Abstract
Background and aims
Activation of the vanilloid receptor subtype 1 (VR‐1) results in release of proinflammatory peptides which initiate an inflammatory cascade known as neurogenic inflammation. We investigated its role in an acute model of surgically induced oesophagitis.
Methods
Oesophagitis was induced by pyloric ligation in wild‐type and VR‐1 deficient mice. A subset of animals were administered the VR‐1 antagonist capsazepine, famotidine, or omeprazole one hour before surgery. Five hours after surgery, myeloperoxidase activity (MPO), histological damage scores, intragastric pH, and immunocytochemical analysis of substance P (SP) receptor endocytosis were determined.
Results
Oesophagitis induced knockout mice exhibited significantly lower levels of MPO activity, histological damage scores, and SP receptor endocytosis than wild‐type mice. Inflammatory parameters were significantly reduced by acid inhibition and capsazepine in wild‐type mice.
Conclusions
We conclude that acute acid induced oesophagitis is reduced in animals lacking VR‐1. This suggests that acid induced oesophagitis may act through VR‐1 and that inhibition of the receptor may reduce inflammation.
Keywords: receptor, substance P, neurogenic inflammation, oesophagitis, vanilloid receptor subtype‐1, capsaicin
Gastro‐oesophageal reflux disease (GORD) is a common disorder affecting an estimated 5–7% of the global population,1 and refers to the backflow of gastric or biliary contents into the oesophagus resulting in inflammation and tissue damage of the oesophagus. Continuous exposure to these compounds may result in precancerous conditions such as Barrett's oesophagus or invasive subsequent cancer. Although symptomatic relief is achieved in a number of patients who are treated with therapies aimed at correcting the altered anatomical or physiological conditions, none of these correct the underlying inflammation.
Previous studies have investigated the mechanisms of neurogenic inflammation in the pancreas2 and intestine3,4,5; however, there has been little research focused on the association between the enteric nervous system and oesophageal diseases. Neurogenic inflammation refers to stimulation of primary sensory neurones which in turn conveys nociceptive information to the spinal cord and exacerbates the inflammatory and immune responses in peripheral tissues via an axon reflex.6,7 This neurogenic response is characterised by plasma extravasation, vasodilatation, neutrophil infiltration, and activation of immune cells.8 The neurogenic arc is initially triggered by an unknown mechanism, which results in release of specific neuropeptides from sensory neurones that act on their respective receptors. Substance P (SP), an 11 amino acid peptide member of the tachykinin family,9 has been extensively studied in its role in neurogenic inflammation. SP is distributed throughout the gastrointestinal tract and in the peripheral and central nervous system where it mediates sensory and motor physiological functions. Although a specific axon reflex has not been conclusively demonstrated in the intestine, proinflammatory neuropeptides such as SP have been implicated in a variety of pathological conditions associated with chronic inflammation and pain.10,11 Specific to the oesophagus, radioimmunoassay studies have found that the gastro‐oesophageal junction region contains a relatively high number of SP containing neurones12 and SP receptors are found in the circular muscle and enteric plexus of the oesophagus. In vitro studies of muscle strips obtained from patients with Barrett's disease have shown that SP receptors are also present in the smooth muscle of these patients.13 These findings are consistent with the proposal that SP acts as a neurotransmitter in mediating certain responses to chemonociceptive stimuli in the region14 and influencing the tone of the lower oesophageal sphincter pressure.15,16
Recent observations in animal models of dermatitis,17 pancreatitis,2 and acute and chronic colitis3,4,5 have suggested that SP is released on stimulation of the vanilloid receptor subtype 1 (VR‐1), also known as TRPV1 (transient receptor potential vanilloid subtype 1), which is present only in primary sensory neurones. VR‐1 is a non‐selective cation channel that is activated by heat, protons, and the exogenous ligand capsaicin. Recently, endogenous ligands such as anandamide and leukotriene B4 have also been shown to stimulate VR‐1 in vitro18,19,20 and in vivo.21 Although the role of these compounds in the oesophagus is not completely understood thus far, these findings are of interest as early experiments have demonstrated the importance of lipoxygenase metabolites in animal oesophagitis models.22 With this background, the present study was designed to test the hypothesis that VR‐1 is responsible, at least in part, for the initial inflammatory process associated with acute oesophagitis. Using both wild‐type and mice deficient in VR‐1, we surgically constructed a model of acute oesophagitis by pyloric ligation, as previously described,22 and examined the effect of inhibition of VR‐1, genetic deletion of VR‐1, or blockade of acid secretion using standard pharmacological agents. To further investigate the association between VR‐1 stimulation and SP release, we performed immunocytochemical analysis of SP receptor (neurokinin 1 (NK‐1)) endocytosis in oesophageal myenteric plexus neurones.
