Abstract
Background and aims
Cellular mediated immunity (CMI) is thought to play a key role in resolution of primary hepatitis C virus (HCV) infection. However, CD4+ and CD8+ T cell responses are also generated during acute infection in individuals who become chronic, suggesting that they developed a defective CMI. The aim of this study was to verify if and when such immune dysfunction is established by measuring the breadth, magnitude, function, and duration of CMI in a large cohort of subjects during the natural course of acute HCV infection.
Methods
CMI was comprehensively studied by prospective sampling of 31 HCV acutely infected subjects enrolled at the onset of infection and followed for a median period of one year.
Results
Our results indicated that while at the onset of acute HCV infection a measurable CMI with effector function was detected in the majority of subjects, after approximately six months less than 10% of chronically infected individuals displayed significant CMI compared with 70% of subjects who cleared the virus. We showed that progressive disappearance of HCV specific T cells from the peripheral blood of chronic patients was due to an impaired ability to proliferate that could be rescued in vitro by concomitant exposure to interleukin 2 and the antigen.
Conclusion
Our data provide evidence of strong and multispecific T cell responses with a sustained ability to proliferate in response to antigen stimulation as reliable pharmacodynamic measures of a protective CMI during acute infection, and suggest that early impairment of proliferation may contribute to loss of T cell response and chronic HCV persistence.
Keywords: hepatitis C virus, CD8+ T cells, CD4+ T cells, protective immunity, immunotherapy
The high rate of chronic hepatitis is one of the striking features of hepatitis C virus (HCV) infection compared with other hepatic infections, and mechanisms leading to failure of viral clearance are still poorly defined. The current opinion is that antiviral cellular mediated immunity (CMI) plays a crucial role in determining the outcome of acute HCV infection.1,2,3,4,5,6 The causal role for T cell responses in HCV clearance was only recently proven in a chimpanzee model by the finding that in vivo depletion of either CD4+ or CD8+ T cells prevents HCV clearance and clinical recovery.7,8 A number of studies employing individual HCV peptides or pools of peptides spanning the entire HCV polyprotein have shown that the majority of infected individuals, independent of disease outcome, mounted virus specific T cell responses in the first months after infection.9,10,11,12,13 We recently reported a cross sectional study of a large cohort of acutely infected subjects in which we detected within the first month of diagnosis of infection a frequency of HCV specific T cell responses (60%) that was twice that of the rate of disease resolution (27%), implying that early induction of T cell response per se is not sufficient to achieve viral clearance.14 Significant differences in the frequencies of HCV specific T cells have been shown between subjects with spontaneous resolution or chronic disease years or even decades following infection.4,5,9,14,15,16,17
The aim of the present study was to investigate the immune mechanisms underlying the establishment of persistent infection during the natural course of acute HCV infection. Thirty one individuals were enrolled after diagnosis of acute infection and followed by prospective sampling for a median period of one year. In this cohort, we sought to define the onset of T cell responses targeting almost the entire HCV genome by sensitive ex vivo assays. We showed that measurable CMI can be detected in the majority of subjects at the onset of acute infection but approximately six months later T cell responses progressively disappear in chronically infected individuals. We showed that HCV specific T cells from peripheral blood of chronic patients have an impaired ability to proliferate that can be rescued in vitro by concomitant exposure to interleukin 2 (IL‐2) and the antigen.
Methods
Study population
The study cohort included 31 patients with acute hepatitis C (table 1). Diagnosis of acute HCV infection in this cohort has been described previously.14 Patients received a medical check up and blood samples were taken for biochemical, virological, and immunological assessment after diagnosis of infection (month 0: start of the observation) and after 1, 3, 6, and 12 months, and then at four month intervals for a total of 24 months or until antiviral therapy. All observations reported in this study refer to the natural history of the infection (that is, in the absence of therapy) unless otherwise specified. Recovery from acute infection has been described previously.14
Table 1 Patients characteristics.
| Patient code | Sex | Age (y) | Risk factor | HLA class I | HLA class II | Outcome | Peak ALT | Peak RNA | Genotype | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| A13 | F | 35 | IVDU | A*01*24 | DRB1*04*0701 DQB1*0302*0303 | Self limited | 1083 | 590 | ND | 17 |
| A24 | M | 57 | Iatrogenic | A*24*33 | DRB1*16*03 DQB1*0502*02 | Self limited | 1728 | 590 | 1b | 12 |
| A25 | F | 58 | Immunoglobulins | A*24*32 | DRB1*16*03 DQB1*0502*02 | Self limited | 1177 | 54 300 | 1b | 27 |
