Abstract
Background
Physical inactivity and obesity increase the risk of colorectal cancer but little is known about whether they influence prognosis after diagnosis.
Methods
Incident cases of colorectal cancer were identified among participants of the Melbourne Collaborative Cohort Study, a prospective cohort study of 41 528 Australians recruited from 1990 to 1994. Participants diagnosed with their first colorectal cancer between recruitment and 1 August 2002 were eligible. At the time of study entry, body measurements were taken and participants were interviewed about their physical activity. Information on tumour site and stage, treatments given, recurrences, and deaths were obtained from systematic review of the medical records.
Results
A total of 526 cases of colorectal cancer were identified. Median follow up among survivors was 5.5 years, and 208 deaths had occurred, including 181 from colorectal cancer. After adjusting for age, sex, and tumour stage, exercisers had an improved disease specific survival (hazard ratio 0.73 (95% confidence interval (CI) 0.54–1.00)). The benefit of exercise was largely confined to stage II–III tumours (hazard ratio 0.49 (95% CI 0.30–0.79)). Increasing per cent body fat resulted in an increase in disease specific deaths (hazard ratio 1.33 per 10 kg (95% CI 1.04–1.71)). Similarly, increasing waist circumference reduced disease specific survival (hazard ratio 1.20 per 10 cm (95% CI 1.05–1.37)).
Conclusions
Increased central adiposity and a lack of regular physical activity prior to the diagnosis of colorectal cancer is associated with poorer overall and disease specific survival.
Keywords: colorectal cancer, exercise, adiposity, survival
A westernised lifestyle has been well established as a risk factor for colorectal cancer (CRC), and some of the likely mechanisms are closely related to physical inactivity and obesity. Over 50 epidemiological studies have examined the relationship between physical activity and the incidence of CRC, with the vast majority showing physical activity to be protective.1 For colon cancer, regular physical activity reduces the incidence by 40–50%, while obesity increases the risk, although there is little evidence that either are related to rectal cancer.1,2,3 Central adiposity appears to be particularly important, and the observed associations are generally stronger in men than in women.3 A number of possible mechanisms have been postulated as to how physical inactivity and obesity might increase the risk of colorectal cancer, including alterations in prostaglandin levels/ratios, changes in insulin‐like growth factors, inducing hyperinsulinaemia, effects on steroid sex hormones and on the immune system, and for physical inactivity, increased bowel transit times,1,4,5,6 but nothing has yet been established.
Despite the large amount of epidemiological data linking the incidence of CRC with physical inactivity and adiposity, there is little concerning any possible effect these factors may have on outcomes of those diagnosed with CRC. In one study of stage II and III colon cancer patients receiving adjuvant treatment, a high body mass index had no effect on overall survival (however, in subgroup analysis, an effect was seen in women but not in men),7 and in patients with rectal cancer, body mass index again did not influence survival.8
Using incident CRC cases from a prospective cohort study, we investigated whether physical activity and obesity influenced the survival of those diagnosed with CRC.
Methods
Study population
The Melbourne Collaborative Cohort Study (MCCS) is a prospective cohort study of 41 528 people (17 049 men) aged between 27 and 75 years at baseline (99.3% were aged 40–69 years).9 Recruitment to the MCCS occurred between 1990 and 1994. Subjects were recruited via the Electoral Rolls (registration to vote is compulsory for Australian adults), advertisements, and community announcements. The study aimed to explore associations between certain epidemiological factors (such as diet, body size, and behaviour) and cancer. The Cancer Council Victoria's Human Research Ethics Committee approved the study protocol. Subjects gave written consent to participate and for the investigators to obtain access to their medical records. For this analysis, subjects were excluded if they had a diagnosis of CRC before baseline.
We identified cases of adenocarcinoma of the colon or rectum that were diagnosed in MCCS participants during follow up to 1 August 2002 by matching participants to the Victorian Cancer Registry. Information on tumour characteristics (site, size, stage, and degree of differentiation), adjuvant treatments, recurrences, and deaths was obtained from medical records. Deaths were also identified by the Victorian Cancer Registry and by linkage to the National Death Index. Victorian deaths were complete to 1 July 2004 and in other states to the end of 2002. Only one participant diagnosed with CRC is known to have left Victoria after diagnosis.
