Short abstract
The Hedgehog pathway was recently shown to antagonise constitutive activity of the Wnt pathway that drives proliferation of colorectal cancer cells. Studies in this issue of Gut refine our understanding of the underlying mechanism and provide evidence for such antagonism in colorectal cancers in vivo
Keywords: Hedgehog, Wnt, colon, cancer
The mucosa of the colon is covered by a single layer of epithelial cells that is replaced once every 3–7 days. New cells are produced by a pool of progenitor cells that lie at the base of small mucosal invaginations called crypts. It has been estimated that these progenitor cells generate approximately 10 billion new cells per day in the human colon.1 Homeostasis in such a massive process of regeneration can only be achieved if the behaviour of cells in the system is not regulated at the level of the individual cell (intrinsic). Instead, the fate of individual epithelial cells must be determined by extra cellular (extrinsic) signals that are generated at the population level. It is now becoming clear that these extrinsic signals are provided by morphogens, a class of molecules that has been identified by developmental biologists who studied patterning events during embryogenesis.2 Morphogens are soluble proteins that form long range concentration gradients and act in a dose dependent manner to induce distinct cellular responses on their target cells. In this way, cellular fate is determined by the distance of a target cell from the source of the morphogen. Several morphogens have now been identified as regulators of cell fate in the adult colon.
The Wnt pathway (fig 1) was the first morphogenetic pathway that was implicated in colonic epithelial homeostasis when it was found that adenomatous polyposis coli (APC) acts as an important regulator of Wnt signalling.3APC had initially been identified as the gene mutated in patients with familial adenomatous polyposis (FAP).4,5 Subsequently, mutations in APC have been found in the majority of sporadic colorectal cancers.6,7 A central role in the Wnt pathway is played by β‐catenin which can translocate from the cytoplasm to the nucleus to form a complex with Tcf transcription factors and regulate Wnt responsive genes. In the absence of Wnts, APC is required to hold together a protein complex that ensures continuous breakdown of β‐catenin and prevents its nuclear translocation. Mutations in APC disrupt this complex and lead to uncontrolled stabilisation and nuclear accumulation of β‐catenin.8 The probable reason for the link between unchecked activity of the Wnt pathway and intestinal tumorigenesis is the essential role of this pathway in the maintenance of intestinal precursor cell phenotype. Substantial evidence exists to show that canonical (β‐catenin‐Tcf mediated) Wnt signalling is required to drive intestinal precursor cell proliferation. Inactivating Wnt signalling by deletion of β‐catenin,9 TCF410 or expression of an inhibitor of soluble Wnts,11 leads to loss of small intestinal precursor cells. Conversely, preventing inhibition of the Wnt pathway by inducible deletion of APC leads to rapid expansion of the small intestinal precursor cell compartment.12 Although almost all experiments have focused on the small intestine, Wnt signalling probably plays a similar role in the colon as nuclear β‐catenin is detected in the precursor cell compartment at the base of the crypts in the colon in vivo and Wnt signalling has been shown to maintain a precursor cell transcriptome in colon cancer cells in vitro.13 Given the importance of Wnt signalling in the maintenance of the precursor cell phenotype, it is understandable that APC mutant cells escape the normal position dependent restraints on proliferation and are capable of clonal expansion even outside the crypt base.
