.6)* |
| Severity of stool problems | 6.3 (0.5) | 6.4 (0.5) | 6.3 (0.6) |
| No of days with loose stools | 2.7 (0.4) | 2.9 (0.4) | 2.6 (0.4) |
| No of days with hard stools | 2.0 (0.4) | 2.0 (0.4) | 2.0 (0.4) |
| No of days with urgency | 2.6 (0.3) | 2.6 (0.4) | 2.4 (0.4) |
| No of days with straining | 2.9 (0.4) | 2.7 (0.4) | 2.5 (0.4)* |
| No of days with incomplete evacuation | 3.3 (0.4) | 3.2 (0.41) | 3.1 (0.4) |
*Significantly different from visit 1 (p<0.05).
Two patients stopped the study after three weeks of initial treatment (one citalopram, one placebo) because of nausea and abdominal discomfort. All other patients participated in the full study protocol as planned.
Colonic barostat study
In two patients, no successful positioning of the colonic barostat assembly into the descending colon was obtained. Twenty one IBS patients (17 women, mean age 37.4 (2.6) years) completed the barostat study of the descending colon prior to the study run in period. They were randomised to receive intravenous citalopram (n = 11) or placebo (n = 10) during the barostat measurements.
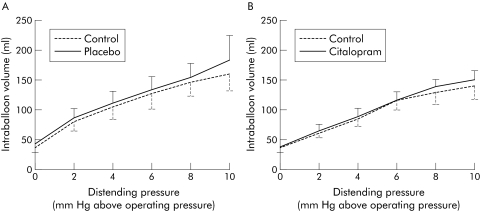
Both placebo and citalopram had no significant effect on colonic compliance (fig 2). Mean intraballoon volume was not significantly altered by placebo (116 (20) v 118 (31) ml; NS) or by citalopram (96 (13) v 91 (19) ml; NS). Placebo had no significant effect on pressure (4.5 (1.5) v 3.5 (1.3) mm Hg above operating pressure; NS) or volume (80 (24) v 75 (22) ml; NS) inducing first perception. Likewise, placebo did not affect pressure (16.3 (2.3) v 15 (2.4) mm Hg above operating pressure; NS) or the corresponding volume (194 (19) v 186 (23) ml; NS) inducing discomfort. Administration of citalopram also had no significant effect on pressures (7.0 (2.8) v 7.0 (2.6) mm Hg above operating pressure; NS) or volumes (104 (27) v 121 (23) ml; NS) inducing first perception, or on pressures (17.4 (2.8) v 16.4 (2.6) mm Hg above operating pressure; NS) or volumes inducing discomfort (177 (34) v 203 (28) ml; NS).
Figure 2 Pressure‐volume relationships before and after administration of placebo (A) and before and after administration of citalopram (B). No significant changes were observed.
Pre‐ and post‐meal intracolonic balloon volumes did not differ significantly after placebo (respectively 116 (14) and 108 (22) ml) or citalopram (respectively 99 (13) and 95 (19) ml) pretreatment. After placebo, ingestion of the meal caused, on average, a 7.6 (13.4) ml decrease in colonic balloon volume during the first 60 postprandial minutes. After citalopram, the decrease in balloon volume was not significantly altered (3.9 (13.8) ml; NS).
IBS symptom questionnaires
All patients had longstanding IBS symptoms (50 (6) months). Symptom severity remained stable throughout the run in period, as revealed by the symptom questionnaire at the beginning and the end of the run in period (table 1), and confirmed by the diaries.
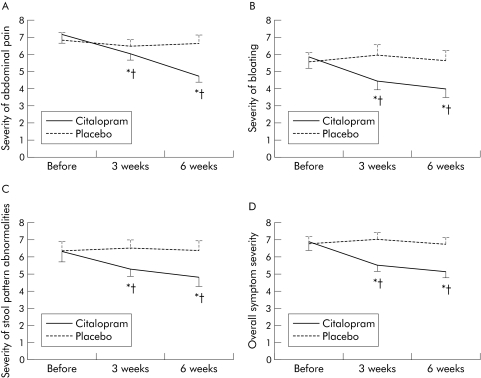
Citalopram significantly improved the number of days per week with abdominal pain after both three and six weeks of treatment compared with baseline while placebo had no significant effect. Similarly, citalopram but not placebo improved the severity of abdominal pain and the severity of the worst episode of abdominal pain (fig 3, table 2). Using a ⩾50% reduction in number of days with abdominal pain at the end of the six week treatment as a response definition, 12 patients responded to citalopram and four patients responded to placebo (intent to treat analysis, dropouts included with non‐responders, p = 0.01).