Assessment of physical activity
All 41 528 participants in the cohort study answered three questions relating to their level of non‐occupational physical activity at study entry (baseline). The first question was “On average (for example, over the last six months) how many times per week did you exercise vigorously for a period of at least 20 minutes?” The second question was “On average (for example, over the last six months) how many times per week did you engage in less vigorous exercise for recreation, sport, or health and fitness purposes, which did not make you sweat or feel out of breath?” Answers were recorded as “none at all”, “once or twice a week”, or “three or more times a week”. The answers to these two questions were combined to separate subjects into “exercisers” or “non‐exercisers”. Those who reported any regular exercise (either “once or twice a week” or “three or more times a week”) were classified as “exercisers”. Those who answered “none at all” to both questions regarding exercise were classified as “non‐exercisers”.
A third question regarding walking was also asked. “On average, over the last six months, how many times a week did you walk for recreation and or exercise?” Again, answers were recorded as “none at all”, “once or twice a week”, or “three or more times a week”. Participants were then classified as “walkers” or “non‐walkers”.
Body composition
Height, weight, and waist circumference were measured at baseline according to written protocols that were based on standard procedures.10 Weight was measured to 100 g using digital electronic scales, height to 1 mm using a stadiometer, and waist and hip circumferences were measured to 1 mm using a two metre metal anthropometric tape. Body mass index (BMI) was calculated as weight in kg divided by the square of height in metres. Waist to hips ratio was also computed. Bioelectrical impedance analysis was performed with a single frequency (50 kHz) electrical current produced by a BIA‐101A RJL system analyser (RJL systems, Detroit, Michigan, USA). Resistance and reactance were measured with subjects in the supine position. Adipose mass, non‐adipose mass, and per cent body fat were calculated from these measurements using formulae that had been developed in Caucasian populations of similar age and BMI distribution to the MCCS participants.11
CRC characteristics
Stage was categorised into four groups based on the American Joint Committee on Cancer (AJCC) staging system: stage I (T1–2, N0, M0), stage II (T3–4, N0, M0), stage III (Tany, N1–2, M0), and stage IV (Tany, Nany, M1). The site of the primary tumour was classified as “right colon” if it was located proximal to the splenic flexure. “Left colon” tumours were located between the splenic flexure and the peritoneal reflection, and rectal cancers below the peritoneal reflection. In the case of synchronous cancers, the characteristics of the tumour with the higher stage (or the larger tumour if they were the same stage) were used. Deaths due to CRC, or as a direct result of treatment for it, were termed “disease specific deaths”.
Statistical analysis
Cox's proportional hazard regression models were used to estimate the hazard ratios for overall survival and disease specific survival associated with each measure at baseline. Follow up began at diagnosis and ended at death or 1 July 2004 (the date that ascertainment of deaths by the Victorian Cancer Registry was complete), whichever came first. All anthropometric measures were fitted as continuous covariates to estimate linear trends on the log hazard scale. BMI was also categorised into normal weight (<25 kg/m2), overweight (25–29 kg/m2), and obese (⩾30 kg/m2) based on WHO recommendations.12 In addition, we also grouped cases into two waist circumference categories: “healthy” (males <94 cm, females <80 cm) and “action level” (males ⩾94 cm, females ⩾80 cm), according to commonly used levels of abdominal fat accumulation.3,13,14 All models were adjusted for age at baseline (as a continuous variable), sex, and stage of disease (as a categorical variable). A separate category was used for cases with missing stage. Further adjustment for tumour grade made virtually no difference to any of the results and therefore these models are not presented. Interactions were also fitted to test for differences in associations by anatomic subsite. Tests based on Schoenfeld residuals and graphical methods using Kaplan‐Meier curves15 showed no evidence that proportional hazard assumptions were violated for any analyses. Kaplan‐Meier methods were used to create survival curves. All statistical analyses and graphs were performed using STATA/SE 8.2 (Stata Corporation, College Station, Texas, USA).