Materials and methods
Animals
These studies were conducted at the Durham Veterans Affairs Medical Center, Durham, North Carolina, USA. All aspects of the research were reviewed and approved by the institution's animal care and use committee. Male 8–12 week C57BL/6 wild‐type (VR‐1 +/+) and VR‐1 deficient (VR‐1 −/−) mice were purchased from an authorised vendor (Charles Rivers Laboratories, Raleigh, North Carolina, USA) or obtained by Dr Sidney Simon and housed under standard laboratory conditions until used. Mice were genotyped using standard polymerase chain reaction (PCR) technique. Genotypic screening of offspring was done as previously described by PCR using tail clip DNA. Briefly, tails were cut into 0.4–0.6 cm pieces and DNA isolated using a QIAamp kit from Qiagen (Valencia, California, USA). Samples were incubated in 180 μl of buffer ATL and 20 μl proteinase K, vortexed, and incubated at 55°C until the tissue was completely lysed. After lysis was completed, a 400 μl buffer AL‐ethanol mixture was added to the sample and mixed vigorously by vortexing. The mixture was then pipetted into a DNeasy mini column setting in a 2 ml collection tube and centrifuged at 6000 g (8000 rpm) for one minute; 500 μl of buffer AW1 and 500 μl of buffer AW2 were mixed with the sample and centrifuged for three minutes at full speed to dry the DNesay membrane. The DNeasy mini column was placed in a clean 2 ml microcentrifuge tube and 200 μl of buffer AE were pipetted directly onto the DNeasy membrane. The final mixture was incubated at room temperature for one minute, and then centrifuged for one minute at 6000 g.
PCR amplification was carried out with primers (0.5 μl), 10 nM dNTPs (0.5 μl), MgCl2 (1.5 μl), and Taq polymerase (0.1 U) for 34 cycles with at an annealing of temperatures of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for one minute. Primers for wild‐type mice were CGA GGA TGG GAA TAA CTC ACT, Mw 7435.8 μg/μmol and GGA TGA TGA AGA CGA CCT TGA AGT, Mw 7466.8 μg/μmol from Invitrogen (Carlsbad, California, USA). Primers for knockout screen consisted of AAT GGG CAG GTA GCC GGA TCA AGC G, Mw 7782.0 μg/μmol and AAC AGA CAA TCG GCT GCT CTG ATG C, Mw 7652.0 μg/μmol from Invitrogen. PCR products were then separated on an agarose gel and products were visualised with ethidium bromide.
Induction of acid reflux oesophagitis
Animals were deprived of food but not water 18 hours prior to the studies. On the day of surgery, animals were weighed and anaesthesia was induced by injecting an intraperitoneal solution containing 90 mg/kg ketamine:10 mg/kg xylazine. Each procedure started once the animal exhibited no response to a toe pinch. Wild‐type and VR‐1 deficient animals received either an acid inducing oesophagitis (n = 6 each genotype) procedure or a sham operation (n = 6 each genotype). The abdominal area was clipped of fur with an electric razor and prepped with three washes of betadine. A small upper midline incision was made and both the pylorus and junction between the forestomach and corpus were ligated as previous reported.24 The non‐glandular corpus region of the mouse stomach is achlorhydric with a pH of 6–7, and therefore to increase the acid contact the forestomach was ligated with an additional suture. For the sham operation, animals had an identical procedure except that the ligasures were loosely applied around the pylorus and corpus, respectively, and not tied. The animals were kept under anaesthesia for five hours, and then the entire oesophagus and stomach were removed and fixed in paraformaldehyde for further examination. The animals were then euthanised by cervial dislocation.