Figure 3 Influence of placebo or citalopram on the severity of abdominal pain (A), bloating (B), severity of stool pattern abnormalities (C), and on overall irritable bowel syndrome symptom severity (D). *p<0.05 compared with before treatment; †p<0.05 compared with placebo.
Table 2 Influence of citalopram and placebo on the severity and frequency of abdominal pain and bloating.
| Before citalopram | Citalopram 3 weeks | Citalopram 6 weeks | Before placebo | Placebo 3 weeks | Placebo 6 weeks | |
|---|---|---|---|---|---|---|
| No of days abdominal pain | 5.3 (0.3) | 4.5 (0.2)*† | 3.3 (0.3)*† | 4.9 (0.3) | 5.0 (0.3) | 5.1 (0.2) |
| Severity of abdominal pain | 7.2 (0.5) | 6.0 (0.3)*† | 4.7 (0.3)*† | 6.8 (0.5) | 6.5 (0.4) | 6.7 (0.4) |
| Worst episode of abdominal pain | 7.8 (0.5) | 6.9 (0.5)*† | 6.0 (0.5)*† | 7.5 (0.5) | 7.7 (0.4) | 7.6 (0.4) |
| No of days bloating | 4.5 (0.4) | 3.8 (0.4)*† | 3.1 (0.3)*† | 4.5 (0.3) | 4.5 (0.3) | 4.2 (0.4) |
| Severity of bloating | 5.8 (0.6) | 4.4 (0.5)*† | 4.0 (0.5)*† | 5.5 (0.5) | 6.0 (0.6) | 5.6 (0.6) |
| Worst episode of bloating | 6.9 (0.6) | 5.8 (0.6)* | 5.4 (0.6)*† | 6.7 (0.6) | 6.6 (0.6) | 7.1 (0.6) |
*Significant compared with run in: p<0.05.
†Significant compared with placebo: p<0.05.
Overall assessment of severity of stool pattern abnormalities was significantly improved during citalopram but not during placebo treatment (table 3, fig 3). With citalopram, significant improvement in urgency and straining was seen at week 6 and of a sense of incomplete evacuation at weeks 3 and 6 (table 3). Citalopram had no significant influence on number of days with hard stools but the number of days with loose stools at weeks 3 and 6 was affected. However, changes in the number of hard or loose bowel movements were modest and did not translate into a significant change in stool frequency in the diaries (see below). It is conceivable that the improvement in some of these items reflects subjective appreciation of bowel movements rather than true stool pattern characteristics. This is in line with the modest changes in numbers of hard and loose stools, lack of a significant change in stool frequency, and improved scores for straining, urgency, and sense of incomplete evacuation.
Table 3 Influence of citalopram and placebo on abnormalities of stool pattern.
| Before citalopram | Citalopram 3 weeks | Citalopram 6 weeks | Before placebo | Placebo 3 weeks | Placebo 6 weeks | |
|---|---|---|---|---|---|---|
| Severity of stool problems | 6.3 (0.6) | 5.3 (0.4)*† | 4.9 (0.5)*† | 6.4 (0.6) | 6.5 (0.5) | 6.4 (0.6) |
| No of days with loose stools | 2.7 (0.4) | 2.1 (0.3)*† | 1.9 (0.3)*† | 2.8 (0.5) | 2.6 (0.4) | 2.3 (0.3) |
| No of days with hard stools | 2.1 (0.4) | 1.9 (0.3) | 1.9 (0.4) | 2.0 (0.4) | 2.0 (0.3) | 1.9 (0.3) |
| No of days with urgency | 2.4 (0.4) | 2.1 (0.3) | 2 (0.3)* | 2.6 (0.4) | 2.5 (0.3) | 2.2 (0.3) |
| No of days with straining | 2.7 (0.4) | 2.5 (0.3) | 2.4 (0.3)*† | 2.5 (0.4) | 3.1 (0.5) | 3.2 (0.5) |
| No of days with incomplete evacuation | 3.0 (0.4) | 2.6 (0.3)*† | 2.5 (0.4)*† | 3.2 (0.4) | 3.2 (0.3) | 3.1 (0.4) |
*Significant compared with run in: p<0.05.