| A26 | F | 28 | ND | A*02*23 | DRB1*15*1101 DQB1*0501*0301 | Self limited | 1044 | 590 | ND | 6 |
| A43 | M | 20 | IVDU | A*01*30 | DRB1*01*1104 DQB1*0501*0301 | Self limited | 1443 | 60 100 | 3a | 12 |
| A45 | M | 57 | Iatrogenic | A*02*32 | DRB1*1301*1302 DQB1*06 | Self limited | 1359 | 96 100 | 1a | 12 |
| A46 | F | 46 | Needle | A*11*33 | DRB1*1101*14 DQB1*0503*0301 | Self limited | 1185 | 223 000 | 1b | 11 |
| A50 | F | 61 | Iatrogenic | A*02*24 B*35*49 | DRB1*04*0701 DQB1*02*0302 | Self limited | 1829 | 2 380 000 | 1b | 12 |
| A1 | M | 25 | Sexual | A*01*66 B*15*35 | DRB1*14*0701 DQB1*0503*02 | Chronic | 2790 | 24 300 | 3a | 26 |
| A3 | M | 25 | IVDU | A*24 B*13*18 | DRB1*0701*1104 DQB1*02*0301 | Chronic | 504 | 2700 | 1a/1b | 13 |
| A5 | M | 20 | IVDU | A*02*03 | DRB1*0101*1301 DQB1*0501*0603 | Chronic | 1113 | 156 000 | 3a | 24 |
| A6 | M | 28 | IVDU | A*02 B*18*40 | DRB1*1101*1104 DQB1*0301 | Chronic | 803 | 919 000 | 3a | 23 |
| A9 | F | 20 | IVDU | A*23*30 B*13*49 | DRB1*0701*1104 DQB1*02*0301 | Chronic | 129 | 1350 | 3a | 23 |
| A11 | M | 31 | IVDU | A*02*26 | DRB1*04*1104 DQB1*0301*0302 | Chronic | 324 | 1 940 000 | 3a | 18 |
| A12 | M | 22 | IVDU | A*01*02 B*13*51 | DRB1*16*0701 DQB1*0502*02 | Chronic | 650 | 900 000 | 3a | 24 |
| A14 | M | 22 | IVDU | A*03*26 | DRB1*15*04 DQB1*0502*0302 | Chronic | 915 | 20 300 | 3a | 13 |
| A16 | M | 23 | IVDU | A*24*30 B*50*52 | DRB1*04*0701 DQB1*02*04 | Chronic | 1071 | 95 700 | 2b | 24 |
| A17 | M | 28 | IVDU | A*01*32 | DRB1*0701*1101 DQB1*0301*0303 | Chronic | 107 | 556 000 | 1b | 7 |
| A18 | M | 27 | IVDU | A*03*68 | DRB1*0701*13 DQB1*02*0603 | Chronic | 474 | 225 000 | 3a | 7 |
| A27 | M | 23 | IVDU | A*02*32 | DRB1*1102*13 DQB1*06*0301 | Chronic | 1072 | 590 | 1a | 6 |
| A28 | M | 56 | Iatrogenic | A*02*25 | DRB1*15*1104 DQB1*0602*0301 | Chronic | 792 | 269 000 | 1b | 15 |
| A29 | M | 34 | IVDU | A*01*26 | DRB1*0701*12 DQB1*02*0301 | Chronic | 816 | 35 600 | 1b | 9 |
| A32 | M | 31 | Iatrogenic | A*02*23 | DRB1*15*13 DQB1*0602*0301 | Chronic | 1515 | 590 | 1b | 21 |
| A33 | F | 33 | IVDU | A*02*11 | DRB1*04*14 DQB1*0503*0302 | Chronic | 351 | 1270 | 1b | 16 |
| A34 | M | 20 | Iatrogenic | A*01*02 | DRB1*15*1104 DQB1*0601*0301 | Chronic | 3276 | 11 100 | 1b | 10 |
| A35 | M | 46 | Iatrogenic | A*01*26 | DRB1*03*1104 DQB1*02*0301 | Chronic | 1330 | 7820 | 1b | 16 |
| A37 | M | 37 | IVDU | A*30*33 | DRB1*01*03 DQB1*0501*0302 | Chronic | 975 | 6630 | 3a | 12 |
| A44 | F | 37 | Needle | A*11*29 | DRB1*01*04 DQB1*0502 | Chronic | 1870 | 108 000 | 1b | 12 |
| A47 | M | 30 | Iatrogenic | A*03*26 | DRB1*1104 DQB1*0301 | Chronic | 568 | 38 300 | 1b | 9 |
| A48 | M | 36 | ND | A*24*30 | DRB1*03*0701 DQB1*02 | Chronic | 2425 | 13 300 | ND | 6 |
| A49 | M | 30 | IVDU | A*02 | DRB1*1104*14 DQB1*0503*0301 | Chronic | 1685 | 960 | 1a | 12 |
All patients gave informed consent before entering the study. The protocol and all procedures were conducted in accordance with the ethics guidelines of the Declaration of Helsinki.
Virological assays
Levels of serum HCV RNA were determined using Amplicor HCV Monitor Kit version 3 (Roche Diagnostic Systems, Branchburg, New Jersey, USA; detection limit 600 UI/ml). In all samples with a viral load <600 UI/ml, the presence of HCV RNA was evaluated by a qualitative polymerase chain reaction (PCR) with a detection limit of 100 copies/ml. RNA extraction and reverse transcription (RT)‐PCR were performed using the protocol and primers previously detailed.14
HCV genotype in PCR positive sample was assessed using a reverse hybridisation line probe assay (Inno‐LiPA HCV Kit; Innogenetics, Ghent, Belgium).
Cell preparation
Peripheral blood mononuclear cells (PBMC) from blood samples were isolated on Ficoll‐Histopaque density gradient (Amersham Pharmacia Biotech, Sweden) at several time points during the course of acute hepatitis and then cryopreserved in media containing 90% fetal bovine serum (Hyclone, Logan, Utah, USA) and 10% DMSO.
Synthetic peptides for T cell analysis
The peptide sequence, spanning core, and NS3‐NS5B regions were derived from the HCV BK strain (genotype 1b).20 Fifteen amino acid long peptides overlapping by 11 amino acids were combined in seven pools covering core, NS3 protease (NS3p), NS3 helicase (NS3h), NS4, NS5A, and NS5B (split into two pools NS5B‐I and NS5B‐II). Pools were tested at a final concentration of 5 μg/ml each.
IFN‐γ ELIspot
Details of this assay have been described previously.14 DMSO and ConA were used as negative and positive controls, respectively. Spots were quantified by an automated ELIspot reader system (ELR03 AID; Elispot Scientific, Strassberg, Germany). Specific signals were evaluated by defining a cut off value based on fold increase over background and overall spot count. PBMC from 20 HCV seronegative subjects were stimulated using individual HCV peptide pools and compared with cells incubated in the absence of peptides (“mock”). Ratios between values measured in the presence and absence represented the fold increase over background. Background ranged between 0 and 55 spots per million cells. Spot counts in this range were not considered antigen specific. Threefold over mock plus at least 55 specific spots/million cells was used as a cut off for a positive response.
