Abstract
Background
The role of positron emission tomography with the glucose analogue [18F] fluoro‐2‐deoxy‐D‐glucose (FDG‐PET) in the initial staging of disease in patients with primary colorectal cancer (CRC) has not been adequately assessed.
Aims
To evaluate the additional value of FDG‐PET as a staging modality, complementary to routine multidetector row computed tomography (MDCT) in patients with CRC.
Methods
Forty four patients with CRC underwent preoperative MDCT and FDG‐PET. The accuracy of intraoperative macroscopic staging was also investigated compared with histopathological diagnosis. All FDG‐PET images were evaluated with respect to detectability of the primary tumour, lymph node involvement, and distant metastases. Both MDCT and FDG‐PET diagnoses and treatment plan were compared with surgical and histopathological results.
Results
Thirty seven patients underwent surgery. Tumour detection rate was 95% (42/44) for MDCT, 100% (44/44) for FDG‐PET, and 100% (37/37) for intraoperative macroscopic diagnosis. Pathological diagnosis of T factor was T1 in five, T2 in four, T3 in 24, and T4 in four cases. Concordance rate with pathological findings of T factor was 57% (21/37) for MDCT and 62% (23/37) for macroscopic diagnosis. Lymph node involvement was pathologically positive in 19 cases. Regarding N factor, overall accuracy was 62% (23/37) for MDCT, 59% (22/37) for FDG‐PET, and 70% (26/37) for macroscopic diagnosis. For all 44 patients, FDG‐PET findings resulted in treatment changes in only one (2%) patient.
Conclusion
FDG‐PET is not superior to routine MDCT in the initial staging of primary CRC.
Keywords: positron emission tomography, colorectal surgery, spiral computed tomography, colorectal neoplasms, neoplasm staging
Colorectal cancer (CRC) is an important cause of morbidity and mortality in Japan as well as in other countries.1 The prognosis of CRC directly relates to extramural tumour spread, ability to achieve surgical clearance, and the presence of lymph node and distant metastases.2,3 Optimal management of individual patients requires detailed assessment of the locoregional and distant extent of disease.
Conventional preoperative staging of CRC has been abdominal computed tomography (CT) and chest radiography to rule out liver, lung, or lymph node metastases and invasion of the surrounding organs, respectively. The introduction of multidetector row CT (MDCT) has provided high resolution imaging and shortened examination time.4 This becomes an effective diagnostic technique in the evaluation of preoperative staging of CRC.
Positron emission tomography with the glucose analogue [18F] fluoro‐2‐deoxy‐D‐glucose (FDG‐PET) is a sensitive diagnostic test that images tumours based on increased utilisation of glucose by tumour cells.5,6 FDG‐PET has been demonstrated to be more sensitive than conventional imaging in the detection of recurrent or metastatic CRC.7,8,9,10,11,12,13 One meta‐analysis revealed an overall sensitivity of 97% and an overall specificity of 76% for FDG‐PET in detecting recurrent CRC.14
However, reports of FDG‐PET in the staging of primary CRC are few.15,16,17,18 Also, these studies had several limitation: patient numbers were small,15,16 or diagnostic accuracy of FDG‐PET was compared with conventional abdominal CT17 or CT was performed at a different hospital.18 Comparison of state of the art FDG‐PET with CT using variable techniques and qualities is not meaningful. Thus the role of FDG‐PET in the initial staging of disease in patients with primary CRC has not been fully investigated to date.19
The purpose of this study was to prospectively evaluate the additional value of FDG‐PET as a staging modality complementary to routine MDCT in patients with primary CRC. In patients undergoing surgery, the accuracy of intraoperative macroscopic staging was also investigated compared with histopathological diagnosis, as well as preoperative imaging results. All studies were performed in one Japanese hospital.
Methods
Patients
Between September 2002 and January 2004, 44 consecutive patients with CRC who approved of this study were enrolled after giving written informed consent in accordance with the regulations of the institutional review board. There were 33 men and 11 women with a mean age of 61.4 years (range 38–82). The primary tumour originated from the right colon (n = 2), sigmoid colon (n = 4), or rectum (n = 38). Histological diagnosis was performed in all patients by colonoscopy. All patients underwent preoperative MDCT and FDG‐PET within one month (median 9 days (range 0–26)).
