Abstract
Background
It remains controversial as to whether delayed gastric emptying in functional dyspepsia is associated with a specific symptom pattern, and it is unknown if gastric emptying in functional dyspepsia is a driver of impaired health related quality of life (HRQOL). We aimed to evaluate the relationship between functional dyspepsia symptoms, gastric emptying, and HRQOL.
Methods
US patients (n = 864; mean age 44 years (range 18–82); 74% female) with functional dyspepsia, as defined by Rome II criteria, were enrolled into one of four clinical trials. All patients had a baseline scintigraphic assessment of gastric emptying of an egg substitute meal, and the trials were stratified on this assessment. Delayed gastric emptying was defined as having at least 6.3% residual volume at four hours. A total of 290 (34%) patients had delayed gastric emptying. HRQOL was assessed by the SF 36 and Nepean dyspepsia index (NDI).
Results
Postprandial fullness was independently associated with delayed gastric emptying but the association was weak (odds ratio (OR) 1.98 (95% confidence interval (CI) 1.02, 3.86); p = 0.04). No independent association was seen with epigastric pain, early satiety, nausea, or bloating. Mean SF 36 physical composite score (PCS) was 42.3 (95% CI 41.6, 43.0) and the mean SF 36 mental composite score (MCS) was 46.8 (95% CI 46.0, 47.5); both mean scores were significantly lower than age and sex adjusted national norms of 50 (p<.0001). Female sex, increasing age, and higher symptom scores for fullness, epigastric pain, and nausea were each independently associated with decreased PCS scores (all p<0.05). Higher baseline nausea symptom score, lower gastric emptying rates at one hour, and lower body mass index were associated with decreased MCS (all p<0.05). Female sex, epigastric pain, and nausea, but not gastric emptying, were associated with an impaired score on the NDI. However, the magnitude of the significant associations were all small.
Conclusions
In patients with functional dyspepsia selected for a clinical trial programme, gastric emptying did not usefully stratify them symptomatically. Quality of life of patients with functional dyspepsia enrolled in this clinical trial programme was significantly impaired but this was not explained by delayed gastric emptying.
Keywords: functional dyspepsia, gastric emptying, quality of life, early satiety
A common clinical syndrome in practice is functional dyspepsia, defined as chronic or recurrent epigastric pain or discomfort.1 In this syndrome, no cause can be identified using conventional diagnostic testing. Many of these patients complain of symptoms related to meals although the pathophysiology remains poorly defined.2,3 Delayed gastric emptying has been reported to occur in a subset of patients with functional dyspepsia, ranging from 25% to 50%.4 A delay in gastric emptying correlates with the presence of antral hypomotility although the underlying cause of the antral hypomotility has not been defined.5 Other potentially important abnormalities identified in functional dyspepsia include impaired fundic accommodation,6 gastric hypersensitivity to distension,7 and Helicobacter pylori infection.8
A continuing controversy in the field is the relationship between gastric emptying and symptoms in functional dyspepsia. Studies from Europe, in particular, have reported that delayed gastric emptying is associated with a specific symptom pattern, namely relevant postprandial fullness and vomiting, particularly in women.9,10,11 Other studies, mainly from the USA, have failed to identify a characteristic symptom profile in patients with delayed gastric emptying compared with those who have normal emptying.12,13 This issue has clinical importance as being able to identify those likely to have delayed gastric emptying may help to target investigations and treatment.14
Another issue of importance in functional dyspepsia is its association with impairment of quality of life.15 While a few studies have suggested that functional dyspepsia is associated with impaired quality of life, a recent systematic review concluded that the impact of functional dyspepsia on quality of life was uncertain.15 In particular, whether delayed gastric emptying reduces quality of life in functional dyspepsia is unknown.
Therefore, our aim was to evaluate whether symptoms could predict delayed gastric emptying in functional dyspepsia in a population of patients recruited for a clinical trial programme. We also assessed the relationship between functional dyspepsia, gastric emptying, and health related quality of life (HRQOL).
Methods
A total of 864 patients in the USA were enrolled into one of four clinical trials of tegaserod versus placebo. The protocol was approved by the local institutional review boards and all patients gave informed consent. Baseline data collected in these trials were analysed.
Subjects
Patients at least 18 years of age with documented functional dyspepsia were eligible to be enrolled. All had to have a history of persistent or recurrent meal related upper abdominal symptoms for at least three months prior to study entry.1 They also had to have a score of at least 2 on a four point ordinal severity scale for any one symptom at least three days per week during the two week drug free baseline observation period prior to randomisation. Patients with epigastric pain alone were not eligible for entry. Patients were required to have negative endoscopy findings defined as no oesophageal, gastric, or duodenal ulcers or erosions, and no evidence of macroscopic tissue damage, with the exception of erythema, verified by oesophagogastroduodenoscopy performed in the week prior to the baseline observation period. However, if an endoscopy had been performed within 30 days prior to study entry, it did not have to be repeated.