BrdU proliferation assay
PBMC (106 in 1 ml of RPMI/10% fetal calf serum) were incubated with peptide pools (5 μg/ml final concentration of each peptide) and anti‐CD28 (10 μg/ml; Becton Dickinson, Franklin Lakes, New Jersey, USA) at 37°C and 5% CO2 for four days. DMSO and ConA were used as negative and positive controls, respectively. For IL‐2 stimulation, the cytokine (ZeptoMetrix Corporation, Buffalo, New York, USA) was added at 10 U/ml final concentration. BrdU (10 μg/ml; Becton Dickinson BrdU proliferation kit) was added for the last 14 hours of incubation. PBMC were washed and stained with surface antibodies, CD3‐APC (Becton Dickinson), CD4‐PerCP‐Cy5 (Becton Dickinson), and CD8β‐PE (Immunotech, Beckman Coulter, Fullerton, California, USA) for 30 minutes at room temperature. After washings, cells were fixed, permeabilised, and treated with Dnase I using the kit reagents and following the manufacturer's protocol. PBMC were then stained with the anti‐BrdU‐FITC antibody (Becton Dickinson). Cells were washed and analysed on a FACS‐Calibur flow cytometer using CellQuest software (Becton Dickinson). To set a threshold for a positive response, PBMC from 20 control (HCV negative) donors were tested by BrdU proliferation assay after stimulation with DMSO (mock) and HCV peptide pools (data not shown). No differences were detected with individual peptide pools. The average of all values plus three times the standard deviation was set as a threshold for a positive response and was 4/10 000 for CD8+ T cells and 5/10 000 for CD4+ T cells. To define a patient as a positive responder in the proliferation assay, the percentage of BrdU+ cells in HCV stimulated PBMC should be above the threshold and three times the value of DMSO stimulated cells.
Tetramer analysis
PBMC (106) were stained with FITC conjugated tetrameric complexes (Proimmune Ltd, Oxford, UK) and antibodies. The following monoclonal antibodies were used: CD3‐APC (Becton Dickinson), CD4‐ PerCP‐Cy5 (Becton Dickinson), and CD8beta‐PE (Immunotech). The following peptides were used: NS31073‐1081 (CINGVCWTV), NS31406‐1415 (KLVALGINAV), NS5b2594‐2602 (ALYDVVTKL), and FluMP (GILGFVFTL). Acquisitions and analyses were done on a FACS Calibur using CellQuest software (Becton Dickinson). PBMC from 20 HCV seronegative individuals were used as controls, to set a threshold for detection that was 0.01% of total CD8+ (data not shown). FACS staining was considered specific only if Tet positive cells formed a cluster distinct from Tet negative CD8+ T cells.
Statistical analysis
The χ2 test and Fisher's exact test were used for comparisons of proportions, and differences were considered significant when p values were <0.05.
Results
T cell response with sustained ability to secrete IFN‐γ is an immunological hallmark of acute/resolving HCV infection
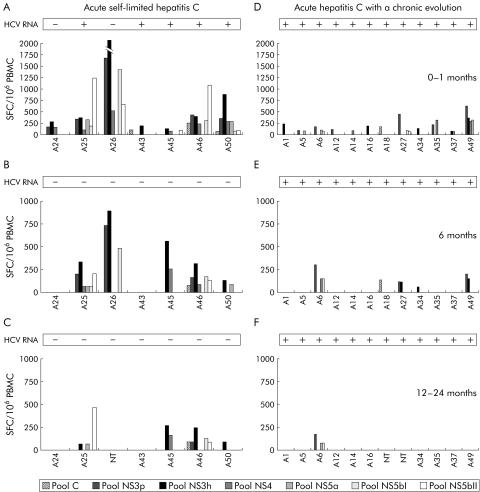
We performed a longitudinal analysis of HCV induced CMI in a cohort of 31 acutely infected subjects (table 1). Eight subjects had a self limiting disease while the others developed persistent infection. The characteristics of this cohort are displayed in table 1 and a detailed description of clinical, epidemiological, and virological parameters of the enrolled subjects has been reported previously.14
T cell responses were measured by ex vivo IFN‐γ ELIspot in PBMC samples collected at the time of diagnosis and after 1, 3, 6, 12 months, and then at four month intervals for a total of 24 months. In fig 1, ELIspot data from responding individuals at three representative time points which will be referred henceforth as early (either 0 or 1 month), intermediate (six months), and late (between 12 and 24 months) are shown. We used a panel of overlapping peptides derived from a 1b HCV isolate corresponding to the core and non‐structural region as representative of the most conserved elements of the HCV polyprotein among different strains. In the early phase of infection, seven of eight subjects with acute self limiting infection displayed HCV specific T cell responses while only half (12 of 23) of individuals who developed a chronic infection showed a measurable T cell response. A broader and stronger CMI was detected in acute self limiting individuals versus chronically evolving patients (fig 1A, D). Notably, two of the acute/resolving individuals (A24 and A26) had already cleared viral RNA at this time point. In the intermediate phase, all acute/resolving subjects displayed a negative HCV RNA test and yet five displayed strong and poly specific CMI by IFN‐γ ELIspot which were still detectable in the late phase (fig 1B, C). In contrast, T cell response measured in chronically evolving patients was not sustained over time as only approximately 20% of these subjects displayed detectable CMI in the intermediate phase, and a single patient remained positive after the first year from diagnosis (fig 1E, F). The difference in the number of subjects responding to HCV peptide pools between chronic and resolving individuals after one year of follow up was statistically significant (p = 0.0002), indicating that long surviving HCV specific T cells are an immunological hallmark of a protective CMI.