MDCT
The diagnosis and location were established by barium enema and/or colonoscopy before CT scanning. The time interval between barium enema and/or colonoscopy and MDCT was ⩽1 week. No specific preparation, such as laxatives, enema, or oral contrast agents, was performed before MDCT examination.
We used an Aquiline 16 CT scanner (Toshiba Medical Systems, Tokyo, Japan). For imaging of the whole body, we used the 16 high resolution central detectors. From these detectors we selected a 2 mm slice thickness and reconstructed the data at 5 mm intervals. Other parameters were a 0.5 second helical rotation time, 135 kVp, and 300 mAs. Iopamidol 100 ml (Iopamiron; Nihon Schering, Tokyo, Japan) was administered through a peripheral venous line at 3 ml/s using a power injector (Autoenhance A‐50; Nemoto Kyorindo, Tokyo, Japan). CT scanning began 120 seconds after the start of injection of the contrast medium and scan data were acquired from the neck to the upper femur within one breathhold in approximately 20 seconds. Multiplanar reformation was reconstructed by a freestanding workstation (ZAIO, Tokyo, Japan) if diagnostic radiologists considered it necessary.
FDG‐PET
Patients fasted for at least four hours before the examination. Patients received an intravenous injection of 200–250 MBq of [18F] fluoro‐2‐deoxy‐D‐glucose and then rested for approximately 60 minutes before undergoing imaging. Image acquisition was performed with use of an Advance NXi (GE Medical Systems, Milwaukee, Wisconsin, USA). Two dimensional emission scanning from the groin to the base of the skull (6–7 bed positions) was performed, lasting five minutes per bed position, in combination with a transmission scan lasting 1.5 minutes per bed position (transmission scanning time was corrected to allow for decay of the transmission sources). Data acquired were reconstructed by iterative ordered subsets expectation maximisation (21 subsets, two iterations).
Image analysis
At first, MDCT images were prospectively evaluated by two radiology physicians in consensus. They were assessed for detectability of the tumour, depth of tumour infiltration (T factor), regional lymph node involvement (N factor), and distant metastasis (M factor).
T factor on MDCT was defined by a modified TNM stage: tumour confined to the bowel wall was classified as T1 or T2. T1 was defined as an intraluminal elevated mass without thickening of the bowel wall. T2 was defined as thickening of the bowel wall (>5 mm) without invasion into the surrounding tissue. Tumour exposed out of the bowel wall but with no extension to the surrounding organs was considered as T3. Tumour infiltration into adjacent organs was considered T4. Lymph nodes were considered positive when the short axis was greater than 1 cm in diameter or there were clusters of three or more smaller nodes (each <1 cm). Lesions in the liver not characteristic of a cyst or haemangioma were considered suspicious of metastases. Also in the lung, pulmonary nodules without calcification were regarded as suspicious of metastases.
All FDG‐PET images were interpreted with knowledge of the patient's medical history and MDCT findings, and were evaluated with respect to detectability of the primary tumour, lymph node involvement, and distant metastases by two nuclear radiology physicians. T factor was not evaluated because the layers of intestinal wall and neighbouring structures cannot be differentiated on FDG‐PET. Uptake higher than background was considered to be increased. Physicians interpreted the FDG‐PET images by visually correlating the FDG‐PET and MDCT images (fig 1). This approach was chosen because it represents the routine practice of combined reading of FDG‐PET and MDCT images in our hospital. On the basis of their visual correlation, physicians assigned a TNM stage on FDG‐PET. Regarding N factor, we chose to analyse the imaging studies on a nodal station bases and not on an individual lymph node basis. It seemed impossible for us to make a precise correlation between individually sampled and mapped lymph nodes on imaging studies.