Patients with a past history of irritable bowel syndrome (IBS) could be included but dyspepsia was required to be the predominant complaint. Patients were subdivided into those with a history of diarrhoea, constipation, or alternating IBS by the clinician. No specific diagnostic criteria were applied.
Excluded were patients with gastro‐oesophageal reflux disease, defined as present if heartburn was the predominant symptom, or defined as the presence of one or more reflux‐like symptoms based on a previously validated gastro‐oesophageal reflux disease (GORD) questionnaire.16 This questionnaire comprises four symptoms: based on previous data, an answer of “yes” to all of these questions yielded an 85% probability of pathological 24 hour oesophageal pH monitoring or erosive oesophagitis on endoscopy, or both.16 Furthermore, those who had been treated with proton pump inhibitors or H2 receptor antagonists within 14 days prior to the screening visit were not eligible for inclusion. A history of response to antisecretory therapy with satisfactory relief of meal related symptoms was also an exclusion criteria. Hence a rigorous approach was taken to exclude GORD. Low dose aspirin was allowed.
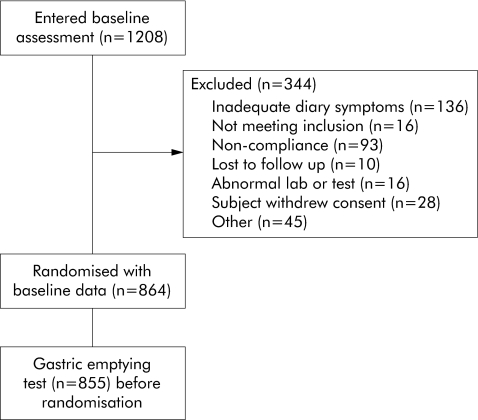
A history of upper gastrointestinal surgery was an exclusion. Patients with a history of persistent diarrhoea, diabetes mellitus, or major psychiatric illness were also excluded. A total of 1208 patients were screened and 344 patients were excluded during screening (25–33% in the different trials) (fig 1).
Figure 1 Patient flow diagram.
Assessment of gastric emptying
This was evaluated by standardised scintigraphy at all sites in the seven days prior to the baseline observation period.17 After eating a standard low fat breakfast of 120 g of Egg‐Beaters (equal to the volume of two large eggs), two slices of toast with strawberry jam (30 g), and drinking water (120 ml), patients were seated and had anterior and posterior images of the gastric region taken using a gamma camera at one minute post‐meal (time 0), 60, 120, and 240 minutes. Delayed gastric emptying was defined as >6.3% residual volume at four hours. This standardised methodology has been previously validated. Central reading of all scintigraphic measurements was performed. In addition, estimated T‐half was performed using a mixed model. A total of 855 patients completed the gastric emptying protocol; 290 patients (34%) had delayed gastric emptying.
Outcome assessments
Self rated dyspepsia symptoms
At baseline, patients recorded each day their symptoms (using a daily diary touchtone telephone system). Patients recorded the maximum severity for early satiety, postprandial fullness (defined as an unpleasant sensation like the persistence of food in the stomach), bloating in the upper stomach (not specifically defined), nausea, and epigastric/stomach pain. This was done using a four point scale: none (not present); mild (awareness of the symptom but easily tolerated); moderate (discomfort sufficient to interfere with normal activities such as work and sleep); and severe (incapacitating with inability to perform normal activities such as work and sleep). In addition, any episodes of vomiting were graded as yes or no. A total dyspepsia symptom severity score was constructed from the baseline two week daily diary card data.
Most bothersome symptom
Patients recorded the most bothersome symptom experienced during the past week on the first day of the two week baseline period.
Health related quality of life
Two specific validated quality of life questionnaires were used to assess this issue at baseline: (1) SF 36 (acute version), as a generic health status questionnaire18; and (2) Nepean dyspepsia index (NDI).19,20 This is a measure of symptom status and quality of life in functional dyspepsia. A 15 item symptom checklist measured the frequency, severity, and bothersomeness of each upper gastrointestinal symptom on Likert scales. A total score for each symptom was created by the addition of the three scales. A total dyspepsia HRQOL score was also derived from the 25 item NDI.
Other measurements
Data on body mass index (BMI) was calculated from the height and weight information obtained by measurement during baseline screening. Helicobacter pylori status was assessed based on testing of blood drawn at the initial visit.
Trial centres
The total number of centres was 67. Centres were subdivided into primary, secondary, and tertiary practices. Primary care was used as the reference group; secondary care consisted of multispecialty groups/gastroenterologist referrals, and tertiary care was considered high level care. Centre type was used as a covariate in the analyses.