Because of our provocative results from these experiments, we elected to further study the effects of antisecretatory agents and a VR‐1 antagonist on two sets of mice. Thus a subset of wild‐type and VR‐1 (−/−) animals were randomised to receive capsazepine (100 µmol/kg, n = 6; Tocris, Ellisville, Missouri, USA), famotidine (10 mg/kg, n = 6; Sigma St Louis, Missouri, USA), or omeprazole (400 µmol/kg, n = 6; Sigma). Administration of drugs was performed in all cases subcutaneously one hour prior to the operation. The dose of each drug was extrapolated from other mouse studies where acid secretion25,26 or VR‐1 inhibition21 were dose dependently studied.
Histological examination
The removed distal oesophagus and proximal stomachs were paraffin embedded and subsequently cut into 5 μm sections. Sections were stained with haematoxylin‐eosin and graded according to a previously validated scale (table 1).27
Table 1 Details of the histological scoring system.
| Score | Surface ulceration |
| 10 | Present |
| 0 | Absent |
| Acute inflammatory infiltrate | |
| Lamina propria | |
| 0 | No polymorphs |
| 1 | Mild infiltrate |
| 2 | Moderate infiltrate |
| 3 | Severe infiltrate |
| Submucosa | |
| 0 | No polymorphs |
| 1 | Mild infiltrate |
| 2 | Moderate infiltrate |
| 3 | Severe infiltrate |
The scale takes into account the presence of mucosal injury as well as infiltration of inflammatory cells within the lamina propia or submucosa. All specimens were scored by observers unaware of the treatment groups.
Intragastric pH measurement
Intragastric content was extracted from the removed specimens and centrifuged at 4°C for two minutes. Intragastric pH was measured using a Corning 430 pH meter (Corning, New York, USA).
Myeloperoxidase (MPO) activity
Segments of the distal oesophagus were removed immediately after euthanasia and stored frozen at −80°C for MPO activity measurement, as previously described.28 Specimens were weighed, placed in a plastic tube on ice, and 0.5% of hexadecyltrimethyllammonium bromide (HTAB) in 50 mM KH2PO4 (pH 6) (HTAB buffer) were added to each sample. Samples were homogenised on ice using a Polytron tissue homogeniser for 15 seconds followed by three cycles of freeze/thawing. All samples were fortified with additional HTAB buffer to equal 1 ml HTAB/50 mg wet weight. Samples were then vortexed and 500 µl of each were transferred to microfuge tubes. Tubes were centrifuged in an Eppendorf microfuge (12 000 g) at 4°C for two minutes and absorbance of each supernatant was read at 460 nm at 0, 30, and 60 seconds after addition of 2.9 ml of 0.167 mg/ml O‐dianisidine dihydrochloride to 0.1 ml of supernatant. One unit of MPO activity was defined as degradation of 1 mol of peroxide per minute at 25°C; the results are expressed in units per gram of protein.
Immunocytochemical analysis of SP release and quantification of NK‐1 receptor endocytosis
SP release was assessed by analysis of NK‐1 receptor endocytosis, as described previously.7 Briefly, portions of distal oesophagus from VR‐1 (+/+), VR‐1 (+/+) treated with famotidine, VR‐1 (+/+) treated with omeprazole, VR‐1 (+/+) treated with capsazepine, and VR‐1 (−/−) mice were fixed overnight in ice cold freshly depolymerised paraformaldehyde (4% in phosphate buffered saline) at 4°C and then placed in ice cold phosphate buffered saline‐30% sucrose for 24 hours. Tissue was then embedded in Tissue Tek OCT (Sakura, Torrance, California, USA), frozen, sectioned at 20 µm, mounted on Superfrost Plus glass slides (Fisher, Pittsburgh, Pennsylvania, USA), and dried with desiccant at room temperature for four hours. After being washed, sections were stained overnight at room temperature using a rabbit antiserum (No 11886‐5 from Steven Vigna) specific for the COOH terminal 15 amino acids of the rat NK‐1 receptor (SPR393–407) at a dilution of 1:3000. Sections were then washed and incubated with cyanine 3‐conjugated donkey antirabbit IgG secondary antibody (Jackson ImmunoResearch, West Grove, Pennsylvania, USA) at a dilution of 1:600 for three hours at room temperature. Sections were washed and coverslipped using one drop of Aquamount (Lerner Laboratories, Pittsburgh, Pennsylvania, USA).