†Significant compared with placebo: p<0.05.
Citalopram significantly improved the severity of bloating after both three and six weeks of treatment while placebo had no significant effect (fig 3). Similarly, citalopram but not placebo improved the number of days per week with bloating and the severity of the worst bloating episode (table 2).
Citalopram, but not placebo, significantly improved the impact of IBS symptoms on daily life and number of days in which daily life was hampered by IBS symptoms (table 4). Overall severity of IBS symptoms was significantly improved during citalopram treatment but was not significantly affected by placebo (fig 3). Number of days in which daily life was hampered by IBS symptom was reduced by ⩾50% during citalopram treatment in 10 patients and during placebo treatment in three patients (p = 0.04).
Table 4 Impact of irritable bowel syndrome symptoms on daily life during treatment with citalopram and placebo.
| Before citalopram | Citalopram 3 weeks | Citalopram 6 weeks | Before placebo | Placebo 3 weeks | Placebo 6 weeks | |
|---|---|---|---|---|---|---|
| Overall severity assessment | 6.9 (0.5) | 5.6 (0.4)*† | 5.2 (0.4)*† | 6.8 (0.4) | 7.1 (0.4) | 6.8 (0.4) |
| No of days with impact on daily life | 4.8 (0.3) | 4.0 (0.3)*† | 3.2 (0.3)*† | 4.6 (0.2) | 4.9 (0.2) | 4.5 (0.2) |
| Severity of impact on daily life | 6.5 (0.5) | 5.8 (0.4)† | 5.2 (0.5)* | 6.5 (0.4) | 6.7 (0.3) | 6.3 (0.4) |
Note: Number of days is expressed as number of days in one week.
*Significant compared with run in: p<0.05.
†Significant compared with placebo: p<0.05.
Carryover effect
The use of crossover studies in functional bowel disorders has been criticised because of failure of symptom severity to return to baseline after the first treatment period. In the present study, severity of abdominal pain, bloating, and overall symptoms were lower at the beginning of the second treatment period compared with the start of the first treatment period, while stool pattern abnormalities were not significantly different (table 1).
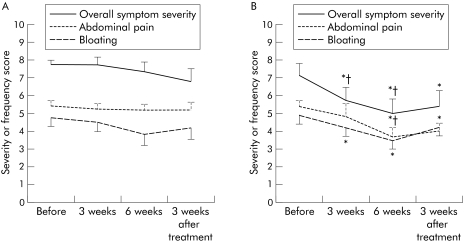
To eliminate any carryover effects, a separate analysis of the first treatment period as a parallel group design was performed. According to this analysis, citalopram provided significant improvement in overall symptom severity, abdominal pain (number of days, severity of abdominal pain, severity of the worst episode), bloating (number of days, severity of bloating), number of days with incomplete evacuation of stools, and number of days that symptoms affected daily life, compared with baseline (table 5). In contrast, placebo only improved the severity of the worst bloating episode. In the parallel group part of the study, citalopram was superior to placebo in improving number of days with abdominal pain, severity of abdominal pain, severity of the worst episode of abdominal pain, and overall symptom severity (fig 4).
Table 5 Analysis of the first part of the study as a parallel‐group design placebo‐controlled study.