Figure 1 Longitudinal analysis of the magnitude and breadth of the T cell response in subjects with acute self limiting infection and subjects with a chronic evolution. (A–F) Peripheral blood mononuclear cell (PBMC) samples collected at three representative time points from diagnosis (0–1 months (A, B); six months (B, E); between 12 and 24 months (C, F)) were tested by interferon γ ELIspot assay against seven peptide pools corresponding to core (C), NS3 protease (NS3p), NS3 helicase (NS3h), NS4, NS5a, and NS5b (split into two pools NS5bI and NS5bII). To simplify, only responses above the threshold defined using hepatitis C virus (HCV) seronegative subjects (see methods) are shown. For all remaining subjects ELIspot responses were negative at all time points tested. Numbers represent spot forming cells (SFC)/106 PBMC. HCV RNA was monitored in serum by quantitative reverse transcription‐polymerase chain reaction (RT‐PCR) and by inhouse RT nested PCR, and results are indicated as + or −. NT, not tested.
We measured the frequencies of CD8+ and Th1 CD4+ T cells producing IFN‐γ by intracellular cytokine staining (ICS) of PBMC from all the patients showing a positive CMI by IFN‐γ ELIspot (data not shown). Both CD8+ and CD4+ virus specific T cells were detected in the peripheral blood of acute patients but no statistically significant correlation was found between the frequencies of the T cell types and disease outcome.
HCV specific CD4+ and CD8+ T cells in chronically evolving patients do not proliferate on antigen re‐encounter in vitro
We showed that peripheral T cell responses were significantly reduced in chronic subjects during the transition from acute to chronic infection (p = 0.0006; fig 1D–F). To understand the mechanism leading to short lasting T cell responses in individuals developing a chronic disease, we analysed proliferation capacity in response to antigen stimulation. HCV peptide pools that gave a positive CMI response by ELIspot were used to stimulate PBMC in an ex vivo cytofluorimetric proliferation assay based on BrdU incorporation which allowed us to study the proliferation capacity of both CD4+ and CD8+ T cells. To validate this assay and to set a threshold for a positive response, we analysed PBMC from 20 seronegative individuals stimulated with HCV peptide pools (see methods).
In table 2, we show the results of the BrdU proliferation analysis performed on six self recovered and six chronically evolving patients' PBMC collected at the moment of diagnosis (time: 0–1 month) and after six months. To allow a comprehensive comparison between the two groups of patients, table 2 includes data on the patient's infecting genotype, the antigen used for ex vivo PBMC stimulations, and IFN‐γ ELIspot data. To simplify the results of the proliferation analysis we have defined a patient's response as positive (+) or negative (−) when the frequencies of BrdU+ T cells were above the assay threshold and three times the mock stimulated control. Strong proliferation responses were observed in all ConA stimulated PBMC (data not shown) indicating that the negative (−) response following HCV peptide stimulation was not a general property of the sampled PBMC.
Table 2 Frequencies of hepatitis C virus (HCV) specific BrdU+ T cells in subjects with acute self limiting infection and those with chronic evolution.
| Subject No | Infecting genotype | Infection outcome | Peptide pool | Time: 0–1 months | Time: 6 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISpot* | BrdU CD4† | CD8‡ | ELISpot* | BrdU CD4† | CD8‡ | ||||||
| A24 | 1b | Self limited | NS3h | 275 | + | 0.09 | 0.05 | 48 | − | 0.00 | 0.00 |
| NS3p | 168 | + | 0.19 | 0.05 | 33 | − | 0.02 | 0.01 | |||
| A45 | 1b | Self limited | NS3h | 123 | − | 0.01 | 0.00 | 558 | + | 0.01 | 0.04 |
| A50 | 1b | Self limited | NS3h | 876 | + | 0.17 | 0.20 | 128 | + | 0.12 | 0.02 |
| A25 | 1b | Self limited | NS3h | 370 | nd | nd | Nd | 30 | + | 0.01 | 0.04 |
| A46 | 1b | Self limited | NS3h | 395 | nd | nd | Nd | 315 | + | 0.04 | 0.14 |
| NS5bI | 300 | nd | nd | Nd | 168 | + | 0.03 | 0.05 | |||
| A43 | 3a | Self limited | NS4 | 193 | + | 0.46 | 0.03 | 48 | + | 0.04 | 0.16 |
| A35 | 1b | Chronic | NS3p | 217 | + | 0.00 | 0.07 | 85 | − | 0.01 | 0.00 |
| NS4 | 313 | − | 0.00 | 0.03 | 88 | − | 0.01 | 0.03 | |||
| A49 | 1a | Chronic | NS3h | 363 | − | 0.03 | 0.03 | 150 | − | 0.03 | 0.01 |
| A34 | 1b | Chronic | NS3h | 133 | − | 0.00 | 0.00 | 58 | − | 0.00 | 0.00 |
| A27 | 1a | Chronic | NS3h | 60 | − | 0.01 | 0.01 | 110 | − | 0.00 | 0.03 |
| A1 | 3a | Chronic | NS3h | 278 | + | 0.04 | 0.38 | 95 | − | 0.01 | 0.01 |
| NS3p | 168 | − | 0.01 | 0.01 | 53 | − | 0.01 | 0.01 | |||
| A18 | 3a | Chronic | Core | 173 | − | 0.00 | 0.01 | 135 | − | 0.01 | 0.01 |
*Spot forming cells (SFC)/106 peripheral blood mononuclear cells (PBMC) (positive values are in bold type).
†%BrdU+CD4+/total CD4+CD3+ (positive values are in bold type).
‡%BrdU+CD8+/total CD8+CD3+ (positive values are in bold type).
nd, not done.