Statistical analysis
Linear regression models were used to evaluate the ability of the individual symptom scores to predict gastric emptying at one, two, or four hours. In addition, a logistic regression analysis examined the ability of the individual symptom scores to discriminate delayed gastric emptying from normal gastric emptying. Linear regression was used to assess whether gastric emptying at one, two, or four hours could predict the overall mean baseline symptom score and the NDI symptom score. All models included age, sex, BMI, and centre type as covariates. Separate models also included a history of IBS. A multiple linear regression model was used to examine the association between physical and mental composite scores on the SF 36 versus symptoms, gastric emptying, age, and sex. These associations are reported using partial r2 values adjusting for all other variables in the model. All p values calculated were two tailed; the α level of significance was set at 0.05.
Results
Demographic and clinical characteristics
A total of 864 patients with a mean age of 44 years (median 43; (range 18–82)) were evaluated. Seventy four per cent of these patients were female and 74% were White. Mean BMI was 26.9 (median 26.0) (table 1). Overall, 16% had a past history of IBS.
Table 1 Demographics data in the study population by each trial and by type of centre.
| Study | Sex (%)* | Age (y)† | BMI† | History of IBS (%)‡ | GE‐1 h¶ (% remaining) | GE‐2 h¶ (% remaining) | GE‐4 h¶ (% remaining) | Physical composite score¶ | Mental composite score¶ | NDI QOL score¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 = Normal +delayed GE (n = 218) | 83 | 41 (18, 82) | 26.1 (12.6, 58.2) | 8, 3, 7 | 79.0 (15.5) [81.0] | 51.7 (21.0) [54.0] | 15.6 (16.0) [11.0] | 41.1 (11.1) [44.0] | 46.8 (11.4) [50.0] | 50.6 (20.2) [49.0] |
| 2 = Normal GE (n = 271) | 71 | 45 (18, 79) | 26.5 (18.0, 47.6) | 7, 2, 5 | 68.3 (17.7) [70.5] | 33.8 (19.5) [32.0] | 2.1 (1.7) [2.0] | 43.5 (10.0) [44.4] | 46.5 (11.5) [48.8] | 47.4 (18.4) [46.8] |
| 3 = Normal +delayed GE (n = 128) | 85 | 41 (18, 78) | 24.5 (15.8, 43.5) | 11, 2, 6 | 80.3 (15.7) [82.0] | 54.5 (20.8) [54.5] | 16.8 (17.8) [11.0] | 39.4 (10.3) [38.2] | 45.8 (11.0) [47.3] | 51.6 (22.4) [49.2] |
| 4 = Normal GE (n = 247) | 63 | 45 (18, 78) | 26.1 (17.7, 58.0) | 8, 2, 6 | 69.0 (17.0) [70.5] | 30.7 (18.4) [29.5] | 2.0 (1.6) [2.0] | 43.4 (9.5) [44.5] | 47.5 (11.4) [50.5] | 47.8 (19.7) [48.0] |
| Total primary centres (n = 26) | 77 | 32.5 (19, 77) | 22.8 (14.5, 41.7) | 4, 0, 4 | 80.2 (16.1) [82.5] | 49.5 (23.2) [53.5] | 9.0 (9.2) [7.0] | 38.1 (10.7) [41.1] | 46.3 (10.2) [48.1] | 54.0 (19.3) [54.7] |
| Total specialist centres (n = 246) | 75 | 42 (18, 79) | 25.5 (12.6, 58.2) | 6, 4, 7 | 74.7 (16.9) [78.0] | 42.3 (23.6) [42.0] | 8.8 (14.6) [3.0] | 40.7 (10.2) [41.3] | 45.8 (11.6) [49.1] | 51.6 (20.1) [50.4] |
| Total academic centres (n = 592) | 73 | 44 (18, 82) | 26.2 (15.6, 58.0) | 9, 2, 6 | 72.0 (17.7) [74.0] | 39.4 (21.5) [38.0] | 7.2 (11.9) [3.0] | 43.1 (10.2) [44.4] | 47.2 (11.3) [50.2] | 47.7 (19.9) [45.8] |
| Total overall (n = 864) | 74 | 43 (18, 82) | 26.0 (12.6, 58.2) | 8, 2, 6 | 73.0 (17.5) [75.0] | 40.5 (22.2) [40.0] | 7.7 (12.7) [3.0] | 42.3 (10.3) [43.5] | 46.8 (11.4) [49.5] | 49.0 (19.9) [47.6] |
*% Females.
†Median (minimum, maximum).
‡% Diarrhoea irritable bowel syndrome (IBS), % constipation IBS, % alternating IBS.
¶Mean (SD) [median].
GE, gastric emptying; NDI, Nepean dyspepsia index quality of life (QOL) score; BMI, body mass index.