| Before citalopram | Citalopram 3 weeks | Citalopram 6 weeks | 3 weeks after citalopram | Before placebo | Placebo 3 weeks | Placebo 6 weeks | 3 weeks after placebo | |
|---|---|---|---|---|---|---|---|---|
| No of days abdominal pain | 5.4 (0.3) | 4.8 (0.4) | 3.7 (0.5)*† | 4.0 (0.4)* | 5.4 (0.3) | 5.3 (0.3) | 5.2 (0.3) | 5.2 (0.4) |
| Severity of abdominal pain | 7.2 (0.6) | 6.2 (0.6) | 4.9 (0.7)*† | 5.4 (0.8)* | 7.8 (0.4) | 7.0 (0.4) | 7.0 (0.6) | 7.2 (0.8) |
| Worst episode of abdominal pain | 7.8 (0.6) | 6.6 (0.6)*† | 4.9 (0.8)*† | 5.4 (0.8) | 8.8 (0.3) | 8.7 (0.4) | 8.5 (0.4) | 7.7 (0.9) |
| No of days with bloating | 4.9 (0.5) | 4.2 (0.5)* | 3.4 (0.4)* | 4.2 (0.5) | 4.8 (0.5) | 4.5 (0.5) | 3.8 (0.6) | 4.2 (0.6) |
| Severity of bloating | 6.5 (0.8) | 5.1 (0.6) | 4.7 (0.7)* | 5.1 (0.7)* | 5.8 (0.7) | 5.4 (0.8) | 4.8 (0.7) | 5.2 (1.0) |
| Worst episode of bloating | 7.2 (0.9) | 6.6 (0.6) | 6.2 (0.5) | 6.1 (0.8) | 7.1 (0.8) | 6.2 (0.9)* | 7.0 (1.0) | 6.4 (0.9)* |
| No of days with incomplete evacuation | 3.8 (0.6) | 3.2 (0.4)* | 3.2 (0.5)* | 4.0 (0.5)† | 2.6 (0.5) | 2.7 (0.4) | 2.5 (05) | 2.3 (0.4) |
| Overall severity assessment | 7.1 (0.7) | 5.7 (0.7)*† | 5.0 (0.8)*† | 5.4 (0.9)* | 7.8 (0.2) | 7.7 (0.4) | 7.3 (0.5) | 6.8 (0.7) |
| No of days with impact on daily life | 5.2 (0.4) | 4.4 (0.3)* | 3.7 (0.5)* | 4.1 (0.5)* | 4.9 (0.2) | 5.0 (0.2) | 4.4 (0.2) | 4.5 (0.4) |
| Severity of impact on daily life | 6.5 (0.6) | 5.7 (0.7)* | 5.1 (0.8)* | 6.0 (0.7) | 6.9 (0.4) | 6.9 (0.4) | 6.4 (0.4) | 6.6 (0.7) |
*Significant compared with run in: p<0.05.
†Significant compared with placebo: p<0.05.
Only those parameters where significance was reached are shown.
Figure 4 Analysis of the first part of the study as a parallel group design placebo controlled study. The effect of initial treatment with placebo (A) or citalopram (B) is shown on overall symptom severity, number of days with abdominal pain, and number of days with bloating. *p<0.05 compared with before treatment; †p<0.05 compared with placebo.
Moreover, a number of symptoms, including number of days with abdominal pain, severity of abdominal pain, number of days that symptoms affected daily life, severity of bloating, and overall symptom severity, did not return to baseline three weeks after the end of the citalopram episode (visit 4) whereas placebo was followed by complete return to baseline at visit 4 for all symptoms except worst episode of bloating (table 5). These findings confirm the symptomatic benefit of citalopram and suggest that the carryover effect is mainly due to prolonged symptom improvement after citalopram.
IBS symptom diaries
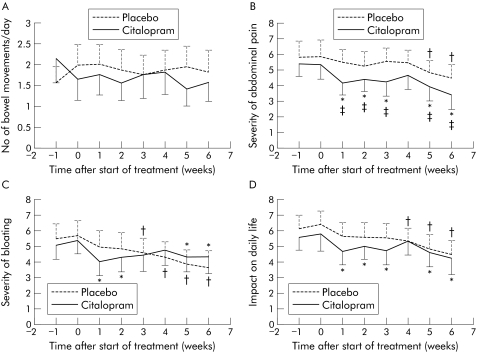
The weekly average diary scores are summarised in fig 5. Analysis of the diaries showed that placebo had no significant effect on daily scores for number of bowel movements and stool consistency (data not shown). During treatment with placebo, daily scores for severity of bloating improved significantly from the third treatment week onwards, and the interference of abdominal symptoms with daily life improved significantly from the fourth week. Daily scores for severity of abdominal pain improved significantly from the fifth week onwards and duration of abdominal pain improved significantly in week 6 (data not shown).