Several additional data are included: patient ID, infecting genotype, outcome of infection, peptide pool used for ex vivo stimulation of PBMC, and frequencies of BrdU+ T cells measured at 0–1 month and at six months. ELIspot data are included to compare the number of SFC/106 PBMC measured in the patient at the corresponding time point and with the corresponding peptide pool. A positive proliferation response is indicated with a + when the frequency of BrdU+ CD8+ or CD4+ T cells was above the threshold set in the assay validation and three times the background of the unstimulated control (see methods).
At the early time point (0–1 month), three of four self recovered individuals (A24, A50, and A43) and two of six chronically evolving individuals (A35 and A1) showed proliferation responses on antigen stimulation; at the late time point (six months), BrdU+ T cells were detected in five of six self recovered and none of chronically evolving individuals (table 2). Differences in frequency of proliferation responses in patients with different disease outcomes six months after diagnosis were statistically significant (p = 0.015), suggesting that the lack and/or loss of T cell proliferation during the acute phase correlates with evolution towards chronic infection. We characterised the phenotype of proliferating T cells and showed that both CD8+ and CD4+ T cells were capable of proliferating in response to antigen stimulation in self recovered individuals. In contrast, only CD8+ proliferating T cells were detected in the two chronically evolving patients showing positive BrdU assay at the early time point (table 2). Interestingly, lack of proliferation capacity of HCV specific T cells in chronic patients was observed despite the presence of measurable IFN‐γ producing T cells by ELIspot assay: at six months, chronic patients A49, A27, and A18 had 150, 110, and SFC/106 PBMC, respectively, and were therefore comparable with resolving patients A50 and A43 (128 and 168 SFC/106 PBMC, respectively). Nevertheless, only T cells from self recovered patients had the ability to proliferate in response to HCV peptide pools. These data strongly suggest that early dysfunction of HCV specific T cells during acute chronic infection leads to impairment in proliferation capacity.
Lack of persistent CMI in chronically evolving patient A49 is due to T cell functional anergy and exhaustion
To test whether failure to detect CD4+ or CD8+ producing IFN‐γ in HCV infected individuals progressing towards chronic disease could be due to functional anergy, T cell exhaustion, or both, we analysed PBMC by tetramer staining technology. Three HLA.A2.01‐peptide tetramers (NS3p, NS3h, and NS5b) were tested on all HLA‐A2 positive acute subjects of our cohort (A5, A27, A34, A45, A49, A50; table 1). Significant frequencies of circulating tetramer positive cells were detected only in patients A49 and A27 (data not shown).
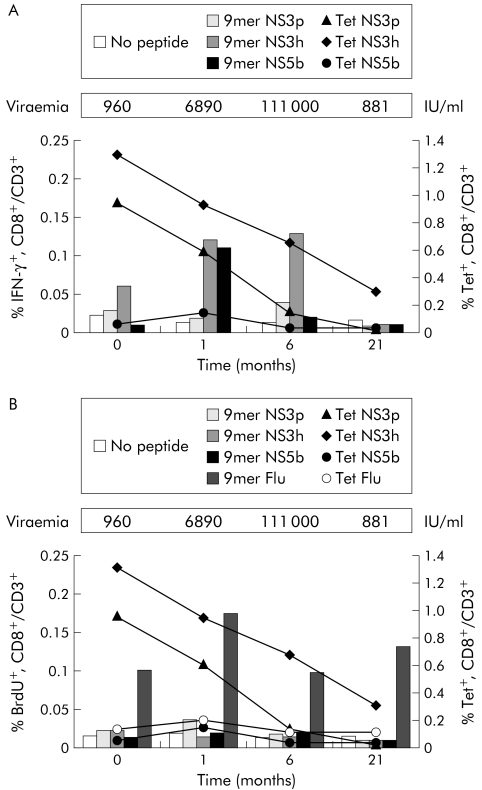
Figure 2A shows a prospective analysis of the HCV tetramer positive (Tet+) T cell frequencies in patient A49 and of secretory capacity, as measured by IFN‐γ ICS after stimulation with the corresponding individual nonamer peptides. NS3h‐Tet+ and NS3p‐Tet+ cells were already relatively abundant at early stages of infection (1.3% and 0.95% of total CD8+ T cells, respectively) but none the less they failed to produce IFN‐γ. In the following weeks their frequency decreased with different kinetics until they became undetectable (NS3p‐Tet) or strongly reduced (NS3h‐Tet) at six and 21 months. This was an HCV specific phenomenon as control influenza virus tetramer positive (Flu‐Tet+) T cells were found at a low but constant frequency in all prospective PBMC samples examined, and they were capable of secreting IFN‐γ when stimulated with the corresponding peptide (data not shown).
Figure 2 Longitudinal analysis of T cell response by intracellular cytokine staining (ICS), BrdU proliferation, and tetramer staining in patient A49. (A) Comparison of the percentages of hepatitis C virus (HCV) specific tetramer positive cells on total peripheral blood mononuclear cells (PBMC) (right axis, lines) and the percentages of interferon γ (IFN‐γ+) cells after stimulation of PBMC with the corresponding nonamer peptide (left axis, bars) during the course of the disease. (B) Comparison of the percentages of HCV specific tetramer positive cells on total PBMC (right axis, lines) and the percentages of BrdU+ T cells after stimulation of PBMC with the corresponding nonamer peptide (left axis, lines) during the course of the disease. Serum levels of viral RNA expressed as UI/ml were monitored by quantitative reverse transcription‐polymerase chain reaction.
These results suggested that loss of HCV specific T cell responses, as measured by both IFN‐γ ELIspot and IFN‐γ ICS assays in the late stages of acute/chronic infection, is mainly due to the paucity of antigen specific T cells. Figure 2B shows a prospective analysis of the proliferation capacity of HCV Tet+ T cells from patient A49. No CD8+ T cell proliferation was detected on stimulation of the cultures with the nonamer peptides, confirming the results already obtained using the HCV peptide pools. In contrast, control Flu‐Tet+ CD8+ cells present in the same PBMC samples from subject A49 were able to proliferate when stimulated with the corresponding peptide from influenza virus (fig 2B).