Results
Patient characteristics
During follow up of the cohort, 536 people were diagnosed with CRC, including 11 with two synchronous tumours. Based on review of their records, seven were excluded because they were found not to have had CRC, and three were excluded because they had a past history of CRC prior to baseline, leaving a total of 526. At 1 July 2004, median follow up (among survivors) since diagnosis was 5.5 years, and 208 (39.5%) deaths had occurred of which 181 were CRC specific deaths. Median time to diagnosis from baseline was 5.3 years (interquartile range (IQR) 2.9–7.2 years). Medical records were reviewed for 520 (99%) cases; there were six cases where we could not access records due to clinician refusal. AJCC stage of disease was unknown for 14 cases and anatomical location of the primary tumour was unavailable for 15 cases.
Descriptive statistics for selected baseline characteristics of the study sample overall and stratified by exercise status and waist circumference groups are shown in table 1. A total of 297 cases reported doing no exercise at baseline, 77 cases participated in regular vigorous exercise, and 194 exercised less vigorously. Some (42 cases) did both vigorous and less vigorous exercise, resulting in 229 that reported participating in some form of regular exercise (that is, “exercisers”). There were 316 “walkers” and 210 “non‐walkers”. Men had a higher median BMI (28 kg/m2 (IQR 26–30 )) than women (26 kg/m2 (IQR 23–30)), and a larger median waist circumference (96 cm (IQR 91–103)) compared with women (81 cm (IQR 74–90)). The per cent of body fat from total weight ranged from 6% to 58%, with men having, on average, lower values than women. Median value for men was 30% (IQR 26–33%) and for women 40% (IQR 36–46%).
Table 1 Demographic and tumour characteristics for all cases, exercisers and non‐exercisers, and “healthy” and “action level” waist circumference groups.
| Characteristic | All cases (n = 526) | Exercisers (n = 229) | Non‐exercisers (n = 297) | “Healthy” waist circumference (n = 214) | “Action level” waist circumference (n = 312) |
|---|---|---|---|---|---|
| Age at diagnosis (y) | |||||
| Median | 66.8 | 68.6 | 67.6 | 66.5 | 68.1 |
| Range | 42.0–79.4 | 42.9–79.6 | 42.0–79.6 | 42.0–79.6 | 44.0–79.2 |
| Sex (No (%)) | |||||
| Male | 270 (51) | 119 (52) | 151 (51) | 101 (47) | 169 (54) |
| Female | 256 (49) | 110 (48) | 146 (49) | 113 (53) | 143 (46) |
| AJCC stage (No (%)) | |||||
| I | 123 (23) | 55 (24) | 68 (23) | 52 (24) | 71 (23) |
| II | 149 (28) | 61 (27) | 88 (30) | 55 (26) | 94 (30) |
| III | 148 (28) | 70 (31) | 78 (26) | 63 (29) | 85 (27) |
| IV | 92 (17) | 38 (17) | 54 (18) | 38 (18) | 54 (17) |
| Unknown | 14 (3) | 5 (2) | 9 (3) | 6 (3) | 8 (3) |
| Site of primary tumour (No (%)) | |||||
| Right colon | 169 (32) | 67 (29) | 102 (34) | 65 (30) | 104 (33) |
| Splenic flexure | 2 (0) | 2 (1) | 0 (0) | 0 (0) | 2 (1) |
| Left colon | 165 (31) | 73 (32) | 92 (31) | 65 (30) | 100 (32) |
| Rectum | 175 (33) | 80 (35) | 95 (32) | 77 (36) | 98 (31) |
| Unknown | 15 (3) | 7 (3) | 8 (3) | 7 (3) | 8 (3) |
| Degree of differentiation (No (%)) | |||||
| Well | 41 (8) | 16 (7) | 25 (8) | 13 (6) | 28 (9) |
| Moderately | 300 (57) | 136 (59) | 164 (55) | 126 (59) | 174 (56) |
| Poorly | 117 (22) | 49 (21) | 68 (23) | 47 (22) | 70 (22) |
| Unknown | 68 (13) | 28 (12) | 40 (13) | 28 (13) | 40 (13) |