Figure 5 Weekly average scores in the daily diaries for number of bowel movements (A), severity of abdominal pain (B), severity of bloating (C), and impact on daily life (D). *p<0.05 after citalopram compared with the run in period. †p<0.05 after placebo compared with the run in period. ‡p<0.05 compared with placebo.
The diaries confirmed the lack of a major effect on stool pattern. Citalopram did not significantly alter daily scores for number of bowel movements or stool consistency (data not shown) throughout the treatment period. The severity of abdominal pain, severity of bloating, and interference of abdominal symptoms with daily life improved significantly from the first treatment week onwards, with the exception of week 4 when no significance difference was seen (fig 5). During weeks 5 and 6, citalopram significantly improved daily scores for duration of abdominal pain (data not shown).
Psychiatric comorbidity
Psychiatric comorbidity was evaluated by the questionnaires mentioned above. Anxiety levels were especially high, as assessed by both the SCL‐90‐R and HAD questionnaires. At baseline, most baseline IBS symptom severity scores were poorly correlated with anxiety and depression levels assessed with either questionnaire. However, number of days with bloating was significantly correlated with anxiety levels assessed by the SCL‐90‐R questionnaire (r = 0.62, p<0.002), depression levels assessed by the SCL‐90‐R questionnaire (r = 0.51, p = 0.02), anxiety levels assessed by the HAD questionnaire (r = 0.44, p = 0.04), and depression levels assessed by the HAD questionnaire (r = 0.50, p = 0.02). Severity of the worst episode of bloating was significantly correlated with anxiety levels assessed by the SCL‐90‐R questionnaire (r = 0.54, p<0.01). Number of days that abdominal symptoms influenced daily life was significantly correlated with anxiety levels on both the SCL‐90‐R and HAD questionnaires (r = 0.48 and r = 0.47, respectively; both p<0.05).
None of the baseline psychosocial variables was related to the outcome of citalopram treatment. Evolution of anxiety and depression levels during treatment is shown in table 6. Citalopram significantly decreased HADS anxiety and depression scores after both three and six weeks of treatment, compared with baseline, whereas only anxiety was significantly decreased by placebo. No significant improvement was seen on the SCL‐90‐R questionnaires. No relationship was found between symptomatic improvement and changes in anxiety or depression scores on SCL‐90‐R and HAD questionnaires (all r2<0.1; NS).
Table 6 Anxiety and depression levels during treatment with citalopram or placebo, assessed using the hospital anxiety and depression scale (HAD) and symptom checklist‐90‐revised (SCL‐90R).
| Before citalopram | Citalopram 3 weeks | Citalopram 6 weeks | Before placebo | Placebo 3 weeks | Placebo 6 weeks | |
|---|---|---|---|---|---|---|
| Anxiety (HAD) | 11.4 (1.1) | 10.7 (1.0)*† | 9.6 (0.8)*† | 13.1 (0.9) | 12.4 (1.0)* | 11.0 (1.1)* |
| Depression (HAD) | 6.0 (0.9) | 3.8 (0.6)*† | 3.7 (0.7)*† | 6.3 (1.0) | 6.3 (0.9) | 6.0 (0.9) |
| Anxiety (SCL‐90R) | 17.7 (1.8) | 15.5 (1.6) | 14.9 (1.6) | 16.8 (1.8) | 16.4 (1.8) | 16.3 (1.9) |
| Depression (SCL‐90R) | 31.5 (3.3) | 29.1 (2.8) | 28.6 (2.9) | 31.2 (3.3) | 32.0 (3.2) | 30.1 (3.3) |
*Significant compared with run in: p<0.05.
†Significant compared with placebo: p<0.05.