Interleukin 2 can rescue antigen dependent proliferation of anergic T cells from chronically evolving subjects
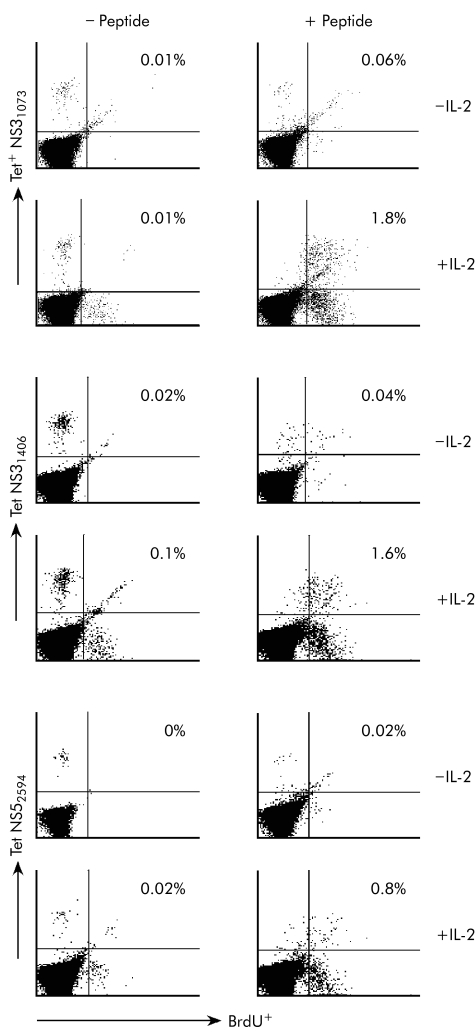
To test whether the impaired proliferation capacity of HCV specific T cells from chronically evolving subjects was a reversible phenotype, the cytokine IL‐2 was added alone or in combination with HCV peptides to PBMC cultures from patient A49 collected at one or six months after diagnosis of infection. This cytokine was able to efficiently rescue antigen dependent proliferation of HCV specific Tet+ CD8+ cells from PBMC samples at both time points (fig 3 and data not shown).
Figure 3 Rescue of proliferation capacity of hepatitis C virus (HCV) specific CD8+ T cells of patient A49 by interleukin 2 (IL‐2). Dot plot analyses of BrdU assays on gated CD8+ T cells stained with HCV tetramers. Peripheral blood mononuclear cells (PBMC) cultures of patient A49 collected six months after the onset of disease were stimulated with the nonamer peptide in the absence and presence of IL‐2. At the end of the culture, cells were stained with the corresponding HCV tetramers. Autofluorescence in the diagonal of the right upper quadrant of the dot blots is due to the presence of death cells during the four day cultures.
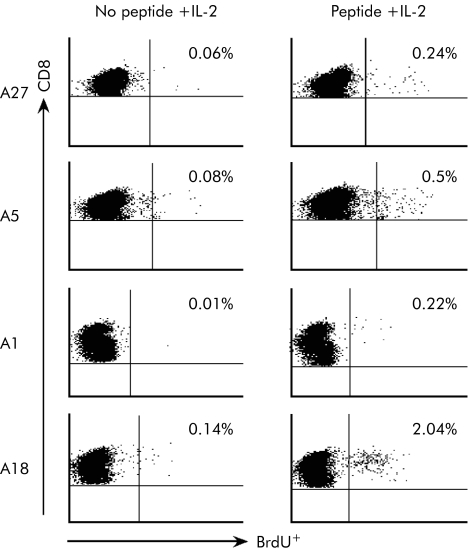
To confirm and extend these observations, we repeated the BrdU proliferation assays in the presence of IL‐2 on PBMC cultures from other chronically evolving subjects: A1, A5, A18, and A27. CD8+ T cells from subject A27 recognised the previously known HLA‐A2 restricted NS3p 1073‐1081 epitope (CINGVCWTV). Subject A18 recognised the previously described HLA‐B7 restricted core 41–49 epitope (GPRLGVRAT; 13). Patient A5 (HLA‐A2.1) recognised a novel epitope present in the 15mer NS3 peptide aa1315 (CDDCHAQDATSILGI). Similarly, CD8+ T cells from subject A1 (HLA‐A1) recognised a previously unknown epitope which maps in the NS3 peptide aa1431 (DVVVVATDALMTGYT).
As shown in fig 4, IL‐2 was able to rescue the proliferation of CD8+ T cells only when added together with the proper HCV antigenic peptide. From these data we conclude that the HCV specific IFN‐γ+ CD8+ T cells induced in chronically evolving patients developed a progressive functional defect that could be overcome by exogenously added IL‐2.
Figure 4 Rescue of proliferation capacity of hepatitis C virus (HCV) specific CD8+ T cells of acute‐chronic patients by interleukin 2 (IL‐2). Dot plot analyses of BrdU proliferation assays on gated CD8+ T cells. Peripheral blood mononuclear cells collected from acute‐chronic patients A27, A5, A1, and A18 at 1, 12, 20, and 3 months after the onset of the disease were cultured with the indicated 15mer peptide in the absence and presence of the cytokine IL‐2.
