Abstract
Background and aims
Endoscopic surveillance of Barrett's oesophagus currently relies on multiple random biopsies. This approach is time consuming, has a poor diagnostic yield, and significant interobserver variability. Elastic scattering spectroscopy is a real time in vivo optical technique which detects changes in the physical properties of cells. The aim of this study was to assess the potential for elastic scattering to detect high grade dysplasia or cancer within Barrett's oesophagus.
Methods
Elastic scattering spectroscopy measurements collected in vivo were matched with histological specimens taken from identical sites within Barrett's oesophagus. All biopsies were reviewed by three gastrointestinal pathologists and defined as either “low risk” (non‐dysplastic or low grade dysplasia) or “high risk” (high grade dysplasia or cancer). Two different statistical approaches (leave one out and block validation) were used to validate the model.
Results
A total of 181 matched biopsy sites from 81 patients, where histopathological consensus was reached, were analysed. There was good pathologist agreement in differentiating high grade dysplasia and cancer from other pathology (kappa = 0.72). Elastic scattering spectroscopy detected high risk sites with 92% sensitivity and 60% specificity and differentiated high risk sites from inflammation with a sensitivity and specificity of 79%. If used to target biopsies during endoscopy, the number of low risk biopsies taken would decrease by 60% with minimal loss of accuracy. A negative spectroscopy result would exclude high grade dysplasia or cancer with an accuracy of >99.5%.
Conclusions
These preliminary results show that elastic scattering spectroscopy has the potential to target conventional biopsies in Barrett's surveillance saving significant endoscopist and pathologist time with consequent financial savings. This technique now requires validation in prospective studies.
Keywords: Barrett's oesophagus, oesophageal neoplasms, dysplasia, spectroscopy
The rapid rise in incidence of oesophageal adenocarcinoma in White men is of great concern in Northern Europe where the mortality rate appears to be among the highest in the world.1 In the UK, the five year survival rate for this cancer is less than 10%.2 Most cases of oesophageal adenocarcinoma arise within a segment of Barrett's columnar lined oesophagus (BO) with intestinal metaplasia. BO is detected, often coincidentally, at endoscopy. Although no visual abnormality is usually seen within the BO segment before cancer develops, there is a series of molecular and histological changes which precede cancer.3,4 The only clinically available marker of risk is the histological presence of either low grade (LGD) or high grade (HGD) dysplasia. HGD carries a cancer risk of 16–59% within five years5,6 but the risk is very much lower in LGD.7,8 In those without dysplasia, fewer than 5% will probably ever develop cancer.
Radical treatment for early oesophageal cancer is associated with long term survival9,10,11 and patients with BO enrol in surveillance programmes, even though there are no data to convincingly show that this is cost effective or that it saves lives. Surveillance, as mandated by the American College of Gastroenterology guidelines,12 relies on regularly spaced but random biopsies being taken from the Barrett's segment. An average endoscopy yields approximately 20 biopsies, most of which are entirely normal. This is time consuming and has a low detection rate, even when abnormalities exist. The problem is compounded by significant interobserver variability between pathologists on the degree of dysplasia present in biopsies from BO, in particular for LGD.13 Furthermore, a major contributory factor to pathologist inaccuracy is the presence of inflammation in the biopsy specimen.14 Not surprisingly, many patients drop out of surveillance programmes.15
New approaches are urgently needed. Ideally, non‐endoscopic methods could be used for population screening. Failing this, better endoscopic techniques are needed for detecting high risk patients which are accurate, easy to use, cheap, and provide results rapidly, preferably without the need to remove tissue. New optical technologies may fulfil this role. Point measurements using an optical probe should accurately analyse tissue without removing it or at least target biopsies to areas likely to contain HGD. This would minimise the number of normal tissue samples taken. Techniques under development include fluorescence spectroscopy, Raman spectroscopy, and elastic scattering spectroscopy (also known as diffuse reflectance spectroscopy).16,17,18,19
Elastic scattering spectroscopy (ESS) is a point measurement which, using an appropriate optical geometry, is sensitive to the size and packing of dense subcellular components such as the nucleus, nucleolus, and mitochondria as well as absorption by haemoglobin.20,21,22 The size and density of these organelles change on transformation to premalignant or malignant conditions. These are precisely the features which pathologists look for in diagnosing dysplasia. The optical probe is passed through the working channel of an endoscope and is placed in direct contact with tissue. A flash of light interrogates a cylinder of tissue approximately 0.5 mm in diameter and 1 mm deep. Results are available within milliseconds. Since the technology uses white light and produces a strong backscattered signal, components are cheap and the system is simple to manufacture. It is also safe as only visible light is used for illumination.