Symptoms
At baseline, epigastric pain, bloating, nausea, vomiting, early fullness, and postprandial fullness were reported as the most bothersome symptom by 21%, 37%, 10%, 2%, 10%, and 20% of patients, respectively. Patients entering the trial scored moderately on the NDI symptom scale (maximum score = 12.5). Mean dyspepsia symptom severity score from the baseline diary card data was 12.1
Gastric emptying
The distribution of residual volume at one, two, and four hours is summarised in table 1. Female sex was associated with delayed gastric emptying in functional dyspepsia (women v men), both univariately (odds ratio (OR) 1.79, p = 0.0009) and adjusted for age, BMI, centre type, individual symptom scores, and NDI score (OR 1.64, p = 0.008). Age was also associated univariately with delayed gastric emptying in the discriminate model (OR 0.98 per one year increment, p = 0.0008). Adjusted for other factors, age was borderline significant (OR 0.99, p = 0.05).
Relationship between symptoms and gastric emptying
A comparison of the prevalence of individual symptoms in those with normal and delayed gastric emptying is summarised in table 2, with the univariate model for each symptom. Each symptom significantly predicted delayed emptying when individually adjusted for age, sex, BMI, and centre type. The results were essentially unchanged when a past history of IBS was adjusted for in the models (data not shown).
Table 2 Symptoms and gastric emptying in functional dyspepsia (univariate analyses).
| Symptom | Level (n)* | No delayed (%) | OR (95% CI) [p value]† | OR (95% CI) [p value]†‡ |
|---|---|---|---|---|
| Early satiety | Yes (385) | 151 (39%) | 1.72 (1.34, 2.21) [<0.001] | 1.71 (1.32, 2.21) [<0.001] |
| No (470) | 139 (30%) | |||
| Postprandial fullness | Yes (533) | 209 (39%) | 1.88 (1.46, 2.42) [<0.001] | 1.85 (1.42, 2.41) [<0.001] |
| No (322) | 81 (25%) | |||
| Bloating | Yes (548) | 205 (37%) | 1.50 (1.20, 1.86) [<0.001] | 1.53 (1.22, 1.92) [<0.001] |
| No (307) | 85 (28%) | |||
| Nausea | Yes (n‐192) | 89 (46%) | 1.55 (1.28, 1.87) [<0.001] | 1.38 (1.13, 1.68) [0.002] |
| No (n = 663) | 201 (30%) | |||
| Epigastric pain | Yes (n = 396) | 150 (38%) | 1.47 (1.19, 1.80) [<0.001] | 1.41 (1.14, 1.75) [0.002] |
| No (n = 459) | 140 (31%) |
*Symptom was indicated as “yes” if the subject reported the symptom as moderate or severe at least 50% of the time during the two week baseline period. Otherwise, symptom was reported as “no” for that individual.
†Mean symptom severity score over the two week baseline period was used in the model, with the OR corresponding to a 1 point average increase in severity for that symptom.
‡Each symptom was adjusted for age, sex, body mass index, and centre type.
OR (95% CI), odds ratio (95% confidence interval).
The association between individual symptom parameters and gastric emptying in functional dyspepsia from the full multiple predictor model is shown in table 3. Only postprandial fullness (OR 1.98 (95% confidence interval (CI) 1.02, 3.86); p = 0.04) was marginally associated with delayed gastric emptying (adjusted for age, sex, BMI, centre type, history of IBS, and other symptoms). There was no association between gastric emptying and epigastric pain, early satiety, bloating, or nausea. In a separate model to predict the two week symptom severity score, female sex, increased BMI, gastric emptying at four hours, and tertiary centre were significant, but all were weak predictors based on partial r2 values (table 4).
Table 3 Final model to predict delayed gastric emptying.
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Age | 0.99 (0.98, 1.00) | 0.04 |
| Female sex | 1.62 (1.13, 2.33) | 0.01 |
| Body mass index | ||
| Underweight | 2.93 (1.03, 8.30) | 0.04 |
| Overweight | 0.87 (0.61, 1.24) | 0.44 |
| Obese | 0.69 (0.47, 1.02) | 0.06 |
| IBS constipation | 1.43 (0.84, 2.43) | 0.18 |
| Diarrhoea | 1.01 (0.38, 2.69) | 0.98 |
| Alternating | 1.23 (0.67, 2.27) | 0.50 |
| Epigastric pain* | 1.02 (0.76, 1.36) | 0.91 |
| Early satiety* | 1.01 (0.61, 1.68) | 0.98 |
| Nausea* | 1.12 (0.88, 1.42) | 0.35 |
| Bloating* | 0.86 (0.54, 1.35) | 0.50 |
| Postprandial fullness* | 1.98 (1.02, 3.86) | 0.04 |
| Centre type | ||
| Primary | 2.43 (1.02, 5.79) | 0.05 |
| Tertiary | 0.97 (0.69, 1.35) | 0.85 |
*Moderate or severe symptom (yes/no).
OR (95% CI), odds ratio (95% confidence interval); IBS, irritable bowel syndrome.
Table 4 Final model to predict two week symptom severity score.
| Variable | Partial r2 | p Value |
|---|---|---|
| Age | 0.3% | 0.14 |
| Female sex | 0.7% | 0.02 |
| Body mass index | 0.9% | 0.006 |
| Gastric emptying | ||
| 1 h | 0.1% | 0.34 |
| 2 h | <0.1% | 0.56 |
| 4 h | 0.7% | 0.01 |
| Centre type | ||
| Primary | 0.1% | 0.27 |
| Tertiary | 3.1% | <0.001 |
Partial r2 values from the multiple linear regression model reflect all variables included in the model.