| Adjuvant chemotherapy (No (%)) | |||||
| Yes | 157 (30) | 70 (31) | 87 (29) | 67 (31) | 90 (29) |
| No | 353 (67) | 152 (66) | 201 (68) | 138 (64) | 215 (69) |
| Unknown | 16 (3) | 7 (3) | 9 (3) | 9 (4) | 7 (2) |
| Adjuvant radiotherapy (No (%)) | |||||
| Yes | 52 (10) | 19 (8) | 33 (11) | 23 (11) | 29 (9) |
| No | 458 (87) | 203 (89) | 255 (86) | 182 (85) | 276 (88) |
| Unknown | 16 (3) | 7 (3) | 9 (3) | 9 (4) | 7 (2) |
| Body mass index (kg/m2) (No (%)) | |||||
| <25 | 163 (31) | 85 (37) | 78 (26) | 139 (65) | 24 (8) |
| 25–30 | 236 (45) | 103 (45) | 133 (45) | 71 (33) | 165 (53) |
| >30 | 127 (24) | 41 (18) | 86 (29) | 4 (2) | 123 (39) |
Risk factors for survival
Table 2 shows adjusted hazards ratios for overall and disease specific survival by age, sex, stage, grade, and adjuvant treatments. Stage and adjuvant chemotherapy were strong prognostic factors. Disease specific unadjusted hazard ratios for grade were 1.37 (95% confidence interval (CI) 0.69–2.73), 2.72 (95% CI 1.34–5.51), and 2.91 (95% CI 1.39–6.09) for cases with moderately, poorly, and unknown differentiated tumours, respectively, compared with cases with well differentiated tumours. Sex, age, and adjuvant radiotherapy had no significant associations with survival (all p>0.5).
Table 2 Hazard ratios for overall survival and disease specific survival*.
| Overall survival (n = 208) | Disease specific survival (n = 181) | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Males | 1 | 1 | ||
| Females | 0.93 (0.70–1.22) | 0.59 | 1.03 (0.76–1.38) | 0.86 |
| Age (per 10 y) | 1.03 (0.86–1.23) | 0.75 | 0.99 (0.82–1.20) | 0.91 |
| AJCC | ||||
| Stage I | 1 | 1 | ||
| Stage II | 2.7 (1.4–5.3) | 2.6 (1.0–6.5) | ||
| Stage III | 6.7 (3.5–12.8) | 11.1 (4.8–25.6) | ||
| Stage IV | 51.4 (26.8–98.5) | 83.7 (36.0–195) | ||
| Unknown | 15.0 (6.3–35.4) | <0.001 | 20.4 (7.1–59.2) | <0.001 |
| Degree of differentiation | ||||
| Well | 1 | 1 | ||
| Moderate | 0.87 (0.46–1.64) | 0.86 (0.43–1.73) | ||
| Poor | 1.38 (0.72–2.66) | 1.38 (0.67–2.82) | ||
| Unknown | 1.39 (0.68–2.83) | 0.02 | 1.41 (0.65–3.05) | 0.03 |
| Any adjuvant chemotherapy† | ||||
| No | 1 | 1 | ||
| Yes | 0.49 (0.30–0.78) | 0.003 | 0.42 (0.25–0.71) | 0.001 |
| Any adjuvant radiotherapy† | ||||
| No | 1 | 1 | ||
| Yes | 0.86 (0.50–1.48) | 0.58 | 0.95 (0.53–1.73) | 0.88 |
*Analyses were adjusted for sex, age, and American Joint Committee on Cancer (AJCC) stage.
†Stage II and III cases only.
HR (95% CI), Hazard ratio (95% confidence interval).
Survival by exercise and body size
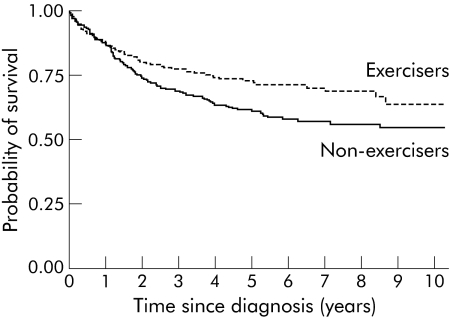
Participants who reported regular exercise before baseline had better overall and disease specific survival than those who did not exercise, after adjustment for age, sex, and stage (table 3). The unadjusted hazard ratios for exercisers compared with non‐exercisers were similar to the adjusted hazard ratios (that is, 0.74 (95% CI 0.56–0.98)) for overall survival and 0.69 (95% CI 0.51–0.94) for disease specific survival. Overall (unadjusted) survival five years after diagnosis was 71% for exercisers and 57% for non‐exercisers. Figure 1 shows that exercise had a similar association with disease specific survival; five year survival proportions were 73% and 61% for exercisers and non‐exercisers, respectively. Walking was not significantly associated with survival.