Using pattern recognition techniques such as multivariate discriminant analysis, algorithms can be developed to classify spectra as premalignant or benign tissues. In this paper, we present our preliminary data using ESS to target biopsies in patients with Barrett's oesophagus undergoing surveillance endoscopy.
Materials and methods
The elastic scattering spectroscopy system
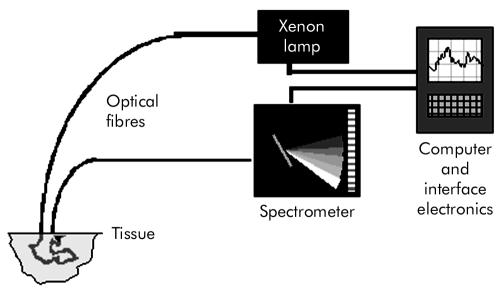
The ESS system contains a pulsed xenon arc lamp, an optical probe, a spectrometer, and a computer to control these components and record the spectra. The arc lamp, spectrometer, and power supply are housed in a portable briefcase size unit to which the laptop computer is connected. Short pulses of white light (320–920 nm) from the xenon arc lamp (Perkin Elmer Inc., Fremont, California, USA) are directed through a flexible optical fibre touching the tissue to be interrogated. Ultraviolet B and C (100–315 nm) light is filtered out to avoid risk to patients. A collection fibre (200 μm diameter), with a fixed separation distance of ∼350 μm from an illumination fibre (400 μm diameter), collects backscattered light from the upper layers of the tissue and propagates it to the spectrometer (S2000 Ocean Optics, Dunedin, Florida, USA). The spectrometer outputs the spectrum to the computer for recording and further analysis (fig 1). The fibre assembly is housed in a plastic sheath (outer diameter 2.0 mm) which can pass into the oesophagus via the biopsy channel of a standard endoscope. Collection and recording of a single spectrum takes approximately 200 ms. The fibre can be sterilised with the endoscope.
Figure 1 Schematic diagram of elastic scattering spectroscopy system.
Patients
This study was approved by the joint University College London/University College London Hospitals (UCLH) ethics committee. Patients referred to our tertiary referral centre for the management of HGD or early cancer in BO from 2000–2003 were enrolled. Informed consent was obtained from all patients prior to their participation in the study. A total of 234 matched optical and histological sites were collected from 81 patients.
Acquisition of elastic scattering spectra and matched histology
A white reference spectrum is recorded from the flat surface of Spectralon (Labsphere Inc., North Sutton, New Hampshire, USA) as its elastic scattering is spectrally flat between 250 and 1000 nm, allowing the system to account for spectral variations in the light source, spectrometer, fibre transmission, and fibre coupling. Immediately before any spectral measurement is made the system automatically records the ambient light within the oesophagus arising from the endoscope light source. In this manner the site specific exogenous light at the moment of measurement is controlled for.
During routine endoscopy, optical measurements were taken, followed immediately by biopsy from the same site (fig 2). The tip of the optical probe was placed in gentle contact with the tissue and the lamp and spectrometer were triggered via a keyboard or foot pedal. The optical probe left a slight mucosal indent enabling the subsequent biopsy to be taken from the same site as the optical measurement. Spectra were only taken from 1–3 sites per patient with a median of four spectra from each site (mean 3.3). Conventional biopsies from these sites were fixed in individual specimen pots for later 1:1 correlation with the optical spectra. Routine quadrantic biopsies were taken every 2 cm as standard practice for management of patients with suspected HGD in BO.
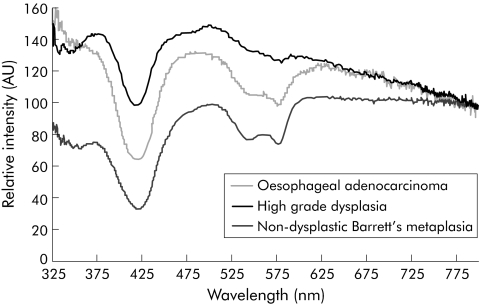
Figure 2 Representative spectra obtained with the elastic scattering spectroscopy. AU, arbitrary units.