Association with quality of life
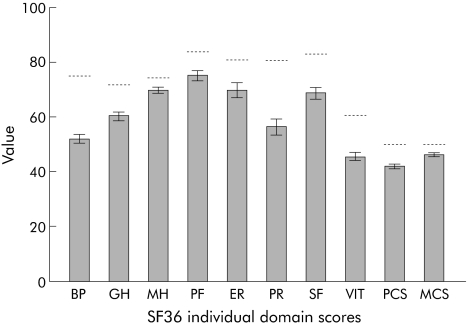
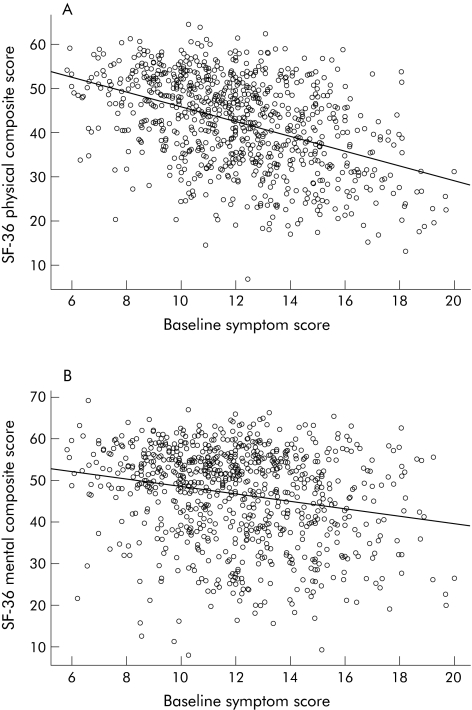
Mean scores on the eight domains of the SF 36 were all significantly lower than published US norms (fig 2). The US norm for the mean SF 36 physical and mental composite score is set at 50. The mean SF 36 physical composite score (PCS) was 42.3 (95% CI 41.6, 43.0) and the mean SF 36 mental composite score (MCS) was 46.8 (95% CI 46.0, 47.5); compared with age and sex adjusted US national norms of 50, mean scores were significantly lower (both p<0.0001). Gastric emptying delay was correlated with a worse PCS (r 2 hours = −0.11; p<0.001) but not MCS (r 2 hours = −0.02; p = 0.52). Higher symptom severity was correlated with worse PCS (r = −0.42; p<0.001) and MCS (r = −0.21; p<0.001) (fig 3).
Figure 2 Mean SF 36 scores for patients with functional dyspepsia and US population norms. BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; ER, emotional role; PR, physical role; SF, social functioning; VIT, vitality; PCS, physical composite score; MCS, mental composite score.
Figure 3 Correlation of two week symptom score and physical composite score (PCS) (A) of the SF 36 (r = −0.44; p<0.0001). Correlation of two week symptom score and mental composite score (MCS) of the SF 36 (B) (r = −0.21; p<0.0001).
Female sex, increasing age, and baseline symptom severity scores for fullness, epigastric pain, and nausea from the two week diary were each independently associated with a decreased PCS score (table 5) in a multiple linear regression model. Higher baseline nausea symptom scores, lower gastric emptying rates (at one hour), and lower BMI were associated with a decreased MCS score (table 6). Female sex, epigastric pain and nausea, but not gastric emptying, were associated with an impaired total score on the NDI HRQOL (table 7). Additionally, postprandial fullness was marginally associated with a worse score on the NDI.
Table 5 Final model to predict the physical composite score of the SF 36.
| Variable | Partial r2 | p Value |
|---|---|---|
| Age | 1.6% | <0.001 |
| Female sex | 1.1% | 0.003 |
| Body mass index | 0.4% | 0.06 |
| Gastric emptying | ||
| 1 h | <0.1% | 0.45 |
| 2 h | 0.1% | 0.32 |
| 4 h | 0.3% | 0.14 |
| Centre type | ||
| Primary | 0.2% | 0.25 |
| Tertiary | <0.1% | 0.82 |
| Postprandial fullness | 0.5% | 0.04 |
| Bloating | 0.1% | 0.32 |
| Early satiety | <0.1% | 0.77 |
| Epigastric pain | 6.5% | <0.001 |
| Nausea | 1.4% | <0.001 |
Partial r2 values from the multiple linear regression model reflect all variables included in the model.