Discussion
Several studies have demonstrated the efficacy of TCAs in the treatment of IBS16,17 but the use of SSRIs, a pharmacologically more selective class of antidepressants, has only been studied in a few trials which yielded inconclusive results. Creed and colleagues19 compared cost effectiveness of psychotherapy, the SSRI paroxetine 20 mg daily, and “usual care” in a large sample of severe IBS patients. After a one year follow up period, severity and frequency of abdominal pain had improved similarly in all groups. However, psychotherapy and paroxetine were superior to usual care in improving the physical component of health related quality of life, and psychotherapy was associated with a significant reduction in health care costs compared with usual care. Kuiken and colleagues20 performed a double blind, randomised, placebo controlled study in 40 non‐depressed IBS patients with 20 mg of the SSRI fluoxetine for six weeks. Rectal sensitivity and rectal compliance, which were the primary end points in this study, were not significantly altered by fluoxetine compared with placebo. Furthermore, abdominal pain, other gastrointestinal symptoms, or global symptom relief did not differ between both groups after six weeks of treatment. Tabas and colleagues21 compared treatment with 10 or 20 mg paroxetine or placebo for 12 weeks in 81 IBS patients that did not respond to fibre. Paroxetine significantly improved overall well being, which was the primary end point, without a relationship to changes in depression or anxiety levels. Paroxetine did not affect abdominal pain or bloating but significantly improved straining, urgency, and feelings of incomplete evacuation paroxetine. Finally, in a very recent controlled study from Iran, fluoxetine was found to be superior to placebo in improving symptoms of pain, bloating, and constipation in constipation predominant IBS.22
In the present study, we demonstrated that the SSRI citalopram was superior to placebo in alleviating several IBS symptoms. Citalopram had a beneficial effect on abdominal pain, bloating, impact of symptoms on daily life, and overall well being. There was only a modest effect on stool pattern. The effect is not secondary to an effect on depression or anxiety as depressed patients were excluded and changes in depression or anxiety scores were not related to symptom improvement. This, however, does not exclude a central analgesic or neuromodulatory role of citalopram. The effect of acute administration of citalopram on colonic sensorimotor function, assessed during a colonic barostat study, did not predict clinical outcome. Although this might have yielded additional important information, all patients declined to undergo a new colonic barostat and manometry examination at the end of the first treatment period.
For most symptom parameters studied on the questionnaire, citalopram was significantly better compared with the run in or compared with placebo, both at week 3 and at week 6 (table 1). This was not only the case for overall severity assessment by the patients but also for severity and frequency of abdominal pain and severity and frequency of bloating. Impact on daily life was clearly favourably influenced by citalopram, as both the number of days with an impact of symptoms on normal functioning and severity of the impairment of normal functioning were significantly improved. Patients also indicated a significant improvement in abnormalities in stool pattern during citalopram but not during placebo treatment. This was mainly related to a decrease in the number of days with urgency, straining, or a sense of incomplete evacuation. There was a modest decrease in the number of days with loose stools, no consistent influence of citalopram on the number of days with hard stools, and there was no significant influence on the overall number of bowel movements. These data suggest that the improvement in abnormalities of bowel habit reflects subjective appreciation of bowel movements, perhaps related to sensory effects of citalopram, rather than true stool pattern characteristics, which may be more driven by underlying colonic motility.
Both anxiety scores and depression scores on validated psychiatric questionnaires improved significantly during treatment with citalopram. However, as patients fulfilling diagnostic criteria for depression were excluded, and as the improvement in IBS symptoms was not correlated with changes in anxiety or depression scores, the effect of citalopram on IBS symptoms is unlikely to occur secondary to an effect on these psychopathological variables. Tolerance of the drug was excellent, and no difference in adverse events was noted between citalopram and placebo. Onset of the effect of citalopram on symptom severity occurred within the first three weeks, which is faster than the usual occurrence of an antidepressant effect.15,16,17 Daily diaries confirmed a symptomatic effect as early as within the first week for severity of abdominal pain, bloating, and impact on daily activities.
Compared with the situation after the first three weeks, substantial further improvement was obtained after six weeks. It is unclear whether this reflects prolongation of the treatment, doubling of the dose of citalopram after three weeks, or both. On the other hand, a transient unfavourable effect of the dose increment after three weeks cannot be excluded, as the diaries showed transient worsening of abdominal pain, bloating, and impact of symptoms on daily life in week 4.
Compared with recent multicentre therapeutic trials in IBS, the placebo effect in the present study was modest. Several factors may play a role in this relatively small placebo effect. Firstly, we selected patients with a longstanding history of stable IBS symptoms. Secondly, a placebo effect of physician visits was minimised by the six week interval after the start of treatment and first clinic visit. Finally, elimination of patients with coexisting major depression or other major psychiatric disorders could, at least in theory, also select a group of patients less likely to show a robust placebo response.