Discussion
To obtain further insight into the immune mechanisms underlying the spontaneous resolution or establishment of persistent HCV infection, we designed a prospective immunological study of the HCV specific CMI in a large cohort of individuals with a diagnosis of acute hepatitis followed for a median period of one year. Subjects enrolled in the study were tested at multiple time points by means of sensitive ex vivo functional immunological assays using a panel of overlapping 15mer peptides spanning 75% of the HCV polyprotein. This approach allowed us to define the breadth and magnitude of HCV specific T cell responses in resolving versus persistent infection, independent of the specific HLA alleles. In contrast, relying on peptides derived from a single prototype strain of HCV, the results likely underestimated the responses against the actual infecting virus. This notwithstanding, the present approach was used in a number of published reports and proved to be effective for identification of HCV specific T cell responses directed against multiple HCV proteins, irrespective of the HLA type or the infecting genotype, with a high diagnostic sensitivity.12,13,14,17,18
The highest numbers of positive responses by IFN‐γ ELIspot were detected during the first two months after diagnosis of infection. Furthermore, individuals with acute self limiting infection displayed a more frequent and broader HCV specific T cell response with respect to subjects with chronic evolution. The main finding arising from this analysis was that patients with a chronic course of disease failed to sustain these responses, and after one year from diagnosis of acute infection, their CMI dropped to undetectable levels. In contrast, in self resolving subjects, HCV specific T cell responses were still detectable, although reduced in magnitude, even after 6–12 months from diagnosis of acute infection when the virus and therefore the antigenic stimulus was not present anymore. These data strengthen recent results from prospective assessment of antigen specific IFN‐γ secretion by CD8+ T cells in HCV acutely infected individuals, demonstrating a faster decline in the magnitude and breadth of CD8+ T cell response in chronically evolving compared with self recovered patients.13 Taken together, these data would suggest that failure to sustain a sufficient number of HCV specific effector T cells in chronic patients correlates with persistence of the virus.
In the present study, we showed that clearance of the virus was associated with the ability of the HCV specific CD4+ and CD8+ T cells to proliferate in response to antigen stimulation. The FACS based proliferation assay which measures DNA incorporation of BrdU in cycling cells on antigen stimulation allowed evaluation of the proliferation capacity of both CD4+ and CD8+ HCV specific T cells during acute HCV infection. At an early stage of infection, only two of six acute/chronic subjects showed positive responses with this proliferation assay. Moreover, a detailed analysis of HCV specific CD8+ T cells from patient A49 showed that these cells are defective in the proliferation capacity despite being present at high frequency in the blood. At a later stage (six months), resolving individuals significantly differentiated from chronic patients in the capacity of their T cells to proliferate ex vivo on antigen restimulation. While helper T cell proliferation responses have been widely investigated in acute and chronic HCV infection,3,5,9,11,19 the proliferation potential of HCV specific CD8+ T cells have concentrated on long term infected patients or under condition of IL‐2 supplementation.17,20
Poor CD8+ proliferation responses to peptide stimulation in the presence or absence of IL‐2 have been demonstrated in established HCV chronic infection by Wedemeyer and colleagues.20 Our data extend previous findings and show impaired proliferation of CD8+ T cells during acute/chronic infection, suggesting that this phenotype might be a cause rather than a consequence of viral persistence. At variance with previous work, we observed that CD8+ T cell proliferation could be rescued by addition of IL‐2. This discrepancy could be explained by differences in the ability of early and late stage HCV specific CD8+ T lymphocytes to respond to IL‐2, although generation of HCV specific CD8+ T cell lines following a single round of peptide stimulation in the presence of IL‐2 have recently been demonstrated in both resolved and persistently infected individuals.17
Finally, we observed that while antigen stimulation of PBMC from self recovered patients resulted in amplification of CD8+ or CD4+ BrdU+ T cells or both, the latter population was never detected in chronic evolving patients. Although our analysis was not extended to a large number of patients, this feature could be explained either by the reduced ability of chronic patients to mount helper T cell responses or by loss of proliferation potential during the acute phase of the infection.2,3,5,19 Indeed, significant loss of IL‐2 secretory capacity in relation to IFN‐γ production has been reported in CD4+ T cells from HCV chronically infected individuals.21 As it is widely assumed that CD4+ T cells are required for the priming, induction, and maintenance of virus specific T cells, it is reasonable to speculate that the proliferative impairment of CD8+ T cells during chronic HCV infection is not due to an intrinsic functional defect of these cells but rather represents a consequence of the progressive loss of IL‐2 secreting HCV specific CD4+ T cells.22
In conclusion, our data indicate strong and multispecific T cell responses with a sustained ability to proliferate in response to antigen stimulation as being reliable pharmacodynamic measures of a protective CMI during acute infection.
Acknowledgements
This work was supported in part by Ministero dell'Istruzione, dell'Università e della Ricerca‐MIUR (project “ Vaccino per l'epatite C”, Legge n. 488/1992), by “Cofin‐MIUR”, projects 2003–2005, by the Viral Hepatitis Project, Istituto Superiore di Sanità (D Leg.vo 30/12/1992 n. 502), and the NIH Founds (Decreto Ministero della Salute 14/10/2003).