Table 6 Final model to predict the mental composite score of the SF 36.
| Variable | Partial r2 | p Value |
|---|---|---|
| Age | 0.2% | 0.25 |
| Sex | <0.1% | 0.93 |
| Body mass index | 1.2% | 0.002 |
| Gastric emptying | ||
| 1 h | 0.5% | 0.04 |
| 2 h | 0.2% | 0.22 |
| 4 h | <0.1% | 0.39 |
| Centre type | ||
| Primary | <0.1% | 0.42 |
| Tertiary | <0.1% | 0.80 |
| Postprandial fullness | <0.1% | 0.87 |
| Bloating | 0.3% | 0.10 |
| Early satiety | <0.1% | 0.91 |
| Epigastric pain | <0.1% | 0.86 |
| Nausea | 1.5% | <0.001 |
Partial r2 values from the multiple linear regression model reflect all variables included in the model.
Table 7 Final model to predict the Nepean dyspepsia index quality of life score.
| Variable | Partial r2 | p Value |
|---|---|---|
| Age | 0.1% | 0.34 |
| Female sex | 0.7% | 0.01 |
| Body mass index | 0.2% | 0.19 |
| Gastric emptying | ||
| 1 h | 0.1% | 0.33 |
| 2 h | <0.1% | 0.52 |
| 4 h | <0.1% | 0.83 |
| Centre type | ||
| Primary | <0.1% | 0.82 |
| Tertiary | <0.1% | 0.36 |
| Postprandial fullness | 0.5% | 0.05 |
| Bloating | <0.1% | 0.38 |
| Early satiety | 0.2% | 0.23 |
| Epigastric pain | 4.5% | <0.001 |
| Nausea | 3.5% | <0.001 |
Partial r2 values from the multiple linear regression model reflect all variables included in the model.
Discussion
The clinical utility of ordering a gastric emptying test in a patient with functional dyspepsia remains controversial.14 Furthermore, whether specific symptom profiles can help identify those with delayed gastric emptying who should be considered for testing is also in dispute.12,13 In this study, we found that postprandial fullness was weakly related to the rate of gastric emptying but the small magnitude of the statistically significant findings is notable; the results suggest that gastric emptying does not usefully stratify patients with functional dyspepsia. The present study also investigated the relationship between functional dyspepsia and HRQOL, as there has been uncertainty in the literature as to what degree this syndrome impairs quality of life.15 We found that patients enrolled in this clinical trial programme had significantly impaired HRQOL both on a generic and disease specific instrument, but this was not explained by delayed gastric emptying. Our data does not support the model that delayed gastric emptying drives symptom severity and impaired quality of life in functional dyspepsia.
The Rome criteria for functional dyspepsia have suggested that patients with this condition can be usefully subdivided into symptom subgroups, and this may change management.1 The criteria used to subdivide patients have varied, although one approach has been to use the predominant symptom.1 However, the relevance of this classification has been questioned. We evaluated whether the predominant symptom could identify those with delayed gastric emptying among a group of patients with meal related functional dyspepsia. We found that patients could rank their predominant upper gastrointestinal symptom; bloating followed by epigastric pain and nausea were the top three predominant symptoms in this clinical trial cohort. Even though patients all had meal related symptoms, postprandial fullness, nausea and vomiting, and early satiety were less frequently reported as the predominant symptom. Notably, none of the predominant symptoms were useful in identifying delayed gastric emptying. Work from Italy has suggested that the severity of dyspeptic symptoms was more predictive than their presence or absence,9,10 and they have argued that this explains the confusion in the literature linking symptoms with delayed gastric emptying. Specifically, Stanghellini et al have reported that relevant postprandial fullness and vomiting as well as female sex were strong predictors for delayed gastric emptying.9,10 We also found in this study that postprandial fullness was a predictor of delayed gastric emptying but it remained a weak association of questionable clinical relevance.
We did observe that women were more likely to have delayed gastric emptying than men as have others,4,21 although sex was not a predictor of the association between symptom scores and delayed gastric emptying. Whether hormonal or other factors account for this association remains obscure. Other research suggests that while gastric emptying does appear to be more delayed in women than in men, this is not accounted for by the phases of the menstrual cycle or plasma concentrations of oestradiol or progesterone.22,23,24
A systematic review has examined the relationship between HRQOL and functional dyspepsia.15 It was concluded that the evidence that HRQOL is substantially affected by functional dyspepsia was remarkably limited despite the high prevalence and enormous cost of this condition. We found that the HRQOL in patients with functional dyspepsia in a clinical trial was significantly impaired and the reductions were of clinical significance, using as a bench mark a reduction of 5 points or more. However, it must be noted that these were patients with meal related dyspepsia and hence the results cannot be generalised to functional dyspepsia patients who do not have meal related symptoms. In the only study to assess dyspepsia subgroups and quality of life using the SF 36, Talley et al found that patients with dysmotility‐like dyspepsia had worse health perception than those with ulcer‐like dyspepsia.25 It is conceivable that comorbid illnesses could completely account for the impaired quality of life in functional dyspepsia rather than the condition itself. Importantly, in this cohort, serious other illnesses, including major psychiatric diseases, were excluded. However, it is still conceivable that psychological distress is the driver of impaired quality of life in functional dyspepsia and, unfortunately, no measurements of psychological distress were obtained to examine this question. No age and sex matched controls were evaluated but rather population normal values were used for the SF 36 comparisons. On the other hand, both disease specific and generic HRQOL assessments were employed.