The use of crossover studies in functional bowel disorders has been criticised because of failure of symptom severity to return to baseline after the first treatment period.29 We nevertheless chose this study design because we wanted to correlate clinical improvement under treatment to the acute effect of a colonic barostat study in as many patients as possible. A carryover effect was indeed obvious in the present study, thereby hampering interpretation of changes in symptom severity during the second treatment period. Analysis of the first treatment period as a double blind parallel arm study confirmed the benefit of citalopram over placebo. Moreover, this analysis revealed that the carryover effect was mainly determined by a prolonged beneficial effect of citalopram on symptoms, which persisted for more than three weeks after cessation of drug intake.
Studies with SSRIs in IBS have shown conflicting results and only one other study demonstrated a significant effect of an SSRI on cardinal IBS symptoms, including abdominal pain, compared with placebo.22 There may be several reasons for these divergent findings. Firstly, different patient samples may have been recruited with respect to sex, previous treatment, psychiatric comorbidity, visceral hypersensitivity, and other variables. Secondly, trial design, methodology, and end points differed significantly between the studies mentioned. Finally, different SSRIs have been used at different doses and there is evidence that they differ slightly in pharmacological profile.15 Paroxetine, for instance, has some anticholinergic properties while citalopram is believed to be the most selective SSRI.15
The mechanism underlying the beneficial effect of citalopram is unclear. We have previously shown that acute administration of citalopram decreases the sensitivity of the colon to distension and inhibits the colonic response to feeding in healthy subjects.23 Although these observations might provide a rationale for use of citalopram in the treatment of IBS, they did not seem to occur in IBS patients in the present barostat studies, although exactly the same protocol was used. It is unclear whether the absence of acute effects of intravenous citalopram administration in IBS could be related to decreased colonic expression of the serotonin reuptake transporter, as was reported in IBS.30 During prolonged administration, SSRIs induce a complex cascade of neuronal adaptive responses, including downregulation and desensitisation of 5‐HT receptors,15 and this might occur both at the central level or at the peripheral level. It is conceivable that patients need more prolonged administration or a higher dose of the drug before the effects on colonic sensorimotor function occur. Alternatively, the drug might exert beneficial effects on visceral sensitivity by a central neuromodulatory or analgesic action. As indicated previously, an effect on anxiety or depression is unlikely to explain the symptomatic benefit.
Based on the present study, the SSRI citalopram is a potentially valuable addition to our therapeutic options in IBS. Citalopram provided symptomatic benefit of rapid onset, was well tolerated, and was not associated with the side effects of tricyclic antidepressants, such as drowsiness or constipation. One drawback of the current study is the selection of patients from a tertiary care centre only. Larger scale studies will be required to study the efficacy of citalopram or other SSRIs in the IBS patient population seen in primary practice and in secondary care.
Acknowledgements
Lukas van Oudenhove is a Research Fellow of the Fund for Scientific Research ‐Flanders (FWO‐Vlaanderen)
Abbreviations
5‐HT - serotonin
SSRIs - selective serotonin reuptake inhibitors
IBS - irritable bowel syndrome
TCA - tricyclic antidepressants
VAS - visual analogue scale
SCL‐90R - symptom checklist‐90‐revised
HADS - hospital anxiety and depression scale
Footnotes
Conflict of interest: None declared.