Abbreviations
HCV - hepatitis C virus
CMI - cellular mediated immunity
IL‐2 - interleukin 2
RT‐PCR - reverse transcription‐polymerase chain reaction
PBMC - peripheral blood mononuclear cells
IFN‐γ - interferon γ
ICS - intracellular cytokine staining
SFC - spot forming cells
Appendix
Other members of the Acute Hepatitis C Italian Study Group were as follows: G Taliani (Institute of Infectious Diseases, University of Florence, Florence, Italy); P Amoroso, S Buonocore, G Lettieri, P Pierri (Cotugno Hospital, Infectious Diseases Unit, Naples, Italy); F Meneghetti (Padova Hospital, Infectious and Tropical Diseases Unit, Padova, Italy); T Stroffolini (San Giacomo Hospital, Liver Unit, Rome, Italy); G. Maio (A Rummo Hospital, Infectious Diseases Unit, Benevento, Italy); R Francavilla (Bisceglie Hospital, Infecious Diseases Unit, Bisceglie, Italy); P Chiriacò (A Perrino Hospital, Infectious Diseases Unit, Brindisi, Italy); U Baldi (Umberto I Hospital, Infectious Diseases Unit, Nocera Inferiore, Italy); P Bellissima (Gravina Hospital, Infection Diseases Unit, Caltagirone, Italy); L Cosco, T Ferraro (Pugliese Ciaccio, Hospital, Infectious Diseases Unit, Catanzaro, Italy); N Petrosillo, P Scognamiglio (National Institute of Infectious Diseases, Lazzaro Spallanzani, Rome, Italy); V Mellace; F Montesano (Soverato, Drug Dependency Unit, ASL 7, Soverato, Italy); G Audino (Drug Dependency Unit ASL 7, Catanzaro, Italy); C De Stefano, M Giustra (Department of the Dependencies ASL 11, Reggio Calabria, Italy); A Caterini (Viterbo Hospital, Infectious Diseases Unit, Viterbo, Italy); V Guadagnino (Institute of Infectious Diseases, University of Catanzaro, Catanzaro, Italy); M Cuccia (Epidemiology and Prevention Service, AUSL 3, Catania, Italy); O Zuccaro (Institute of Hygiene, University of Rome Tor Vergata, Rome, Italy); L Laurenti (Department of Cell Biotechnology and Haematology, University “La Sapienza”, Rome, Italy); C Scottà (Department of Cellular and Development Biology, University “La Sapienza”, Rome, Italy); M Capobianchi (Laboratory of Virology, National Institute for Infectious Diseases, L Spallanzani, IRCCS, Rome, Italy).
Footnotes
Conflict of interest: None declared.
References
- 1.Diepolder H M, Zachoval R, Hoffmann R M.et al Possible mechanism involving T‐lymphocyte response to non‐structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 19953461006–1007. [DOI] [PubMed] [Google Scholar]
- 2.Gerlach J T, Diepolder H M, Jung M C.et al Recurrence of hepatitis C virus after loss of virus‐specific CD4+ T‐cell response in acute hepatitis C. Gastroenterology 1999117933–941. [DOI] [PubMed] [Google Scholar]
- 3.Missale G, Bertoni R, Lamonaca V.et al Different clinical behaviors of acute hepatitis C virus infection are associated with different vigour of the anti‐viral cell‐mediated immune response. J Clin Invest 199698706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechner F, Wong D, Dunbar R.et al Analysis of successful immune response in persons infected with HCV. J Exp Med 200091499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thimme R, Oldach D, Chang K M.et al Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 20011941395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grüner N H, Gerlach T J, Jung M C.et al Association of hepatitis C virus‐specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis 20001811528–1536. [DOI] [PubMed] [Google Scholar]
- 7.Shoukry N A, Grakoui A, Houghton M.et al Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med 20031971645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grakoui A, Shoukry N A, Woolard D J.et al HCV persistence and immune evasion in the absence of memory T cell help. Science 2003302659–662. [DOI] [PubMed] [Google Scholar]
- 9.Chang K M, Thimme R, Melpolder J J.et al Differential CD4 and CD8 T cell responsiveness in hepatitis C virus infection. Hepatology 200133267–274. [DOI] [PubMed] [Google Scholar]
- 10.Lechner F, Gruener N H, Urbani S.et al CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol 2000302479–2487. [DOI] [PubMed] [Google Scholar]
- 11.Kamal S M, Ismail A, Graham C.et al Pegylated interferon alfa therapy in acute HCV: relation to HCV‐specific T cell response kinetics. Hepatology 2004391721–1731. [DOI] [PubMed] [Google Scholar]
- 12.Rahman F, Heller T, Sobao Y.et al Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology 20044087–97. [DOI] [PubMed] [Google Scholar]
- 13.Cox A L, Mosbruger T, Lauer G M.et al Comprehensive analyses of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology 200542104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spada E, Mele A, Berton A.et al Multi‐specific T‐cell response and negative HCV RNA tests during acute HCV infection are early prognostic factors of spontaneous clearance. Gut 2004531673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takaki A, Wiese M, Maertens G.et al Cellular immune response persists and humoral responses decrease two decades after recovery from a single‐source outbreak of hepatitis C. Nat Med 20006578–582. [DOI] [PubMed] [Google Scholar]
- 16.Wertheimer A M, Miner C, Lewinsohn D M.et al Novel CD4+ and CD8+ T‐cell determinants within the NS3 protein in subjects with spontaneously resolved HCV infection. Hepatology 200337577–589. [DOI] [PubMed] [Google Scholar]
- 17.Lauer G M, Barnes E, Lucas M.et al High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 2004127924–936. [DOI] [PubMed] [Google Scholar]
- 18.Takamizawa A, Mori C, Fuke I.et al Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol 1991651105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulsenheimer A, Gerlach J T, Gruener N H.et al Detection of functionally altered hepatitis C virus‐specific CD4+ T cells in acute and chronic hepatitis C. Hepatology 2003371189–1198. [DOI] [PubMed] [Google Scholar]
- 20.Wedemeyer H, He X S, Nascimbeni M.et al Impaired effector function of hepatitis C virus‐specific CD8 T cells in chronic hepatitis C virus infection. J Immunol 20021693447–3458. [DOI] [PubMed] [Google Scholar]
- 21.Semmo N, Day C L, Ward S M.et al Preferential loss of IL‐2‐secreting CD4+ T helper cells in chronic HCV infection. Hepatology 2005411019–1028. [DOI] [PubMed] [Google Scholar]
- 22.Lichterfeld M, Kaufmann D E, Yu X G.et al Loss of HIV‐1‐specific CD8+ T cell proliferation after acute HIV‐1 infection and restoration by vaccine‐induced HIV‐1‐specific CD4+ T cells. J Exp Med 200420701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]