Table 3 Hazard ratios for overall survival and disease specific survival by exercise, walking, and body size measures*.
| Overall survival (n = 208) | Disease specific survival (n = 181) | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Exercise | 0.77 (0.58–1.03) | 0.08 | 0.73 (0.54–1.00) | 0.05 |
| Walking | 1.09 (0.82–1.46) | 0.55 | 1.03 (0.75–1.41) | 0.86 |
| Per cent fat (per 10%) | 1.25 (0.99–1.58) | 0.06 | 1.33 (1.04–1.71) | 0.02 |
| Weight (per 10 kg) | 1.11 (1.00–1.25) | 0.05 | 1.15 (1.02–1.29) | 0.02 |
| BMI (per 5 kg/m2) | 1.12 (0.96–1.31) | 0.15 | 1.15 (0.98–1.35) | 0.10 |
| Waist circumference (per 10 cm) | 1.16 (1.02–1.31) | 0.02 | 1.20 (1.05–1.37) | 0.008 |
*Analyses were adjusted for sex, age, and American Joint Committee on Cancer (AJCC) stage.
BMI, body mass index; HR (95% CI), Hazard ratio (95% confidence interval).
Figure 1 Kaplan‐Meier curves for disease specific survival by exercise group (log rank p = 0.02).
Conversely, disease specific survival was worse for participants with greater per cent body fat, waist circumference, and/or weight (table 3). BMI was not significantly associated with outcome. The disease specific hazard ratio for participants with “action level” waist circumference compared with those with “healthy” waist circumference was 1.35 (95% CI 1.00–1.84). To illustrate this further, five year survival proportions for a 66 year old (average aged) male with stage II disease were 98% and 87% for “healthy” and “action level” waist circumferences, respectively; for a 66 year old male with stage III disease, the corresponding proportions were 60% and 49%. All hazards ratios for body size measures remained virtually unchanged after further adjustment for exercise, and vice versa (data not shown).
Analyses by AJCC stage
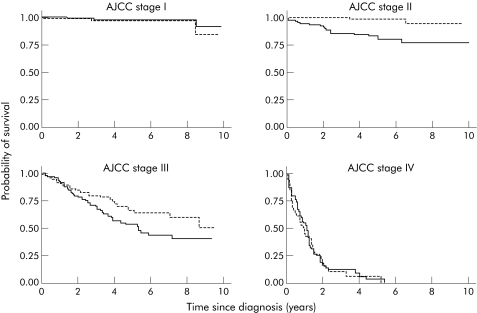
Exercise had virtually no association with disease specific survival in stage I or IV cases but quite strong associations in patients with cancers that were stage II or III at diagnosis (interaction p = 0.01; fig 2). In these patients, the hazard ratio for disease specific survival, comparing exercisers with non‐exercisers, was 0.49 (95% CI 0.30–0.79). The corresponding hazard ratio for overall survival was 0.61 (95% CI 0.41–0.92). There was no persuasive evidence that associations between survival and walking or any of the body size measures differed by stage (data not shown).
Figure 2 Kaplan‐Meyer curves of disease specific survival with respect to exercise by American Joint Committee on Cancer (AJCC) stage.
Analyses by primary site
Detrimental associations with a large waist circumference and high per cent body fat were mostly confined to patients with tumours in the left colon or rectum (interaction p = 0.06 for both variables; table 4). Conversely, the better outcome in exercisers was mainly restricted to patients whose cancers originated in the right colon (table 4) although the test for interaction was large (p = 0.20).