Immunostained sections were analysed using a Zeiss LSM‐410 inverted krypton‐argon confocal laser scanning system coupled to a Zeiss Axiovert 100 microscope. Optical sections (0.5 µm) of 512×512 pixels were obtained and processed using Adobe PhotoDeluxe. Quantification of NK‐1 receptor endocytosis was performed by analysing 10 NK‐1 receptor immunoreactive oesophageal myenteric plexus neurones per mouse and determining the number of these cells containing >50 NK‐1 receptor immunoreactive endosomes. Cytoplasmic endosomes were distinguished from plasma membrane associated NK‐1 receptor immunoreactivity by ensuring that the nucleus of the cell was in the same optical section as the NK‐1 receptor immunoreactive endosomes.
Statistical analysis
Data are presented as mean (SEM), except where indicated. Statistical analyses were performed using ANOVA followed by Dunnett's or Tukey‐Kramer post tests as indicated. Statistical analysis was done using GraphPad Instat version 3.00 for Windows 95 (GraphPad Software, San Diego, California, USA). Significance was assumed at p<0.05.
Results
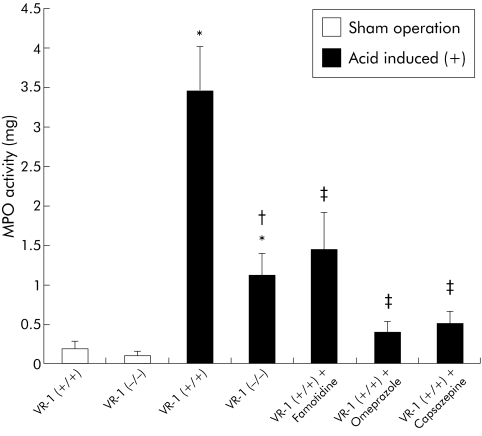
The acid induction model used produced significant levels of inflammation characterised by increased MPO activity, pathological scores, and histology compatible with oesophagitis. Both wild‐type and VR‐1 deficient mice exposed to the acid induced operation exhibited significantly higher levels of MPO activity compared with those within the sham operation group (fig 1). Figure 1 also illustrates the significantly higher MPO activity in VR‐1 (+/+)compared with VR‐1 (−/−) mice in acid exposed animals.
Figure 1 Myeloperoxidase (MPO) activity in sham operated and acid induced animals given famotidine, omeprazole, and capsazepine one hour prior to surgery. MPO activity was significantly increased in both vanilloid receptor subtype 1 (VR‐1) (+/+) and VR‐1 (−/−) acid exposed groups compared with sham operated animals (*p<0.05). VR‐1 (−/−) mice exhibited lower levels of MPO compared with VR‐1 (+/+) (†p<0.05). MPO activity was also significantly reduced by famotidine, omeprazole, and capsazepine (‡p<0.05). Data are presented as mean (SEM).
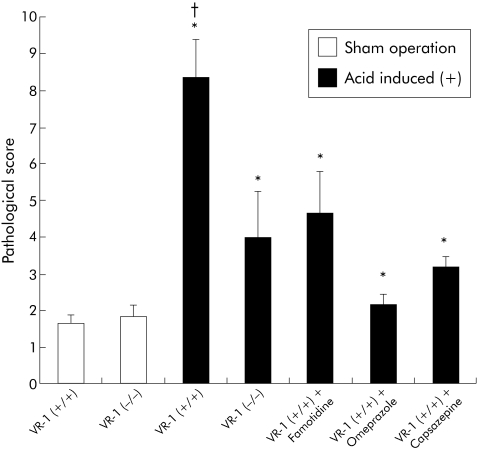
Similar to MPO activity, pathological scores were significantly increased by the acid induced operation in both wild‐type and knockout mice (fig 2) but VR‐1 (−/−) mice demonstrated significantly less pathological damage than VR‐1 (+/+) animals.