Figure 1 A simplified model of hedgehog (Hh) and Wnt signalling and its interaction. Hh signalling. Gli proteins are the mediators of Hh signalling. In the absence of a Hh signal, full length Gli is phosphorylated by protein kinase A (PKA), casein kinase I (CKI), and glycogen synthase kinase 3β (GSK3β), leading to proteolytic processing into a truncated form that represses gene transcription of Hh target genes. Hh binds to the Hh binding receptor patched (Ptc), relieving its inhibition of the Hh signalling receptor smoothened (Smo). Smo then inhibits the proteolytic processing of Gli through Cos2 and fused kinase (Fu). Stabilised full length Gli is subsequently capable of activation of expression of Hh target genes. Wnt signalling. In the absence of Wnt signalling, β‐catenin is phosphorylated by GSK3β and CKI leading to its ubiquitination and subsequent breakdown. Wnt binds a complex of LDL receptor related protein and frizzled, leading to inhibition of β‐catenin proteolysis whereupon stabilised β‐catenin can enter the nucleus, form a complex with Tcf transcription factors, and regulate transcription of Wnt target genes. Hh‐Wnt interaction. As Akiyoshi and colleagues22 show in this issue of Gut, interaction between both pathways occurs downstream of Gli and downstream of β‐catenin phosphorylation.
Another morphogenetic pathway that regulates homeostasis of gastrointestinal epithelial cells is the hedgehog (Hh) pathway (fig 1).14 Similar to the Wnt pathway, the Hh pathway was initially identified in genetic screens for patterning genes in Drosophila.15 Three Hh genes have been identified in most vertebrates that have been named Sonic, Indian, and Desert hedgehog. Hhs act via a receptor system that consists of two different proteins, a Hh binding receptor patched (Ptc), expression of which is itself upregulated by Hh signalling, and a Hh signalling receptor smoothened (Smo). In the absence of Hh, Ptc inhibits Smo via a mechanism that remains to be resolved. When Hh binds Ptc, this inhibitory interaction is relieved and Smo signalling activated. Signalling downstream of Smo is still only partially understood but involves a complex of proteins that regulates the activity of downstream transcription factors Gli1–3. These Glis exist both in a full length activator and a truncated repressor form that is generated by proteolytic processing. Smo signalling inhibits the constitutive proteolytic processing of Glis leading to stabilisation and nuclear translocation of full length Glis and transcriptional activation of Hh target genes. Analysis of the phenotype of Shh, Ihh, and Gli1‐3 mutant mice suggest that Gli2 and Gli3 are the most important mediators of Hh signalling in the gut.16 Gli1 mutant mice do not seem to have a phenotype17 but Gli1 can rescue many aspects of loss of Gli218 indicating that Gli1 function is similar but redundant. In the normal adult colon, Sonic hedgehog (Shh) is expressed by a few precursor cells at the base of the crypts19 whereas Indian hedgehog (Ihh) and Hh receptor Ptc are expressed by differentiated absorptive enterocytes at the luminal end of the crypt.20,21 Akiyoshi and colleagues22 now show in this issue of Gut that Gli1, both mediator and transcriptional target of Hh signalling, also localises to the differentiated colonic enterocytes at the top of the crypt (see page 991). These expression patterns are consistent with a major role of Hh signalling in cell fate regulation of colonic enterocytes at the top of the crypt. This is supported by the findings that Hh signalling regulates colonic enterocyte differentiation in HT29 colon cancer cells in vitro and in the rat colon in vivo.21
As is often found for morphogenetic pathways, Wnt and Hh signalling are not independent regulatory mechanisms in colonic epithelial homeostasis. Ihh expression is lost from adenomatous polyps in patients with FAP,21 suggesting that Ihh expression is negatively regulated by Wnt signalling. This hypothesis was corroborated by blocking Wnt pathway activity in DLD1 colon cancer cells and showing that this reconstituted Ihh expression in these cells.21 A study by Berman and colleagues23 found that Hh signalling is indeed silenced in colon cancer cells in vitro. Berman et al found that an Hh reporter construct showed constitutive Hh pathway activity in oesophageal, gastric, biliary, and pancreatic cancer cell lines, but no activity was detected in DLD1 or HCT116 colon cancer cell lines. Indeed, in contrast with other gastrointestinal cancer cell lines, the Hh inhibitor cyclopamine did not affect viability of these colon cancer cells. The exact role of the different hedgehogs in colorectal carcinogenesis needs further examination as it was found that in contrast with downregulation of Ihh, expression of Shh may actually be upregulated in adenomatous polyps and adenocarcinomas.24 Although this finding needs to be confirmed by in situ hybridisation, it seems plausible that the domain of Shh expression by colonic precursor cells expands as this compartment expands during colorectal carcinogenesis.