We considered collecting a larger number of paired data sets from each patient but did not do so as it would have increased the procedure time in patients who were often elderly and frail. Taking matched data sets requires inserting the optical probe and biopsy forceps alternatively through the endoscope for each site, with individual processing for each biopsy. The statistics were based on the number of study pairs obtained and, in our view, small numbers of paired biopsies from a large number of patients would give a statistically more robust final algorithm to work with.
Histological assessment
Following an initial meeting to confirm histological criteria, each biopsy was assessed by three pathologists for the presence and extent of dysplasia and inflammation. The first pathologist (MRN, based at UCLH) reported as per normal clinical practice based on the Vienna classification system.23 The second and third pathologists (SD and MOD based at Cambridge University) worked together to assess all biopsies blindly and a consensus was reached. The two histological diagnoses obtained were compared and a further meeting held to agree the diagnosis for any difficult slides.
A total of 234 matched optical and histological biopsy sites from normal and neoplastic columnar lined oesophagus were examined; 16 were excluded due to acquisition artefacts. Only spectra from biopsy sites where all of the pathologists agreed were used to train and test the ESS algorithm. Where they disagreed the integrity of the gold standard was compromised and these 37 further sites were excluded. This left 181 sites for analysis, corresponding to 595 spectra.
Only sites with extensive dysplasia or cancer (present in more than 50% of the surface epithelium) were used to train the algorithm. Focal cancer lying in extensive LGD was not used in the algorithm development as it would limit the precision of the pattern recognition methods but focal sites were subsequently tested. Thirty three biopsies classified as high risk had extensive abnormalities and 12 had focal abnormalities.
Intervention is only offered to patients who have HGD or cancer so the most useful application of ESS would be to accurately detect these patients. High risk patients were defined as those with HGD or cancer and low risk patients as those with LGD or no dysplasia. Development of ESS to make this distinction is vital for it to be a clinically useful tool for targeting biopsies in the surveillance of BO. We included the small number of sites which were finally defined as “indefinite for dysplasia” in the low risk group.
Statistical analysis
Interpathologist agreement was analysed using the kappa statistic.
Model generation
The ESS spectra were cleaned before analysis. Initially, each spectrum was made up of 1801 intensity points spanning the wavelength range 320–920 nm. Spectra were smoothed using a linear filter (Savitsky‐Golay method, quadratic model, span 7 below 620 nm and span 20 above 620 nm, where noise was greater). Between 320 and 370 nm and between 890 and 920 nm, the signal to noise ratio of the spectra was too low as the Xenon arc lamp has a low light output at these wavelengths. Only the window between 370 and 890 nm was used in the analysis. The spectra were then normalised by setting the mean intensity of each spectrum to zero and the variance to one, allowing for more accurate shape comparison.
The number of points in a spectrum is much larger than the number of spectra in the training set. Using all points in the spectrum to train the model would result in overfitting of the model. Consequently, the data were compressed to 30 values using principal component analysis with virtually no loss of information. Linear discriminant analysis was then applied to these 30 principal components to maximise the discrimination between the two groups.
It would be optimal to use the entire data set to calculate the algorithm as the larger the training set the more accurate the algorithm becomes. However, assessing the accuracy of a classification algorithm is best done using a novel test set. Two data analysis methods were therefore used. Leave one out cross validation analysis trains the algorithm on all the data except one point which is then tested. This is repeated until all points have been tested and an overall model accuracy is determined. The alternative analysis, which we refer to as block validation, uses 60% of the data to train and 40% to test the model. To ensure statistical robustness, this process was repeated 100 times with different randomly resampled training and test sets. This resulted in 100 sensitivity and specificity values being generated. These are, therefore, presented as averages with their standard deviation.
Model validation
All spectra from a single site were kept together, in either the training set or the testing set, to prevent bias being introduced through non‐independence of data. Using the algorithm created with linear discriminant analysis, the canonical score for each test spectrum was determined. This allows the spectrum to be assigned to one group or the other. The “cut off” score between the groups can be adjusted to favour sensitivity or specificity or to optimise overall accuracy. A receiver operating characteristics (ROC) curve is obtained by plotting the sensitivity and 1−specificity of the algorithm for the whole range of “cut off” canonical scores. The area under the ROC curve represents the overall accuracy that can be expected from an algorithm.