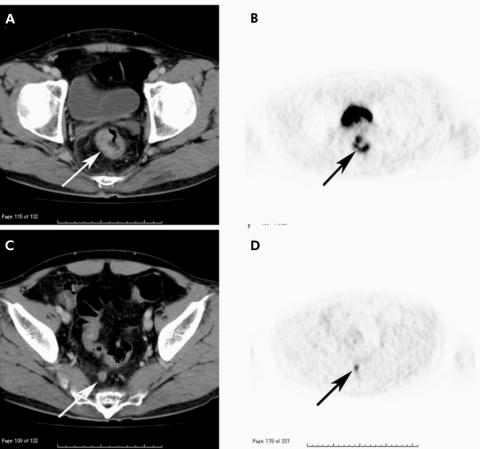
Figure 1 (A) Primary rectal tumour was exposed to the rectal wall but no extension to the pelvic side walls on computed tomography (CT) (arrow). (B) At the same level as (A), avid uptake was demonstrated on positron emission tomography (PET) (arrow). (C) A superior rectal lymph node was greater than 1 cm on CT (arrow). (D) At the same level as (C), PET showed uptake corresponded to the lymph node demonstrated on CT (arrow). This case was preoperatively diagnosed as TNM stage T3N1 and confirmed at surgery and on histopathological examination.
Preoperative staging decision
Both MDCT and FDG‐PET results were presented at the colorectal cancer conference, comprising surgeons, medical oncologists, endoscopists, nuclear radiology physicians, and radiation oncologists. All conference members confirmed the MDCT and FDG‐PET findings. When a clear differentiation between different tumour stages on MDCT and FDG‐PET was not possible, both stages were noted and confirmed after surgery. Based on the consensus of the conference, patients were divided into two groups. Patients considered as unresectable were referred to the Division of Gastrointestinal Medical Oncology, where chemotherapy, chemoradiotherapy, or best supportive care was performed. If unresectable factors were negative, the patient was admitted to a surgical ward and curative resection was attempted. In our hospital, neoadjuvant therapy was not routinely performed. These decisions on diagnosis and treatment plan were recorded and compared with surgical and pathological results.
Macroscopic diagnosis
For the 37 patients who proceeded to surgery, detection of the primary tumour, its depth of invasion, lymph node status, and liver metastases were macroscopically diagnosed either during surgery or through a node collection and classification procedure immediately after resection. These procedures were performed with knowledge of the preoperative imaging findings.
Data analysis
Resected specimens were examined by pathologists without knowing the preoperative MDCT and FDG‐PET findings. The diagnostic accuracy of MDCT, FDG‐PET, and macroscopic diagnosis of T and N factors were assessed using the histopathological findings as the gold standard. Comparison of diagnostic and pathological parameters was performed using the McNemar test. The level of statistical significance was determined at 5% in all cases.
Results
All 44 patients tolerated both MDCT and FDG‐PET examinations without any complications. Based on both MDCT and FDG‐PET, 10 lesions of distant metastases were revealed in five patients and defined as unresectable: three bone metastases, three lung metastases, two liver metastases, and two distant lymph node metastases. MDCT showed eight of these 10 lesions; one each of bone and distant lymph node metastasis were missed. FDG‐PET showed nine of the 10 lesions; one lung metastasis was missed. These five patients did not undergo surgical resection. Two patients were refused any anticancer treatment and left our hospital although their tumours were potentially resectable. Thus the remaining 37 patients were defined as resectable and underwent surgery. As expected, all lesions were resected with regional lymph node dissection.
The tumour detection rate was 95% for MDCT, 100% for FDG‐PET, and 100% for intraoperative macroscopic diagnosis. The two cases which were not detected on MDCT were 0.7 cm and 1.8 cm adenocarcinomas, both limited to the submucosal layer. Regarding T factor, concordance rate with pathological findings was 57% for MDCT and 62% for macroscopic diagnosis (table 1). The difference was not significant (p = 0.813). In three of seven cases, tumours were diagnosed as T4 at surgery but histopathologically with no evidence of invasion to the adjacent organ. In contrast, in one case, MDCT showed no evidence of invasion to the adjacent organ but the tumour was found to have invaded the vagina at surgery and combined resection was performed. Invasion was confirmed histopathologically.
Table 1 Comparisons of MDCT and macroscopic diagnosis in staging depth of tumour invasion with colorectal carcinoma.
| Pathological staging | MDCT diagnosis | Macroscopic diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tx | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |
| T1 | 2 | 0 | 2 | 1 | 0 | 1 | 4 | 0 | 0 |
| T2 | 0 | 0 | 1 | 3 | 0 | 1 | 0 | 3 | 0 |
| T3 | 0 | 0 | 5 | 17 | 2 | 0 | 3 | 18 | 3 |
| T4 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 4 |
MDCT, multidetector row computed tomography.