Table 4 Hazard ratios for disease specific survival by exercise, walking, and body size measures, by anatomical subsite*.
| Right colon (n = 171) | Left colon (n = 165) | Rectum (n = 175) | p Value† | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Exerciser | 0.50 (0.27–0.90) | 0.82 (0.46–1.44) | 1.00 (0.59–1.69) | 0.20 |
| Walker | 1.27 (0.75–2.15) | 1.01 (0.54–1.89) | 0.88 (0.52–1.52) | 0.64 |
| Per cent fat (per 10%) | 1.02 (0.71–1.47) | 1.59 (1.08–2.35) | 1.65 (1.17–2.31) | 0.06 |
| Weight (per 10 kg) | 1.06 (0.87–1.29) | 1.34 (1.09–1.65) | 1.15 (0.95–1.39) | 0.24 |
| BMI (per 5 kg/m2) | 0.98 (0.73–1.32) | 1.46 (1.09–1.96) | 1.20 (0.89–1.63) | 0.17 |
| Waist circumference (per 10 cm) | 1.03 (0.84–1.25) | 1.44 (1.15–1.79) | 1.21 (0.94–1.55) | 0.06 |
*Analyses were adjusted for sex, age, and American Joint Committee on Cancer (AJCC) stage.
†Test of interaction between sub sites.
BMI, body mass index; HR (95% CI), Hazard ratio (95% confidence interval).
Other analyses
Results were similar when absolute adipose mass and waist to hip ratio were used instead of per cent fat and waist circumference, respectively (not shown). Height and fat free mass showed little relationship with survival (data not shown). Exclusion of cases diagnosed in the first two years of follow up (90 cases) made no material difference to any of the results—the hazard ratio for disease specific survival for exercisers was 0.71 (95% CI 0.51–1.00) while for waist circumference the hazard ratio per 10 cm increase was 1.20 (95% CI 1.04–1.39).
Discussion
We found that obesity and inactivity were associated with poorer overall and disease specific survival in patients diagnosed with CRC. The associations were more compelling for obesity, specifically waist circumference, per cent body fat, and weight than for exercise. In particular, those participants with an “action level” waist circumference had a 35% reduction in disease specific survival compared with those with a “healthy” waist circumference. No effect on survival was seen for BMI or regular walking. Our study found that exercisers, compared with non‐exercisers, had an absolute improvement in five year overall survival of 14% (12% for disease specific survival), a result that is clinically relevant. The beneficial effects of exercise were confined to cases with stage II or III tumours at diagnosis, with exercise resulting in a 39% reduction in all cause mortality and a 51% reduction in disease specific mortality within this subgroup. This improvement in survival is at least as large as is achieved with adjuvant chemotherapy.16,17
One of the strengths of our study relates to the accuracy of our body size measurements. Each case had precise measurements taken by trained staff rather than self reporting of height and weight. In addition, we had near complete data on the tumour characteristics and treatments received for our cases and identification of all deaths that occurred within the follow up period. It could be argued that more aggressive tumours might impact on a person's ability to regularly exercise by causing lethargy or pain and in so doing favorably bias the survival of exercisers. Similarly, weight can certainly be affected by an underlying malignancy. In order to exclude the possibility that undiagnosed (but possibly symptomatic) cancers could have influenced exercise patterns or body size and potentially bias the findings, we performed our analyses excluding cases diagnosed within two years of enrolment into the cohort study. The associations between exercise, adiposity, and survival were unaltered after we excluded these cases.
The main weakness in our study relates to the accuracy of the exercise measurement. This was self reported and we have no data on the duration of an individual's activity habits. Nor can we make any comment on the length of time an individual exercised per week. However, any biases introduced by this would most likely attenuate the associations. Because only a small proportion of cases reported “regular vigorous” exercise, we are unable to say whether the vigorousness of exercise is important. Similarly, our study is not powered to demonstrate an association between the frequency of exercise and survival.