Results
Histological assessment
Visible nodules were seen in three of 35 sites with HGD and in nine of 10 sites with invasive cancer. All other sites appeared endoscopically to be normal columnar mucosa. Histological assessments of the columnar lined biopsies are shown in table 1. Kappa statistics were used to determine whether the agreement between UCLH and Cambridge pathologists was greater than expected by chance alone. This was found to be very much better for slides demonstrating cancer, HGD, and non‐dysplastic BO than for LGD or indefinite for dysplasia. The overall kappa value suggested good agreement among these pathologists for the Vienna classification as a whole (kappa = 0.68, table 2).
Table 1 Distribution of columnar lined biopsy sites, as assessed by the three pathologists (independent diagnosis at UCLH and consensus between two pathologists in Cambridge).
| Cambridge consensus diagnosis | UCLH independent diagnosis | Sites agreed | Spectra available for analysis | |
|---|---|---|---|---|
| Vienna 5 (Cancer) | 12 | 14 | 10 | 32 |
| Vienna 4 (HGD) | 37 | 35 | 35 | 103 |
| Vienna 3 (LGD) | 28 | 16 | 5 | 18 |
| Vienna 2 (indefinite for dysplasia) | 15 | 16 | 2 | 8 |
| Vienna 1 (no dysplasia) | 130 | 147 | 129 | 434 |
| Inadequate | 8 | 0 | ||
| Total | 218 | 218 | 181 | 595 |
LGD low grade dysplasia; HGD, high grade dysplasia.
Table 2 Summary of inter‐agreement kappa values for comparison of pathologists in the analysis of oesophageal biopsies.
| Calculated kappa | 95% CI | Strength of agreement | |
|---|---|---|---|
| Cancer (Vienna 5) | 0.76 | 0.74–0.79 | Good |
| High grade dysplasia (Vienna 4) | 0.66 | 0.61–0.70 | Good |
| Low grade dysplasia (Vienna 3) | 0.22 | 0.19–0.24 | Fair |
| Indefinite for dysplasia (Vienna 2) | −0.02 | −0.04–0.00 | None |
| BO without dysplasia (Vienna 1) | 0.61 | 0.53–0.69 | Good |
| Total | 0.68 | 0.61–0.74 | Good |
95% CI, 95% confidence interval; BO, Barrett's oesophagus.
Of much greater practical relevance is defining an individual's cancer risk. When patients were considered as either high or low risk (for ESS algorithm development), the pathologists demonstrated even better agreement for this classification (kappa = 0.72 (95% confidence interval (CI) 0.66–0.78)).
Spectral analysis and algorithm development
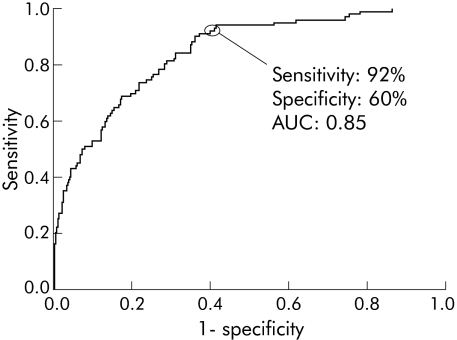
Results of the leave one out and block validation analyses for detection of extensive HGD or cancer are shown in table 3. The “cut off” canonical score between the groups was adjusted in order to obtain the best balance between sensitivity and specificity using an ROC curve. Sensitivity of 92% and specificity of 60% for the leave one out analysis was obtained for detecting extensive HGD or cancer in a biopsy. The block validation analysis produced a sensitivity of 87% (95% CI 86–88%) and specificity of 59% (95% CI 59–60%) (fig 3).
Table 3 Summary of average algorithm sensitivities and specificities for differentiating between low risk (normal and low grade dysplasia) and high risk (high grade dysplasia and cancer) sites.
| Leave one out | Block validation | ||
|---|---|---|---|
| Mean | 95% CI | ||
| Sensitivity (%) | 92% | 87% | 86–88% |
| Specificity (%) | 60% | 59% | 59–60% |
| Area under the curve (%) | 85% | 82% | 81–83% |
Figure 3 Receiver operator characteristics of the leave one out analysis for detection of high risk sites in columnar lined oesophagus using elastic scattering spectroscopy. AUC, area under the curve.