Figure 2 Pathological scores in sham operated and acid induced animals given famotidine, omeprazole, and capsazepine one hour prior to surgery. Scores were significantly increased in acid induced operated animals compared with sham operated mice (*p<0.05). In acid induced operated mice, vanilloid receptor subtype 1 (VR‐1) (+/+) scores were significantly higher than in VR‐1 (−/−) mice, famotidine treated animals, omeprazole treated animals, and capsazepine treated mice (†p<0.05). Data are presented as mean (SEM).
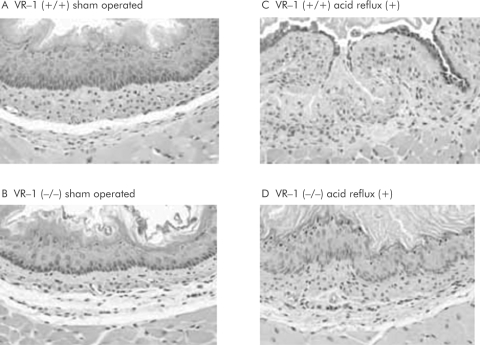
Microscopic architecture was maintained and only a few inflammatory cells were observed in the lamina propia and mucosa of animals in the sham operated group (fig 3A, B) in both VR‐1 (+/+) and (−/−) mice. Conversely, VR‐1 (+/+) mice exposed to acid by the surgical ligation technique exhibited consistent erosions with infiltration of inflammatory cells, and disorganised architecture (fig 3C). However, VR‐1 (−/−) mice exposed to the acid induction operation showed histological patterns similar to those seen in the sham operated group, with minimal to no inflammatory changes (fig 3D).
Figure 3 Haematoxylin‐eosin preparations of lower oesophageal section (70×). (A) Normal histology in wild‐type animals exposed to a sham operation. The findings observed in wild‐type animals were similar to those in the vanilloid receptor subtype 1 (VR‐1) (−/−) mice, also subjected to the same procedure (B). When acidic reflux was stimulated in wild‐type mice, erosion, disorganised histoarchitecture, and infiltration of inflammatory cells were noted (C). These changes however were absent in VR‐1 (−/−) mice (D), in which the microscopic morphology was maintained. All photomicrographs were at 70×.
To test the effect of acid suppression in this model of acute oesophagitis, a subset of wild‐type and VR‐1 deficient mice were pretreated with famotidine, a histamine antagonist, or omeprazole, a proton pump inhibitor. Additionally, pretreatment with capsazepine, a VR‐1 antagonist, was compared with animals in which antisecretory therapy was administered. Figure 1 illustrates MPO activity in sham operated mice and mice instrumented with an acid induction operation treated with capsazepine, famotidine, or omeprazole. MPO activity was significantly increased in wild‐type mice exposed to acid; MPO levels were significantly reduced by pharmacological inhibition of acid with famotidine or omeprazole as well as the VR‐1 antagonist capsazepine. The inhibition in MPO activity levels observed after treatment with capsazepine was statistically similar to that present in omeprazole treated animals. Similar to MPO, the pathological damage score was also significantly reduced with famotidine, omeprazole, or capsazepine pretreatment in wild‐type animals (fig 2). There was no significant difference in pathological score between VR‐1 (−/−) animals and wild‐type animals treated with omeprazole, famotidine, or capsazepine.
Intragastric pH was also measured in all groups tested (table 2).
Table 2 Intragastric pH in mice with either a sham operation or oesophagitis.
| Genotype | Operation | Drug | Intragastric pH |
|---|---|---|---|
| VR‐1 (+/+) | Sham | 6.12 (0.89) | |
| VR‐1 (−/−) | Sham | 5.98 (0.95) | |
| VR‐1 (+/+) | Oesophagitis | 2.15 (0.43) | |
| VR‐1 (−/−) | Oesophagitis | 2.28 (0.33) | |
| VR‐1 (+/+) | Oesophagitis | Famotidine | 4.23 (1.53)** |
| VR‐1 (+/+) | Oesophagitis | Omeprazole | 5.81 (0.35)** |
| VR‐1 (+/+) | Oesophagitis | Capsazepine | 4.33 (0.80)** |
VR‐1, vanilloid receptor subtype 1.
A subset of mice were administered famotidine, omeprazole, or capsazepine. Note that sham operated mice did not have a significant reduction in intragastric pH, while both (+/+) and (−/−) mice with oesophagitis had a significant reduction in pH. Note the increase in pH levels following antisecretory and VR‐1 antagonism therapy.