Another potential criticism of our study could be that our measurement of physical activity and body size predated the diagnosis of CRC. We did not assess whether exercise habits were maintained during the period of follow up nor was body size and shape reassessed after cancer diagnosis. Thus it is not clear whether weight loss or exercise following detection of CRC would influence the outcome. It is well established that CRC development occurs over many years and involves multiple genetic mutations,18 and our findings suggest that exercise and adiposity may influence this process in its early stages. Our findings are also consistent with prospective cohort studies that have shown exercise (in the premorbid period) reduced the risk of developing colon cancer,19,20,21,22,23,24,25 as well as cohort studies that demonstrated an increased incidence of colon cancer with rising waist circumference, particularly in men.20,26,27,28
At first inspection, our findings of improved survival with regular exercise and reduced adiposity may seem obvious due to the well known health advantages of exercise and weight loss in reducing cardiovascular mortality. However, there were only 27 deaths (13% of all deaths) not attributable to CRC (14 occurring in non‐exercisers and 13 in exercisers) while 181 deaths were due to CRC. If our findings of improved survival were due to reductions in non‐CRC deaths (such as deaths due to heart disease, stroke, or diabetes) one would expect to see less of an effect on disease specific survival compared with overall survival. In contrast, we found the associations between survival and both regular exercise and low adiposity to be, if anything, larger for disease specific survival than overall survival (table 3). This suggests that the improved survival seen was a result of a direct effect on tumour biology.
With respect to premorbid body size measurements, the strongest effects were seen with per cent body fat and waist circumference. For every 10% increase in body fat we found a 33% decrease in disease specific survival, while a 10 cm increase in waist circumference resulted in a 20% reduction in disease specific survival. Slightly weaker reductions were observed for overall survival. The probability of survival did not appear to vary by measures of BMI, as has been found by others,7,8 or fat free mass. As BMI can be elevated by an increase in muscle bulk rather than body fat, this suggests that a more accurate measure of adiposity, and in particular central adiposity, is more important for determining survival from CRC than BMI. The hypothesis that waist circumference may be a more important indicator than BMI is consistent with our previous work that investigated the incidence of colon cancer in men.27
Whether or not the detrimental effects of being overweight or the beneficial effects of regular exercise differ by the site of the primary tumour is not clear from our study although some trends were observed (table 4). It is well known that the molecular characteristics of CRCs vary, and tumours arising in the right colon differ in this respect to either left colon or rectal cancers, a classic example being the prevalence of microsatellite instability.29 Even though the effects of exercise and obesity were independent, it is likely however that the mechanisms involved are similar. If this were so, obesity and a sedentary lifestyle should both negatively impact on survival for tumours originating in the same locations, a finding not supported by our study. Although there are many studies demonstrating a reduced risk of developing colon cancer in more physically active people, this has not been shown for rectal cancer. We found no effect of exercise on survival for rectal cancer.
In conclusion, we have observed improved survival for patients with CRC who participated in regular exercise prior to diagnosis. This effect was stronger for right sided colon cancers, and stage II–III disease. We have also observed a detrimental effect on disease specific survival associated with increased adiposity prior to diagnosis, this effect being greatest for cancers distal to the splenic flexure and independent of exercise. Although the precise mechanisms are unknown, should the veracity of these observations be confirmed, maintenance of ideal body weight and promotion of physical activity could form the basis of interventions to reduce not only the incidence of CRC but also mortality. Such interventions are also likely to have beneficial effects on cardiovascular disease and metabolic disorders such as type II diabetes.
Acknowledgements
This study was made possible by the contribution of many people, including the original investigators and the diligent team who recruited the participants and who continue working on follow up. Finally, we would like to express our gratitude to the many thousands of Melbourne residents who continue to participate in the study.
Abbreviations
AJCC - American Joint Committee on Cancer
BMI - body mass index
CRC - colorectal cancer
MCCS - Melbourne Collaborative Cohort Study
Footnotes
Cohort recruitment was funded by VicHealth and The Cancer Council Victoria. This study was funded by grants from the National Health and Medical Research Council (126402, 209057, 170215, 251533) and VicHealth (1999‐0227; 1998‐0406) and was further supported by infrastructure provided by The Cancer Council Victoria.
Conflict of interest: None declared.