Detecting focal dysplasia
When the algorithm was applied to biopsies with focal HGD/cancer lying within otherwise normal mucosa, sensitivity for detecting the abnormality was 85% using the leave one out analysis and 80% using block validation. Specificity remained at 60%.
Differentiating between inflammation and dysplasia
A common problem pathologists face is differentiating HGD from inflammation. Fifty six sites (197 spectra) were classified as having moderate or severe inflammation but no dysplasia. These were compared with the 45 HGD/cancer sites. Using a leave one out analysis, sensitivity and specificity for discriminating high risk lesions from inflammation were both 79%. Using the block validation analysis, sensitivity was 77% and specificity 76%.
Predicting the value of ESS in detecting HGD and cancer during endoscopic surveillance
Once the sensitivity and specificity of ESS optical measurements have been calculated, it becomes possible to estimate the number of biopsies required to detect HGD or cancer at surveillance endoscopy. This number is dependent on the prevalence of HGD and cancer in the population being endoscoped.
We reviewed the number of biopsies showing HGD or intramucosal cancer in a series of 20 other patients who were referred to us for management. The average number of biopsies taken was 17 per endoscopy with a mean of 1.8 showing extensive HGD or cancer and a further 0.7 biopsies showing focal HGD or cancer.
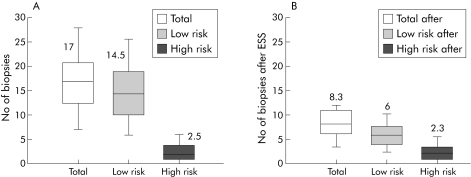
Of crucial interest to a patient enrolling for surveillance endoscopy is that if this algorithm were translated to clinical practice, then quadrantic biopsies every 2 cm would no longer be required. Instead, for example, 17 optical measurements could be taken and the number of biopsies necessary for histological examination would fall from a mean of 17 to 8 per endoscopy. There would be a small reduction in the number of sites of HGD or cancer detected but each patient has on average 2.5 sites containing HGD or cancer, so the chances of missing all of the sites is very much lower. Figure 4 illustrates this possible reduction in the number of biopsies required with ESS guidance compared with biopsies taken without guidance (random biopsies).
Figure 4 Estimated need for conventional biopsies with and without elastic scattering spectroscopy (ESS) measurement. (A) Number of biopsies taken at surveillance endoscopy using four quadrant large cup biopsies every 2 cm throughout the length of Barrett's oesophagus (BO). Biopsy sites were classified as high risk if they included high grade dysplasia or cancer, and low risk if they did not. For each measurement, the box shows the median and interquartile range. The bars represent 95% confidence intervals. Out of an average of 17 biopsies, only about eight would show ESS algorithms requiring targeted biopsies. (B) If ESS were used to target biopsies, the number of low risk biopsies would be reduced by 60% to a median of 6 but almost all of the high risk sites would still be detected.
We can estimate the performance of our system if it were used to guide conventional endoscopic surveillance by measuring the reduction in the number of physical biopsies taken and whether HGD or cancer would be missed during surveillance. Using the optimum values for sensitivity and specificity, in a standard surveillance population where the prevalence of HGD or cancer is 1%, we calculate that virtually no HGD sites would be missed and a histopathologist would, on average, look at 60% less “low risk” biopsies. Furthermore, a negative standard surveillance endoscopy performed with the assistance of ESS would have a negative predictive value in excess of 99.5%. Using our system, we found the mean time taken to collect quadrantic optical measurements every 2 cm in the oesophagus in 10 consecutive patients was 2.2 minutes.
Discussion
Surveillance endoscopies yield large numbers of normal biopsies in low risk patients. Histology is not an ideal gold standard as patients do develop cancer without dysplasia having been detected previously. It may well be that in these patients, dysplasia was present at the time of a previous endoscopy but was not detected. Furthermore, there is incomplete agreement between pathologists on the diagnosis of dysplasia. A robust reliable tool is therefore needed which is simple to use and is available in the clinical setting.