References
- 1.Thompson W G, Longstrech G F, Drossman D A.et al Functional bowel disorders and functional abdominal pain. Gut 199945(suppl 2)ii43–ii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johanson J F. Options for patients with irritable bowel syndrome: contrasting traditional and novel serotonergic therapies. Neurogastroenterol Motil 200416701–711. [DOI] [PubMed] [Google Scholar]
- 3.Talley N J. Antidepressants in IBS: are we deluding ourselves? Am J Gastroenterol 200499921–923. [DOI] [PubMed] [Google Scholar]
- 4.Crowell M D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol 20041411285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakai A, Diksic M, Kumakura Y.et al The effects of the 5‐HT3 antagonist, alosetron, on brain serotonin synthesis in patients with irritable bowel syndrome. Neurogastroenterol Motil 200517212–221. [DOI] [PubMed] [Google Scholar]
- 6.Berman S, Chang L, Suyenobu B.et al Condition‐specific deactivation of brain regions by 5‐HT(3) receptor antagonist Alosetron. Gastroenterology 2002123969–977. [DOI] [PubMed] [Google Scholar]
- 7.Mayer E A, Berman S, Derbyshire S W G.et al The effect of the 5‐HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther 2002161357–1366. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Northcutt A R, Kong S.et al Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo‐controlled trial. Lancet 20003551035–1040. [DOI] [PubMed] [Google Scholar]
- 9.Friedel D, Thomas R, Fisher R. Ischemic colitis during treatment with alosetron. Gastroenterology 2001120557–560. [DOI] [PubMed] [Google Scholar]
- 10.Novick J, Miner P, Krause R.et al A randomized, double‐blind, placebo‐controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002161877–1888. [DOI] [PubMed] [Google Scholar]
- 11.Lydiard R B. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry 200162(Suppl 8)38–45. [PubMed] [Google Scholar]
- 12.Mayer E A, Craske M, Naliboff B D. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry 200162(Suppl 8)28–36. [PubMed] [Google Scholar]
- 13.Stahl S M. Gut feelings about irritable bowel syndrome. J Clin Psychiatry 200162590–591. [DOI] [PubMed] [Google Scholar]
- 14.Mertz H R. Irritable bowel syndrome. N Engl J Med 20033492136–2146. [DOI] [PubMed] [Google Scholar]
- 15.Stahl S.Essential. psychopharmacology: neuroscientific basis and practical applications, 2nd edn. Cambridge: Cambridge University Press, 2000
- 16.Jackson J L, O'Malley P G, Tomkins G.et al Treatment of functional gastrointestinal disorders with antidepressant medications: a meta‐analysis. Am J Med 200010865–72. [DOI] [PubMed] [Google Scholar]
- 17.Drossman D A, Toner B B, Whitehead W E.et al Cognitive‐behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 200312519–31. [DOI] [PubMed] [Google Scholar]
- 18.Morgan V, Pickens D, Gautam S.et al Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 200554601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creed F, Fernandes L, Guthrie E.et al The cost‐effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003124303–317. [DOI] [PubMed] [Google Scholar]
- 20.Kuiken S, Tytgat G, Boeckxstaens G. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: A double blind, randomized, placebo‐controlled study. Clin Gastroenterol Hepatol 20031219–228. [DOI] [PubMed] [Google Scholar]
- 21.Tabas G, Beaves M, Wang J.et al Paroxetine to treat irritable bowel syndrome not responding to high‐fiber diet: a double‐blind, placebo‐controlled trial. Am J Gastroenterol 200499914–920. [DOI] [PubMed] [Google Scholar]
- 22.Vahedi H, Merat S, Rashidioon A.et al The effect of fluoxetine in patients with pain and constipation‐predominant irritable bowel syndrome: a double‐blind randomized‐controlled study. Aliment Pharmacol Ther 200522381–385. [DOI] [PubMed] [Google Scholar]
- 23.Tack J, Broekaert D, Corsetti M.et al Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther 200623265–274. [DOI] [PubMed] [Google Scholar]
- 24.First M B, Spitzer R L, Gibbon M.et alStructured Clinical Interview for DSM‐IV Axis I Disorders—Patient Edition (SCID‐I/P, version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute, 1996
- 25.Derogatis L.The SCL‐90‐R manual‐II: scoring, administration and procedures for the SCL‐90‐R, 2nd edn. Towson, MD: Clinical Psychometric Research, 1992
- 26.Zigmond A S, Snaith R P. The hospital anxiety and depression scale. Acta Psychiatr Scand 198367361–370. [DOI] [PubMed] [Google Scholar]
- 27.Bjelland I, Dahl A A, Haug T T.et al The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res 20025269–77. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz N, Hartkamp N, Franz M.et al Properties of the symptom check list (SCL‐90‐R) in a psychosomatic consultation‐liaison setting. Psychol Rep 2002901201–1207. [DOI] [PubMed] [Google Scholar]
- 29.Veldhuyzen van Zanten S J O, Talley N J, Bytzer P.et al Design of treatment trials for functional gastrointestinal disorders. Gut 199945(suppl 2)69ii77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coates M D, Mahoney C R, Linden D R.et al Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 20041261657–1664. [DOI] [PubMed] [Google Scholar]