The interaction between Hh and Wnt signalling is a two way interaction (fig 2) as Hh signalling also antagonises Wnt signalling in the adult colon. Hh signalling acts to restrict expression of Wnt targets to the base of the crypt in vivo21 and transfection of APC mutant DLD1 colon cancer cells with Ihh leads to breakdown of nuclear β‐catenin/TCF4 complexes and downregulation of expression of Wnt targets in vitro.21 Akiyoshi et al now confirm the inhibitory effect of Hh signalling on Wnt signalling in vitro. The authors take this line of research an important step further by showing that this effect can be mediated by Gli1. The data therefore show that antagonism occurs at a very downstream level in the Hh pathway. It is not yet clear if the negative regulation of Wnt signalling involves Gli protein directly or is the result of the up or downregulation of a transcriptional target of Hh signalling. Two colon cancer cell lines were used in their experiments with different genetic mutations that lead to constitutive Wnt signalling. SW480 cells have mutated APC whereas HCT116 cells are known to harbour a stabilising mutation in β‐catenin. Wnt pathway activity was measured in these cells using a reporter construct for β‐catenin‐Tcf signalling. The authors show that Gli1 diminishes nuclear levels of β‐catenin and dose dependently inhibits constitutive Wnt signalling in both cell lines, even after augmenting Wnt pathway activity by cotransfection with Wnt1 or additional mutant β‐catenin. These data show that Gli1 inhibits Wnt signalling at a level below β‐catenin phosphorylation (fig 1). This inhibitory effect on Wnt signalling suggests that the role of Hh signalling in colorectal carcinogenesis may be antioncogenic and thus distinct from that in other gastrointestinal cancers, as was also suggested by the study of Berman and colleagues.23 In their current study, Akiyoshi et al provide additional, more indirect evidence for the antagonism between Hh and Wnt signalling in vivo by showing that expression of Gli1 is inversely related to nuclear accumulation of β‐catenin in colorectal cancers.
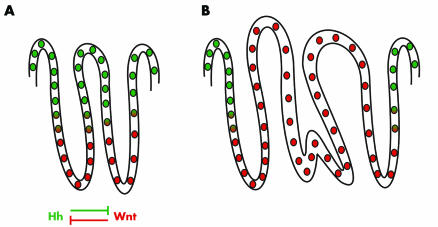
Figure 2 A model of the interaction of hedgehog (Hh) and Wnt signalling in the adult colon. (A) In the normal colon, cells at the base of the crypt are exposed to Wnt signals which maintain precursor cell phenotype whereas cells at the top of the crypt receive Indian hedgehog (Ihh) which induces differentiation. Wnt negatively regulates expression of Ihh and Hh signalling restricts Wnt to the base of the crypt. (B) In colon cancer, Wnt signalling is constitutively active, leading to loss of Ihh expression and loss of differentiation.
In conclusion, morphogenetic pathways play a critical role in the maintenance of homeostasis of adult gastrointestinal epithelia. Genetic mutations found in colorectal cancer often compromise the correct interpretation of these morphogenetic signals by mutating their receptors or intermediate signalling molecules. This may enable clonal growth of cancerous cells by helping them to escape the normal position dependent growth restraints that morphogenetic pathways impose. The classic examples are mutations in the Wnt pathway. The constitutive activity of Wnt signalling that results from these mutations can be inhibited by Hh signalling in colon cancer cells. Akiyoshi et al now provide the first evidence for an antagonistic interaction between the Hh and Wnt pathways in colorectal cancers in vivo and show that this interaction probably occurs at a downstream level in both pathways.
Footnotes
Conflict of interest: None declared.