Our preliminary data suggest that we may have such a tool. Optical biopsy only requires cheap components, is easy to use, and in its current configuration allows for accurate targeting of biopsies. The optical probe is placed in direct contact with the mucosa and measurements which suggest the absence of high grade dysplasia or cancer throughout an entire Barrett's segment have an accuracy of over 99.5%. The probe does not need to be removed from the endoscope unless a physical biopsy is taken. This makes it possible to survey a 10 cm Barrett's segment without dysplasia in two minutes, which is a time saving of at least 70%. Our modelling also suggests that ESS will decrease the number of low risk biopsies the pathologist reviews by 60% and will be associated with almost no loss in accuracy. Areas of extensive inflammation, which pathologists often find difficult to correctly classify, are less likely to be biopsied as our tool correctly detects these as non‐dysplastic. Furthermore, the results of our system could be made available to the clinician in real time which would enable immediate identification of an area where a biopsy was needed.
The accuracy of our model is based on our finding that “on average” a patient at high risk will have 2.5/17 biopsies with HGD when quadrantic samples are taken every 2 cm. This is consistent with Cameron and Carpenter's findings.24 As optical spectra are easy and quick to collect and analyse, we would envisage that clinicians might wish to take measurements every 1 cm instead of every 2 cm leading to a further increase in the accuracy of detecting dysplasia with no more than a couple of minutes added to the procedure time.25
Other optical biopsy techniques have been evaluated. Laser induced fluorescence spectroscopy was shown to accurately classify 90–96% of normal and HGD Barrett's mucosa16 and adenomatous and hyperplastic polyps in the colon.26,27 In both studies the groups were small and clear separation of test and training sets was not carried out, which will have led to overestimation of the accuracy of the technique. Raman (inelastic) spectroscopy has the advantage of interrogating tissue biochemistry and is very accurate.17 Unfortunately, it requires powerful lasers and is too expensive and slow to be used currently in clinical practice.
A true cost‐benefit analysis cannot be done using the present data as conventional biopsies were taken from all of the sites examined with ESS. This important question will need to be answered in a future prospective trial in which the equipment costs are balanced against savings in endoscopy and histopathology time when conventional biopsies are only taken when the ESS spectrum is suspicious of dysplasia or cancer.
ESS seems a good alternative because of its simplicity. It measures the physical properties of cells such as nucleus size and shape and cellular packing density, which are relevant to development of dysplasia.22 In our work, light is scattered multiple times before being detected, which makes it difficult to correlate changes in individual parameters and the optical spectra. The correlation requires complex statistics.
Others have tried to understand the correlation between the physical properties of cells and optical spectra by observing light which only undergoes a single scattering event. This is done using a polarised optical probe. Backman et al elegantly demonstrated that alteration of single scattering events can be correlated with changes in nuclear size18 and Wallace et al demonstrated in a small patient group the value of observing “single light scattering” events to detect dysplasia in Barrett's oesophagus.19 Combining optical biopsy approaches is likely to be better than using any single technique alone.28 We could speculate that, when incorporating Raman spectroscopy becomes feasible, the accuracy will be so enhanced that we will no longer need to take conventional biopsies at all!
ESS has been studied previously in a number of tissues as a minimally invasive diagnostic technique where access to the tissue is achieved by either direct topical access or mediated by endoscopy, including the colon and bladder.29,30,31,32,33 We have also successfully used ESS in the assessment of breast tissue and axillary nodes,34 head and neck lymph nodes,35 and bony resection margins in oral cancer.36
As scattering is induced by gradients of the optical index of refraction, ESS spectral signatures will also be altered if the refractive index of nuclei or organelles changes, for example due to changes in the amount of granularity of the chromatin.21,37 As aneuploidy is of prognostic importance in the development of oesophageal adenocarcinoma, it is interesting to speculate whether ESS could be used to detect it in vivo.38
There are limits to this technology. It is a point measurement rather than an imaging technique but it is simple and fast to use. Accuracy is less than 100% but even three expert gastrointestinal pathologists did not always agree on the degree of dysplasia, a phenomenon which is well recognised.39 As we used their consensus to train our algorithm, we cannot expect optical biopsy to be completely accurate. Our results, however, show that there is a good opportunity for the partial automation of histopathology in this difficult area. We have demonstrated elsewhere that accuracy is greater when measurements are taken ex vivo40 and patient movement during endoscopy means that there is less than perfect coregistration between optical measurements and biopsies. This will increase the error, as will the slightly different amount of tissue that is examined. ESS interrogates a volume of tissue approximately 1 mm3 whereas a conventional biopsy is larger than this. Finally, statistical analysis is open to many errors, although we were careful to use independent testing and training sets to minimise the risk of bias.
We would like to enhance the system by combining information from light that is singly or multiply scattered. Most importantly, however, we need to prospectively test our algorithm in patients.