Values are mean (SD).
**p<0.01 compared with VR‐1 (+/+).
Intragastric pH was significantly lower in both VR‐1 (+/+) and VR‐1 (−/−) mice undergoing surgically induced oesophagitis. However, wild‐type mice pretreated with famotidine, omeprazole, or capsazepine had significantly elevated pH levels in the oesophagitis model. Capsazepine pretreatment resulted in intragastric pH levels comparable with those of antisecretory therapy.
Confocal microscopy and SP release
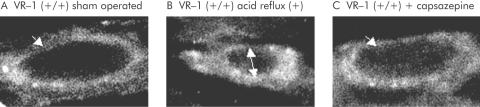
Confocal microscopy of the myenteric plexuses of the lower oesophagus demonstrated normal distribution of the NK‐1 receptor localised in the plasma membrane of neurones in animals in the sham operated group (fig 4A). However, in wild‐type mice exposed to acid, the NK‐1 receptor was observed in multiple intracellular cytoplasmic endosomes, indicating SP release and NK‐1 receptor binding and subsequent internalisation (fig 4B). We have previously demonstrated that this represents accurate SP release and receptor activation.7 Capsazepine pretreatment inhibited SP release and subsequent endocytosis, as seen by the majority of NK‐1 receptors found on the plasma membrane (fig 4C).
Figure 4 Confocal microscopy of sham operated (A), acid exposed (B), and acid exposed plus capsazepine treated (C) vanilloid receptor subtype 1 (VR‐1) (+/+) mice. Endosomal internalisation of neurokinin 1 receptor (NK‐1R) was observed in myenteric neurones of acid exposed animals while sham operated and capzasepine treated wild‐type mice had the receptors confined to the cell membrane (white arrows). The dark inner area of (B) (double arrow) represents the nucleus. Internalisation of NK‐1R was observed in myenteric neurones of acid exposed animals. This phenomenon was inhibited by pretreatment with capsazepine (1000×).
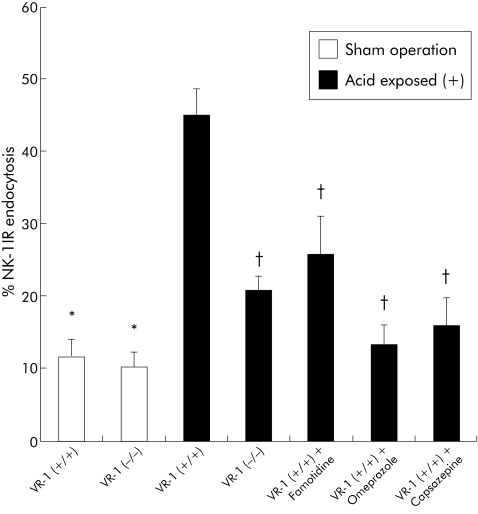
Quantification of internalisation was performed using a previously established method of internalised NK‐1 receptor positive endosomes (fig 5). Minimal endocytosis was found in sham operated animals but significant elevation in NK‐1 endocytosis was seen in surgically induced oesophagitis. However, VR‐1 (−/−) animals and VR‐1 (+/+) animals with oesophagitis pretreated with famotidine, omeprazole, and capsazepine demonstrated a significant reduction in NK‐1 endocytosis in comparison with sham operated wild‐type animals.
Figure 5 Immunocytochemical analysis of substance P (SP) release and quantification of neurokinin 1 (NK‐1) receptor endocytosis. Quantification of SP release in sham operated and acid exposed animals. Exposure to acid produced a significant release in SP (*p<0.05). SP release was reduced in vanilloid receptor subtype 1 (VR‐1) (−/−), VR‐1 (+/+) plus famotidine treated animals, VR‐1 (+/+) plus omeprazole treated animals, and VR‐1 (+/+) plus capsazepine treated animals (†p<0.05). Data are presented as mean (SEM).