Other limitations of the present study need to be considered. It is clear that the cohorts assembled for this clinical trial programme are not necessarily representative of all patients with functional dyspepsia. Furthermore, patients with ulcer‐like dyspepsia alone, in particular, were not included, although patients had the broad range of symptoms typical of the disorder. Hence whether these results can be extended to those with non‐meal related symptoms is unclear. On the other hand, it would be surprising if delayed gastric emptying predicted non‐meal related symptoms. Indeed, one could argue that enrichment of the population with meal related symptoms would tend to bias it towards observing an association with gastric emptying which was not found. The test meal used in the present study lacks lipid. This is a limitation, as many patients with functional dyspepsia report that fatty meals aggravate their meal related symptoms.26 Although lipids aggravate symptoms, it is unclear whether a fat containing meal would show delayed gastric emptying in a larger or a different subset of patients. The lipid component has particularly been associated with symptoms of nausea,27,28 and absence of lipids from the test meal may also contribute to the lack of an association of delayed emptying with nausea in the present study.
Patients with a past history of IBS were included, although they comprised only 16% of cases and dyspepsia had to be the major complaint. Notably, IBS was a clinical diagnosis and rigorous criteria were not evaluated. While it is possible that overlapping IBS might partially mitigate any symptom association with gastric emptying, we found essentially the same results when IBS was and was not controlled for in the models, and therefore believe IBS is unlikely to have affected the observations.
Another limitation is that mechanisms aside from gastric emptying were not able to be considered in these analyses. For example, impaired fundic accommodation and antral stiffness may be important pathogenic abnormalities in functional dyspepsia which could modulate the associations between gastric emptying and symptoms.6,29 However, the previous positive studies from Europe also did not assess any of these possible mechanisms,9,10,30 so this is unlikely to explain the current findings. We cannot ascertain whether the current data can be extrapolated to patients with mild symptoms or non‐meal related symptoms.
The current study also has some important strengths. We studied a large cohort of patients who were very well characterised. A number of different standardised symptom assessments were used and all yielded similar results, suggesting the findings are robust in terms of the association between gastric emptying and symptoms. The method used to quantify gastric emptying also had considerable strengths; this test had been validated in a previous multicentre study,17 the same technique was used in all centres, and central reading of scintigraphic measurements was undertaken. This is in contrast with another large clinical trial dataset that assessed the association between gastric emptying and symptoms, but used the C13 octanoic acid breath test12; this test may be less accurate than scintigraphy and has led to criticism of the reported results.31 Another strength of the present study was the strict exclusion of patients with overlapping GORD. Not only were patients required to have a normal endoscopy but they also could not have predominant reflux symptoms nor could they have any reflux symptom measured by a validated questionnaire.
In conclusion, in a large population of patients with functional dyspepsia recruited for randomised clinical trials who had meal related dyspepsia, we found that delayed gastric emptying was only weakly associated with symptoms. Hence it appears unlikely that the presence or severity of specific dyspeptic symptoms is explained by delayed gastric emptying among patients with meal related functional dyspepsia. Similarly, while there was significant impairment of quality of life in functional dyspepsia, this was not explained by delayed gastric emptying.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
Supplementary Material
Abbreviations
HRQOL - health related quality of life
NDI - Nepean dyspepsia index
PCS - physical composite score
MCS - mental composite score
IBS - irritable bowel syndrome
GORD - gastro‐oesophageal reflux disease
BMI - body mass index
OR - odds ratio
Footnotes
Supported by a grant to the Mayo Foundation (Dyspepsia Center) from Novartis.
Conflict of interest: declared (the declaration can be viewed on the Gut website at http://www.gutjnl.com/supplemental).