In conclusion, elastic scattering spectroscopy offers the hope of a simple reliable tool to accurately target biopsies during surveillance endoscopy for Barrett's oesophagus.
Acknowledgements
We acknowledge the National Cancer Institute Network for Translational Research into Optical Imaging (U54‐CA104677‐02)
Abbreviations
BO - Barrett's oesophagus
LGD - low grade dysplasia
HGD - high grade dysplasia
ESS - elastic scattering spectroscopy
ROC - receiver operating characteristic
Footnotes
Conflict of interest: None declared.
References
- 1.Jankowski J A, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett's metaplasia has regional variations in the west. Gastroenterology 2002122588–590. [DOI] [PubMed] [Google Scholar]
- 2.Toms J R. ed. CancerStats Monograph 2004. London: Cancer Research UK, 2004
- 3.Reid B J, Blount P L, Rubin C E.et al Flow‐cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology 19921021212–1219. [PubMed] [Google Scholar]
- 4.Reid B J, Prevo L J, Galipeau P C.et al Predictors of progression in Barrett's esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. Am J Gastroenterol 2001962839–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnell T G, Sontag S J, Chejfec G.et al Long‐term nonsurgical management of Barrett's esophagus with high‐grade dysplasia. Gastroenterology 20011201607–1619. [DOI] [PubMed] [Google Scholar]
- 6.Reid B J, Levine D S, Longton G.et al Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low‐ and high‐risk patient subsets. Am J Gastroenterol 2000951669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skacel M, Petras R E, Gramlich T L.et al The diagnosis of low‐grade dysplasia in Barrett's esophagus and its implications for disease progression. Am J Gastroenterol 2000953383–3387. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery E, Goldblum J R, Greenson J K.et al Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow‐up study based on 138 cases from a diagnostic variability study. Hum Pathol 200132379–388. [DOI] [PubMed] [Google Scholar]
- 9.van Sandick J W, van Lanschot J J, Kuiken B W.et al Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut 199843216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Headrick J R, Nichols FC I I I, Miller D L.et al High‐grade esophageal dysplasia: long‐term survival and quality of life after esophagectomy. Ann Thorac Surg 2002731697–1702. [DOI] [PubMed] [Google Scholar]
- 11.Holscher A H, Bollschweiler E, Schneider P M.et al Early adenocarcinoma in Barrett's oesophagus. Br J Surg 1997841470–1473. [PubMed] [Google Scholar]
- 12.Sampliner R E. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol 2002971888–1895. [DOI] [PubMed] [Google Scholar]
- 13.Skacel M, Petras R E, Gramlich T L.et al The diagnosis of low‐grade dysplasia in Barrett's esophagus and its implications for disease progression. Am J Gastroenterol 2000953383–3387. [DOI] [PubMed] [Google Scholar]
- 14.Baak J P. Routine morphometrical analysis can improve reproducibility of dysplasia grade in Barrett's oesophagus surveillance biopsies. J Clin Pathol 200255910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald C E, Wicks A C, Playford R J. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ 20003211252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panjehpour M, Overholt B F, VoDinh T.et al Endoscopic fluorescence detection of high‐grade dysplasia in Barrett's esophagus. Gastroenterology 199611193–101. [DOI] [PubMed] [Google Scholar]
- 17.Kendall C, Stone N, Shepherd N.et al Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett's oesophagus. J Pathol 2003200602–609. [DOI] [PubMed] [Google Scholar]
- 18.Backman V, Wallace M B, Perelman L T.et al Detection of preinvasive cancer cells. Nature 200040635–36. [DOI] [PubMed] [Google Scholar]
- 19.Wallace M B, Perelman L T, Backman V.et al Endoscopic detection of dysplasia in patients with Barrett's esophagus using light‐scattering spectroscopy. Gastroenterology 2000119677–682. [DOI] [PubMed] [Google Scholar]
- 20.Lovat L B, Bown S G. Elastic scattering spectroscopy for detection of dysplasia in Barrett's esophagus. In: van Dam J, ed. Gastrointestinal endoscopy clinics of North America: optical biopsy, vol 14. Amsterdam: Elsevier, 2004507–517. [DOI] [PubMed]