Discussion
In this report we investigated the role of VR‐1 in an acute model of oesophagitis. Our results indicate that acid induced oesophagitis is significantly attenuated in animals lacking VR‐1 or by pharmacologically antagonising the receptor in wild‐type mice. The magnitude of the reduction in inflammation was similar to that produced by standard antisecretory therapy. Additionally, reduction of SP release and subsequent NK‐1 receptor activation (as measured by NK‐1 receptor endocytosis) mirrored the decrease in inflammation indexes in mice either deficient in VR‐1 or wild‐type mice provided with antisecretory drugs. These results strongly suggest that in this model of oesophagitis, acid exposure stimulates VR‐1, which subsequently releases SP to initiate an inflammatory cascade.
VR‐1 is a non‐selective cation channel with six transmembrane domains that is found on primary sensory neurones and provides sensory information of chemical, physical, and inflammatory input.24 The receptor has been localised using immunohistochemistry in numerous locations throughout the gastrointestinal tract, including the oesophagus,27 stomach, small intestine, and colon.29 VR‐1 immunoreactivity is found predominantly in nerve fibres of the myenteric plexus, with lesser staining in the lamina propria and nerve endings that innervate the mucosa.30 Interestingly, such nerve endings seem not to penetrate the entire extent of the mucosa, suggesting that the luminal stimulants that act on VR‐1 either pass across the mucosa or enhance the production of secondary mediators to operate on the receptor. Recent studies indicate that VR‐1 containing primary sensory neurones are present in the lower portions of the oesophagus31 and that the number of immunoreactive fibres increase in patients with oesophagitis.27
Stimulation of VR‐1 containing primary sensory neurones release neuropeptides, including SP, that convey nociceptive information to the spinal cord and also modulate inflammation in peripheral tissues via an axon reflex. Known agonists of VR‐1 include heat, acid, and capsaicin. Additionally, a variety of endogenous ligands have emerged as potential agonists of VR‐1. Work from our group and others have demonstrated that inhibition of VR‐1 using selective antagonists, or surgical sensory denervation, attenuates several animal models of intestinal inflammation.5,9 Other investigators have reported similar findings in models of pancreatitis2 and cystitis.32 In addition to the reduction in inflammatory parameters to basal levels in these models, sensory nerve ablation or inhibition of VR‐1 using selective VR‐1 antagonists also significantly decreased NK‐1 receptor internalisation, indicating diminution of SP release. This suggested that in these models, sensory nerves containing VR‐1 are stimulated which results in SP release and subsequent inflammation.
In this study we intended to examine the effect of endogenous acid, a known agonist of VR‐1, in mice either deficient in VR‐1 or mice provided with an antagonist to VR‐1 prior to acid exposure. In this model, ligation of the pylorus and forestomach results in rapid infusion of acid into the oesophagus (as demonstrated by the significant reduction in intragastric pH (table 2)) and in a robust inflammatory response with a significant increase in MPO activity and pathological damage. Mice genetically deficient in VR‐1 (VR‐1 (−/−)) demonstrated a significant reduction in MPO and pathological damage compared with wild‐type animals (figs 1–2). This reduction in MPO and pathological scoring in VR‐1 (−/−) mice was similar to mice that were preoperatively treated with standard antisecretory agents, famotidine and omeprazole, indicating that acid was the causative agent in initiating inflammation. Intragastic pH was elevated in these animals, strongly suggesting that inhibition of acid production prevented inflammation. Furthermore, pretreatment with capsazepine, an antagonist of VR‐1, in VR‐1 (+/+) animals, demonstrated a nearly identical inhibition of inflammation as famotidine and omeprazole. Interestingly, capsazepine pretreatment resulted in an increase in intragastric pH. To date, there is no reported effect of capsazepine on acid secretion. The cause of this is not clear but several reports indicate that capsazepine may inhibit nicotinic acetylcholine receptors and non‐specifically block voltage activated calcium channels.33,34
We also examined the effects of genetic deletion or antagonism of VR‐1 on SP receptor internalisation in acid exposed mice. Quantification of the SP receptor using confocal microscopy has been shown to correspond to SP release from sensory neurones and has been shown to directly correlate with inflammation. Similar to previous work, SP release (as measured by SP receptor internalisation) mirrored the inflammatory parameters of MPO and pathological damage (figs 1, 2). Wild‐type mice and mice pretreated with famotidine, omeprazole, and capsazepine demonstrated a significant reduction in SP receptor internalisation, suggesting that without acid, VR‐1 is not stimulated to release SP. Furthermore, VR‐1 deficient animals with acid exposure (pH 2.15) demonstrated a significant reduction in NK‐1 endocytosis, indicating that without VR‐1, acid cannot stimulate VR‐1 to release SP.