Regarding N factor, overall accuracy was 62% for MDCT, 59% for FDG‐PET, and 70% for macroscopic diagnosis (table 2). Sensitivity, specificity, positive predictive value, and negative predictive value were calculated as 58%, 67%, 65%, and 60%, respectively, for MDCT; 37%, 83%, 70%, and 43%, respectively, for FDG‐PET; and 68%, 72%, 72%, and 68%, respectively, for macroscopic diagnosis. Macroscopic diagnosis showed a slightly higher accuracy but values were not significantly different between these modalities (p = 0.624 for MDCT v macroscopic diagnosis; p = 0.466 for FDG‐PET v macroscopic diagnosis).
Table 2 Comparisons of MDCT, PET, and macroscopic diagnosis in staging lymph node metastasis with colorectal carcinoma.
| Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value(%) | Accuracy (%) | |
|---|---|---|---|---|---|
| MDCT | 58 (11/19) | 67 (12/18) | 65 (11/17) | 60 (12/20) | 62 (23/37) |
| PET | 37 (7/19) | 83 (15/18) | 70 (7/10) | 43 (15/27) | 59 (22/37) |
| Macroscopic diagnosis | 68 (13/19) | 72 (13/18) | 72 (13/18) | 68 (13/19) | 70 (26/37) |
MDCT, multidetector row computed tomography; PET, positron emission tomography.
Of the 44 patients, FDG‐PET findings resulted in treatment changes in only one (2%) patient who had bone and distant lymph node metastases detected only by FDG‐PET. Although MDCT detected lung metastases that were not demonstrated on FDG‐PET in one case, the patient had other distant metastases and the treatment plan was not influenced by the MDCT findings.
Discussion
CT examination is an established method for staging colorectal carcinoma. However, recent studies have shown low accuracy rates due to considerably low sensitivity for detection of lymph node metastases and for local tumour extension.20,21,22,23 MDCT is expected to improve diagnostic accuracy by scanning a wider region with better resolution. To evaluate the usefulness of FDG‐PET, we compared the diagnostic accuracy of FDG‐PET with this up to date technology.
The efficacy of imaging techniques is usually evaluated by retrospective reading by radiologists blinded to the clinical information. However, this is not practical and many physicians often feel that actual diagnostic results are different from the results reported. We wished to evaluate the usefulness of FDG‐PET in the clinical setting. Recently, integrated PET‐CT scanners have been introduced into the clinical situation.24 Using this technique, PET, CT, and integrated PET‐CT images are displayed together on the monitor. This type of PET scanner and reading style will become routine. Thus in this study, FDG‐PET images were interpreted with knowledge of the patient's medical history and MDCT images.
For detection of a primary tumour, FDG‐PET was positive in all 44 lesions but MDCT gave two false negative lesions. We did not use any preparation before the MDCT studies. If sufflation of air or water into the bowel cavity and administration of antiperistaltic drug had been performed, the detection rate might have been improved. In contrast, FDG‐PET was positive in all lesions, including those that were negative with MDCT. Such high sensitivity confirms the results of previous reports.15,16,17 The injected dose that we used was lower than the conventional dose reported. However, we had confirmed in our preliminary study that image quality with this dose did not deteriorate. This may have been due to differences in the physique of Japanese and Western patients. We should try to reduce radiation exposure while preserving diagnostic accuracy.
CT studies over the last decade showed accuracy rates of 41–82% in T staging.20,21,22,23 Our result (57%) was comparable with these reports. Even with the improved imaging resolution of MDCT, it is still difficult to discriminate bowel wall layers as conventional single detector spiral CT. MDCT did not demonstrate satisfactory results for diagnosis of N factor, as reported in previous studies.25,26,27 Microscopic metastasis or uninvolved swelling of lymph nodes results in misdiagnosis. As long as a diagnosis is made based on the size of lymph nodes, a certain percentage of false positive and negative lymph nodes is unavoidable. In this study, FDG‐PET had low sensitivity (37%) and high specificity (83%), as reported in previous studies.16,17,18 FDG‐PET was no better than MDCT. The high false negative rate was attributed to limited spatial resolution, which was a disadvantage in detecting micrometastases, and the proximity of the dose to the primary tumour to lymph node metastases.