References
- 1.Potten C S, Booth C, Pritchard D M. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 199778219–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabata T, Takei Y. Morphogens, their identification and regulation. Development 2004131703–712. [DOI] [PubMed] [Google Scholar]
- 3.Korinek V, Barker N, Morin P J.et al Constitutive transcriptional activation by a beta‐catenin‐Tcf complex in APC−/− colon carcinoma. Science 19972751784–1787. [DOI] [PubMed] [Google Scholar]
- 4.Nishisho I, Nakamura Y, Miyoshi Y.et al Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991253665–669. [DOI] [PubMed] [Google Scholar]
- 5.Kinzler K W, Nilbert M C, Su L K.et al Identification of FAP locus genes from chromosome 5q21. Science 1991253661–665. [DOI] [PubMed] [Google Scholar]
- 6.Luchtenborg M, Weijenberg M P, Roemen G M.et al APC mutations in sporadic colorectal carcinomas from The Netherlands Cohort Study. Carcinogenesis 2004251219–1226. [DOI] [PubMed] [Google Scholar]
- 7.Konishi M, Kikuchi‐Yanoshita R, Tanaka K.et al Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology 1996111307–317. [DOI] [PubMed] [Google Scholar]
- 8.Gregorieff A, Clevers H. Wnt signalling in the intestinal epithelium: from endoderm to cancer. Genes Dev 200519877–890. [DOI] [PubMed] [Google Scholar]
- 9.Ireland H, Kemp R, Houghton C.et al Inducible Cre‐mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta‐catenin. Gastroenterology 20041261236–1246. [DOI] [PubMed] [Google Scholar]
- 10.Korinek V, Barker N, Moerer P.et al Depletion of epithelial stem‐cell compartments in the small intestine of mice lacking Tcf‐4. Nat Genet 199819379–383. [DOI] [PubMed] [Google Scholar]
- 11.Pinto D, Gregorieff A, Begthel H.et al Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003171709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansom O J, Reed K R, Hayes A J.et al Loss of Apc in vivo immediately perturbs Wnt signalling, differentiation, and migration. Genes Dev 2004181385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Wetering M, Sancho E, Verweij C.et al The beta‐catenin/TCF‐4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002111241–250. [DOI] [PubMed] [Google Scholar]
- 14.Lees C, Howie S, Sartor R B.et al The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology 20051291696–1710. [DOI] [PubMed] [Google Scholar]
- 15.Nusslein‐Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980287795–801. [DOI] [PubMed] [Google Scholar]
- 16.de Santa Barbara P, van den Brink G R, Roberts D J. Molecular etiology of gut malformations and diseases. Am J Med Genet 2002115221–230. [DOI] [PubMed] [Google Scholar]
- 17.Park H L, Bai C, Platt K A.et al Mouse Gli1 mutants are viable but have defects in SHH signalling in combination with a Gli2 mutation. Development 20001271593–1605. [DOI] [PubMed] [Google Scholar]
- 18.Bai C B, Joyner A L. Gli1 can rescue the in vivo function of Gli2. Development 20011285161–5172. [DOI] [PubMed] [Google Scholar]
- 19.van den Brink G R, Hardwick J C, Nielsen C.et al Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut 200251628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen C M, Williams J, van den Brink G R.et al Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab Invest 2004841631–1642. [DOI] [PubMed] [Google Scholar]
- 21.van den Brink G R, Bleuming S A, Hardwick J C.et al Indian Hedgehog is an antagonist of Wnt signalling in colonic epithelial cell differentiation. Nat Genet 200436277–282. [DOI] [PubMed] [Google Scholar]
- 22.Akiyoshi T, Nakamura M, Koga K.et al Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving Wnt signalling activation. Gut 200655991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman D M, Karhadkar S S, Maitra A.et al Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003425846–851. [DOI] [PubMed] [Google Scholar]
- 24.Oniscu A, James R M, Morris R G.et al Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol 2004203909–917. [DOI] [PubMed] [Google Scholar]