- 21.Mourant J R, Canpolat M, Brocker C.et al Light scattering from cells: the contribution of the nucleus and the effects of proliferative status. J Biomed Opt 20005131–137. [DOI] [PubMed] [Google Scholar]
- 22.Mourant J R, Hielscher A H, Eick A A.et al Evidence of intrinsic differences in the light scattering properties of tumorigenic and nontumorigenic cells. Cancer 199884366–374. [PubMed] [Google Scholar]
- 23.Schlemper R J, Riddell R H, Kato Y.et al The Vienna classification of gastrointestinal epithelial neoplasia. Gut 200047251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron A J, Carpenter H A. Barrett's esophagus, high‐grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol 199792586–591. [PubMed] [Google Scholar]
- 25.Reid B J, Blount P L, Feng Z.et al Optimizing endoscopic biopsy detection of early cancers in Barrett's high‐grade dysplasia. Am J Gastroenterol 2000953089–3096. [DOI] [PubMed] [Google Scholar]
- 26.Kapadia C R, Cutruzzola F W, O'Brien K M.et al Laser‐induced fluorescence spectroscopy of human colonic mucosa. Detection of adenomatous transformation. Gastroenterology 199099150–157. [DOI] [PubMed] [Google Scholar]
- 27.Kara M, DaCosta R S, Wilson B C.et al Autofluorescence‐based detection of early neoplasia in patients with Barrett's esophagus. Dig Dis 200422134–141. [DOI] [PubMed] [Google Scholar]
- 28.Georgakoudi I, Jacobson B C, Van Dam J.et al Fluorescence, reflectance, and light‐scattering spectroscopy for evaluating dysplasia in patients with Barrett's esophagus. Gastroenterology 20011201620–1629. [DOI] [PubMed] [Google Scholar]
- 29.Bigio I J, Bown S G, Briggs G.et al Diagnosis of breast cancer using elastic‐scattering spectroscopy: preliminary clinical results. J Biomed Opt 20005221–228. [DOI] [PubMed] [Google Scholar]
- 30.Ge Z F, Schomacker K T, Nishioka N S. Identification of colonic dysplasia and neoplasia by diffuse reflectance spectroscopy and pattern recognition techniques. Appl Spectrosc 199852833–839. [Google Scholar]
- 31.Judith R M, Irving J B, James D B.et al Elastic scattering spectroscopy as a diagnostic tool for differentiating pathologies in the gastrointestinal tract: preliminary testing. J Biomed Opt 19961192–199. [DOI] [PubMed] [Google Scholar]
- 32.Mourant J R, Bigio I J, Boyer J.et al Spectroscopic diagnosis of bladder cancer with elastic light scattering. Lasers Surg Med 199517350–357. [DOI] [PubMed] [Google Scholar]
- 33.Dhar A, Johnson K S, Novelli M R.et al Optical biopsy of colonic lesions: Comparison of elastic scattering spectroscopy with histology. Gastrointest Endosc 200663257–261. [DOI] [PubMed] [Google Scholar]
- 34.Johnson K S, Chicken D W, Pickard D C.et al Elastic scattering spectroscopy for intraoperative determination of sentinel lymph node status in the breast. J Biomed Opt 200491122–1128. [DOI] [PubMed] [Google Scholar]
- 35.Jerjes W, Swinson B, Pickard D.et al Detection of cervical intranodal metastasis in oral cancer using elastic scattering spectroscopy. Oral Oncol 200440673–678. [DOI] [PubMed] [Google Scholar]
- 36.Jerjes W, Swinson B, Johnson K S.et al Assessment of bony resection margins in oral cancer using elastic scattering spectroscopy: a study on archival material. Arch Oral Biol 200550361–366. [DOI] [PubMed] [Google Scholar]
- 37.Fang H, Ollero M, Vitkin E.et al Noninvasive sizing of subcellular organelles with light scattering spectroscopy. IEEE J Selected Top Quantum Electron 20039267–276. [Google Scholar]
- 38.Reid B J, Levine D S, Longton G.et al Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low‐ and high‐risk patient subsets. Am J Gastroenterol 2000951669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid B J, Haggitt R C, Rubin C E.et al Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol 198819166–178. [DOI] [PubMed] [Google Scholar]
- 40.Samuel D S, Clark B, Dhar A.et al Optical biopsy of colonic lesions: comparison of elastic scattering spectroscopy performed in vivo and ex vivo. Gastroenterology 2005128(suppl 2)S1766 [Google Scholar]