The accuracy of intraoperative macroscopic diagnosis was superior but not significantly different from that of MDCT and FDG‐PET. By palpation and inspection, lymph nodes in the immediate vicinity of the primary tumour could be differentiated more easily than by MDCT or FDG‐PET. Moreover, macroscopic diagnosis was made with knowledge of the MDCT and FDG‐PET findings. Nevertheless, accurate diagnosis of lymph node metastasis is difficult, even at surgery.
FDG‐PET has the advantage of studying the whole body at one examination and synchronous tumours have been identified on FDG‐PET. However, MDCT can also scan the whole body in a shorter time than FDG‐PET. In this study, distant metastases revealed only 10 lesions in five patients. While patient numbers were too small to compare the usefulness of the diagnostic modalities, MDCT and FDG‐PET showed various corresponding metastatic lesions.
In assessing the influence of FDG‐PET findings on clinical management, changes in therapeutic decision making were made in only 2% (1/44) of cases, which is less than in other investigations. The incidence of management alterations due to FDG‐PET was reported as 16–50%.8,13,18,28,29 The reason may be selection of patients in the other studies as many were already known to have advanced disease and FDG‐PET was performed to detect recurrences or metastases.
The results of this study suggest that the diagnostic accuracy of FDG‐PET for the initial staging of CRC was not superior to routine MDCT and was not influential in terms of patient management. We believe routine evaluation of patients with a suspicion of CRC by FDG‐PET is not necessary; it should be performed on selected patients who have suggestive but inconclusive metastatic lesions with other modalities.
Acknowledgements
This work was supported by the Foundation for Promotion of Cancer Research in Japan.
Abbreviations
PET - positron emission tomography
FDG‐PET - [18F] fluoro‐2‐deoxy‐D‐glucose‐positron emission tomography
CRC - colorectal cancer
CT - computed tomography
MDCT - multidetector row computed tomography
Footnotes
Conflict of interest: None declared.
References
- 1.Nomura K, Sobue T, Honma I.et al Mortality from malignant neoplasms by age group and sex in Japan. In: The editorial board of the cancer statistics in Japan. Cancer statistics in Japan 2003. Tokyo: Foundation for Promotion of Cancer Research, 200340–41.
- 2.Adam I J, Mohamdee M O, Martin I G.et al Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994344707–711. [DOI] [PubMed] [Google Scholar]
- 3.de Haas‐Kock D F, Baeten C G, Jager J J.et al Prognostic significance of radial margins of clearance of rectal cancer. Br J Surg 199683781–785. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, He H D, Foley W D.et al Four multidetector‐row helical CT: image quality and volume coverage speed. Radiology 200021555–62. [DOI] [PubMed] [Google Scholar]
- 5.Som P, Atkins H L, Bandoypadhyay D.et al A fluorinated glucose analog, 2‐fluoro‐2‐deoxy‐D‐glucose (F‐18): nontoxic tracer for rapid tumor detection. J Nucl Med 198021670–675. [PubMed] [Google Scholar]
- 6.Flier J S, Mueckler M M, Usher P.et al Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 19872351492–1495. [DOI] [PubMed] [Google Scholar]
- 7.Topal B, Flamen P, Aerts R.et al Clinical value of whole‐body emission tomography in potentially curable colorectal liver metastases. Eur J Surg Oncol 200127175–179. [DOI] [PubMed] [Google Scholar]
- 8.Arulampalam T, Costa D, Visvikis D.et al The impact of FDG‐PET on the management algorithm for recurrent colorectal cancer. Eur J Nucl Med 2001281758–1765. [DOI] [PubMed] [Google Scholar]
- 9.Delbeke D, Vitola J V, Sandler M P.et al Staging recurrent metastatic colorectal carcinoma with PET. J Nucl Med 1997381196–1201. [PubMed] [Google Scholar]