References
- 1.Talley N J, Stanghellini V, Heading R.et al Functional gastroduodenal disorders: a working team report for the Rome II consensus on funtional gastrointestinal disorders. Gut 199945(suppl 2)II37–II42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley N J, Zinsmeister A R, Schleck C D.et al Dyspepsia and dyspepsia subgroups: a population‐based study. Gastroenterology 19921021259–1268. [PubMed] [Google Scholar]
- 3.Castillo E J, Camilleri M, Locke G R.et al A community‐based, controlled study of the epidemiology and pathophysiology of dyspepsia. Clin Gastroenterol Hepatol 20042985–996. [DOI] [PubMed] [Google Scholar]
- 4.Quartero A O, de Wit N J, Lodder A C.et al Disturbed solid‐phase gastric emptying in functional dyspepsia: a meta‐analysis. Dig Dis Sci 1998432028–2033. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Brown M L, Malagelada J R. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology 19869194–99. [DOI] [PubMed] [Google Scholar]
- 6.Tack J, Piessevaux H, Coulie B.et al Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 19981151346–1352. [DOI] [PubMed] [Google Scholar]
- 7.Holtmann G, Geobell H, Talley N J. Impaired small intestinal peristaltic reflexes and sensory thresholds are independent functional disturbances in patients with chronic unexplained dyspepsia. Am J Gastroenterol 199691485–491. [PubMed] [Google Scholar]
- 8.Moayyedi P, Deeks J, Talley N J.et al An update of the Cochrane systematic review of Helicobacter pylori eradication therapy in nonulcer dyspepsia: resolving the discrepany between systematic reviews. Am J Gastroenterol 2003982621–2626. [DOI] [PubMed] [Google Scholar]
- 9.Stanghellini V, Tosetti C, Paternic'o A.et al Predominant symptoms identify different subgroups in functional dyspepsia. Am J Gastroenterol 1999942080–2085. [DOI] [PubMed] [Google Scholar]
- 10.Stanghellini V, Tosetti C, Paternic'o A.et al Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology 19961101036–1042. [DOI] [PubMed] [Google Scholar]
- 11.Tack J, Bisschops R. Mechanisms underlying meal‐induced symptoms in functional dyspepsia. Gastroenterology 20041271844–1847. [DOI] [PubMed] [Google Scholar]
- 12.Talley N J, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility‐like dyspepsia? Am J Gastroenterol 2001961422–1428. [DOI] [PubMed] [Google Scholar]
- 13.Bredenoord A J, Chial H J, Camilleri M.et al Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol 20031264–272. [PubMed] [Google Scholar]
- 14.Camilleri M, Talley N J. Pathophysiology as a basis for understanding symptom complexes and therapeutic targets. Neurogastroenterol Motil 200416135–142. [DOI] [PubMed] [Google Scholar]
- 15.El‐Serag H B, Talley N J. Systematic review: health‐related quality of life in functional dyspepsia. Aliment Pharmacol Ther 200318387–393. [DOI] [PubMed] [Google Scholar]
- 16.Johnsson F, Roth Y, Damgaard Pedersen N E.et al Cimetidine improves GERD symptoms in patients selected by a validated GERD questionnaire. Aliment Pharmacol Ther 1993781–86. [DOI] [PubMed] [Google Scholar]
- 17.Tougas G, Eaker E Y, Abell T.et al Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000951456–1462. [DOI] [PubMed] [Google Scholar]
- 18.Ware J E, Jr, Sherbourne C D. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 199230473–483. [PubMed] [Google Scholar]
- 19.Talley N J, Haque M, Wyeth J W.et al Development of a new dyspepsia impact scale: the Nepean dyspepsia index. Aliment Pharmacol Ther 199913225–235. [DOI] [PubMed] [Google Scholar]
- 20.Talley N J, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean dyspepsia index. Am J Gastroenterol 1999942390–2397. [DOI] [PubMed] [Google Scholar]
- 21.Talley N J, Shuter B, McCrudden G.et al Lack of association between gastric emptying of solids and symptoms in nonulcer dyspepsia. J Clin Gastroenterol 198911625–630. [DOI] [PubMed] [Google Scholar]
- 22.Hutson W R, Roehrkasse R L, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 19899611–17. [DOI] [PubMed] [Google Scholar]
- 23.Caballero‐Plasencia A M, Valenzuela‐Barranco M, Martin‐Ruiz J L.et al Are there changes in gastric emptying during the menstrual cycle? Scand J Gastroenterol 199934772–776. [DOI] [PubMed] [Google Scholar]
- 24.Degnen L P, Phillips S F. Variability of gastrointestinal transit in healthy women and men. Gut 199639299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talley N J, Weaver A L, Zinsmeister A R. Impact of functional dyspepsia on quality of life. Dig Dis Sci 199540584–589. [DOI] [PubMed] [Google Scholar]
- 26.Feinle‐Bisset C, Vozzo R, Horowitz M.et al Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol 200398170–181. [DOI] [PubMed] [Google Scholar]
- 27.Barbera R, Feinle C, Read N W. Nutrient‐specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Dig Dis Sci 1995401636–1641. [DOI] [PubMed] [Google Scholar]
- 28.Feinle C, Grundy D, Otto B.et al Relationship between increasing duodenal lipid doses, gastric perception, and plasma hormone levels in humans. Am J Physiol Regul Integr Comp Physiol 2000278R1217–R1223. [DOI] [PubMed] [Google Scholar]
- 29.Caldarella M P, Azpiroz F, Malagelada J R. Antro‐fundic dysfunctions in functional dyspepsia. Gastroenterology 20031241220–1229. [DOI] [PubMed] [Google Scholar]
- 30.Sarnelli G, Caenepeel P, Geypens B.et al Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol 200398783–788. [DOI] [PubMed] [Google Scholar]
- 31.Choi M G, Camilleri M, Burton D D.et al [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology 19971121155–1162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.