References
- 1.Friedenreich C M, Orenstein M R. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr 2002132(11 suppl)3456–64S. [DOI] [PubMed] [Google Scholar]
- 2.Colditz G A, Cannuscio C C, Frazier A L. Physical activity and reduced risk of colon cancer: implications for prevention. Cancer Causes Control 19978649–667. [DOI] [PubMed] [Google Scholar]
- 3.Vainio H, Bianchini F. eds. IARC Handbooks of Cancer Prevention, Vol. 6,Weight control and physical activity Lyon, IARC Press, 2002. *
- 4.Westerlind K C. Physical activity and cancer prevention—mechanisms. Med Sci Sports Exerc 2003351834–1840. [DOI] [PubMed] [Google Scholar]
- 5.Quadrilatero J, Hoffman–Goetz L. Physical activity and colon cancer. A systematic review of potential mechanisms. J Sports Med Phys Fitness 200343121–138. [PubMed] [Google Scholar]
- 6.Greenwald P. Cancer prevention clinical trials. J Clin Oncol 200220(18 suppl)14–22S. [PubMed] [Google Scholar]
- 7.Meyerhardt J A, Catalano P J, Haller D G.et al Influence of body mass index on outcomes and treatment‐related toxicity in patients with colon carcinoma. Cancer 200398484–495. [DOI] [PubMed] [Google Scholar]
- 8.Meyerhardt J A, Tepper J E, Niedzwiecki D.et al Impact of body mass index on outcomes and treatment‐related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol 200422648–657. [DOI] [PubMed] [Google Scholar]
- 9.Giles G G, English D R. The Melbourne Collaborative Cohort Study. IARC Sci Publ 200215669–70. [PubMed] [Google Scholar]
- 10.Lohman T, Roche A, Martorell R.Anthropometric Standardization Reference Manual. Champaign: Kinetics Books, 1988, * (IL)
- 11.Roubenoff R, Baumgartner R N, Harris T B.et al Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci 199752M129–M136. [DOI] [PubMed] [Google Scholar]
- 12.WHO Expert Committee Physical Status. The Use and Interpretation of Anthropometry. Geneva: WHO, 1995, * [PubMed]
- 13.Lean M E, Han T S, Morrison C E. Waist circumference as a measure for indicating need for weight management. BMJ 1995311158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molarius A, Seidell J C, Sans S.et al Varying sensitivity of waist action levels to identify subjects with overweight or obesity in 19 populations of the WHO MONICA Project. J Clin Epidemiol 1999521213–1224. [DOI] [PubMed] [Google Scholar]
- 15.Collett D.Modelling survival data in medical research. Boca Raton: Chapman & Hall/CRC, 1999, * (FL)
- 16.Moertel C G, Fleming T R, Macdonald J S.et al Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990322352–358. [DOI] [PubMed] [Google Scholar]
- 17.Wolmark N, Fisher B, Rockette H.et al Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C‐01. J Natl Cancer Inst 19888030–36. [DOI] [PubMed] [Google Scholar]
- 18.Vogelstein B, Fearon E R, Hamilton S R.et al Genetic alterations during colorectal‐tumor development. N Engl J Med 1988319525–532. [DOI] [PubMed] [Google Scholar]
- 19.Ballard‐Barbash R, Schatzkin A, Albanes D.et al Physical activity and risk of large bowel cancer in the Framingham Study. Cancer Res 1990503610–3613. [PubMed] [Google Scholar]
- 20.Giovannucci E, Ascherio A, Rimm E B.et al Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995122327–334. [DOI] [PubMed] [Google Scholar]
- 21.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer 1996731134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez M E, Giovannucci E, Spiegelman D.et al Leisure‐time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst 199789948–955. [DOI] [PubMed] [Google Scholar]
- 23.Nilsen T I, Vatten L J. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer 200184417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Severson R K, Nomura A M, Grove J S.et al A prospective analysis of physical activity and cancer. Am J Epidemiol 1989130522–529. [DOI] [PubMed] [Google Scholar]
- 25.Thun M J, Calle E E, Namboodiri M M.et al Risk factors for fatal colon cancer in a large prospective study. J Natl Cancer Inst 1992841491–1500. [DOI] [PubMed] [Google Scholar]
- 26.Moore L L, Bradlee M L, Singer M R.et al BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord 200428559–567. [DOI] [PubMed] [Google Scholar]
- 27.MacInnis R J, English D R, Hopper J L.et al Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev 200413553–559. [PubMed] [Google Scholar]
- 28.Schoen R E, Tangen C M, Kuller L H.et al Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999911147–1154. [DOI] [PubMed] [Google Scholar]
- 29.Kim H, Jen J, Vogelstein B.et al Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994145148–156. [PMC free article] [PubMed] [Google Scholar]