- 10.Flamen P, Stroobants S, Van Cutsem E.et al Additional value of whole‐body positron emission tomography with fluorine‐18‐2‐fluoro‐2‐deoxy‐D‐glucose in recurrent colorectal cancer. J Clin Oncol 199917894–901. [DOI] [PubMed] [Google Scholar]
- 11.Johnson K, Bakhsh A, Young D.et al Correlating computed tomography and positron emission tomography scan with operative findings in metastatic colorectal cancer. Dis Colon Rectum 200144354–357. [DOI] [PubMed] [Google Scholar]
- 12.Lai D T, Fulham M, Stephen M S.et al The role of whole‐body positron emission tomography with [18F]fluorodeoxy‐glucose in identifying operable colorectal cancer metastases to the liver. Arch Surg 1996131703–707. [DOI] [PubMed] [Google Scholar]
- 13.Ogunbiyi O A, Flanagan F L, Dehdashti F.et al Detection of recurrent and metastatic colorectal cancer: comparison positron emission tomography and computed tomography. Ann Surg Oncol 19974613–620. [DOI] [PubMed] [Google Scholar]
- 14.Huebner P H, Park K C, Shepherd J E.et al A meta‐analysis of the literature for whole body FDG PET detection of recurrent colorectal cancer. J Nucl Med 2000411177–1189. [PubMed] [Google Scholar]
- 15.Gupta N C, Falk P M, Frank A L.et al F‐18 fluorodeoxyglucose (FDG) PET for preoperative staging of colorectal carcinoma (abstr). J Nucl Med 199233975 [Google Scholar]
- 16.Abdel‐Nabi H, Doerr R J, Lamonica D M.et al Staging of primary colorectal carcinomas with fluorine‐18 Fluorodeoxyglucose whole‐body PET: correlation with histopathologic and CT findings. Radiology 1998206755–760. [DOI] [PubMed] [Google Scholar]
- 17.Mukai M, Sadahiro S, Yasuda S.et al Preoperative evaluation by whole‐body 18F‐fluorodeoxyglucose positron emission tomography in patients with primary colorectal cancer. Oncol Rep 2000785–87. [PubMed] [Google Scholar]
- 18.Kantorová I, Lipská L, Bělohlávek O.et al Routine 18F‐FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med 2003441784–1788. [PubMed] [Google Scholar]
- 19.Arulampalam T H A, Costa D C, Loizidou M.et al Positron emission tomography and colorectal cancer. Br J Surg 201 88176–189. [DOI] [PubMed] [Google Scholar]
- 20.Thoeni R F. Colorectal cancer: radiological staging. Radiol Clin N Am 199735457–458. [PubMed] [Google Scholar]
- 21.Hundt W, Braunschweig R, Reiser M. Evaluation of spiral CT in staging of colon and rectum carcinoma. Eur J Radiol 1999978–84. [DOI] [PubMed] [Google Scholar]
- 22.Angelelli G, Macarini L, Lupo L.et al Rectal carcinoma: CT staging with water as contrast medium. Radiology 1990177511–514. [DOI] [PubMed] [Google Scholar]
- 23.Chiesura‐Corona M, Muzzio P C, Giust G.et al Rectal cancer: CT local staging with histopathologic correlation. Abdom Imaging 200126134–138. [DOI] [PubMed] [Google Scholar]
- 24.Cohade C, Osman M, Leal J.et al Direct comparison of 18F‐FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med 2003441797–1803. [PubMed] [Google Scholar]
- 25.Matsuoka H, Nakamura A, Masaki T.et al Preoperative staging by multidetector‐row computed tomography in patients with rectal carcinoma. Am J Surg 2002184131–135. [DOI] [PubMed] [Google Scholar]
- 26.Klinna C, Eibel R, Matzek W.et al Staging of rectal cancer: diagnostic potential of multiplanar reconstructions with MDCT. AJR Am J Roentgenol 2004183421–427. [DOI] [PubMed] [Google Scholar]
- 27.Flippone A, Ambrosini R, Fuschi M.et al Preoperative T and N staging of colorectal cancer: accuracy of contrast‐enhanced multidetector‐row CT colonography‐initial experience. Radiology 200423183–90. [DOI] [PubMed] [Google Scholar]
- 28.Boykin K N, Zibari G B, Lilien D L.et al The use of FDG‐positron emission tomography for the evaluation of colorectal metastases of the liver. Am Surg 1999651183–1185. [PubMed] [Google Scholar]
- 29.Ruers T J, Langenhoff B S, Neeleman N.et al Value of positron emission tomography with (F‐18)fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol 200220388–395. [DOI] [PubMed] [Google Scholar]