Clinically, these results may provide insight into the pathophysiology of the presence of chronic cough and asthma in patients suffering form reflux disease. Previously, this association has been explained by several physiopathological mechanisms, including: (1) a vagal‐oesophageal‐bronchial reflex; (2) sensitive bronchial reactivity; and (3) microaspiration of gastric contents (reviewed by Harding35). Hamamoto and colleagues36 proposed an alternative pathway in which C fibres contained in the vagal nerve elicit tachykinins release that contract the bronchial smooth muscle, increase bronchial secretion,37 and increase vascular permeability.38,39 The use of specific tachykinin receptor antagonists has been used to prevent bronchoconstriction and airway oedema in different animal models of acid induced reflux disease.40,41,42 To date, the involvement of VR‐1 on this oesophageal‐pulmonary circuit is unknown but the availability of potent VR‐1 antagonists and animals deficient in VR‐1 may clarify the role of the receptor in this inflammatory pathway.
Although these results are provocative, direct clinical correlation with GORD patients must be tempered on the basis of two arguments. Firstly, the animal model used in our experiments represents an acute (five hours) oesophagitis model that might not reflect the chronic process present in the majority of patients presenting with severe oesophagitis. Secondly, we used a model in which only acid was refluxated into the oesophagus. Although acid is a primary factor in the inflammatory process associated with oesophagitis, the role of biliary components in oesophagitis has been emphasised by many authors.39 Further studies are warranted to determine the effect of VR‐1 inhibition in chronic acid exposure or alkaline reflux. Finally, the effects of genetic deletion of VR‐1 or receptor antagonism do not to completely inhibit the effects of acid induced inflammation. As demonstrated in figs 1, 2, and 5, VR‐1 (−/−) animals exposed to acid were found to have a significant reduction in inflammatory parameters and NK‐1 endocytosis compared with wild‐type animals but levels did not return to sham operated levels, indicating that other inflammatory processes are occurring.
In conclusion, this study presents direct evidence that acid induced oesophagitis is attenuated in mice deficient in VR‐1. The reduction in inflammatory indices and pathological scoring mirrored the effects of standard antisecretory therapies. Additionally, SP release (and receptor endocytosis) was also reduced in VR‐1 (−/−) mice and wild‐type mice given capsazepine, indicating that acid stimulates VR‐1 containing sensory neurones which in turn release SP. These results suggest a link between VR‐1 and acid induced oesophagitis that might help elucidate the physiopathology of oesophagitis and provide a novel therapeutic target for patients with gastro‐oesophageal reflux disease.
Abbreviations
VR‐1 - vanilloid receptor subtype 1
GORD - gastro‐oesophageal reflux disease
MPO - myeloperoxidase
NK‐1 - neurokinin 1
SP - substance P
PCR - polymerase chain reaction
HTAB - hexadecyltrimethyllammonium bromide
Footnotes
Conflict of interest: None declared.
References
- 1.Nebel O T, Fornes M F, Castell D O. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 197621953–956. [DOI] [PubMed] [Google Scholar]
- 2.Nathan J D, Patel A A, McVey D C.et al Capsaicin vanilloid receptor‐1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 2001281G1322–G1328. [DOI] [PubMed] [Google Scholar]
- 3.Kihara N, de la Fuente S G, Fujino K.et al Vanilliod‐1 receptor containing primary sensory neurons mediate dextran sulfate sodium‐induced chronic colitis in rats. Gut 20035713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castagliuolo I, LaMont J T, Letourneau R.et al Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 1994107657–665. [DOI] [PubMed] [Google Scholar]
- 5.Mantyh C R, Pappas T N, Lapp J A.et al Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 19961111272–1280. [DOI] [PubMed] [Google Scholar]
- 6.Bozic C R, Lu B, Hopken U E.et al Neurogenic amplification of immune complex inflammation. Science 19962731722–1725. [DOI] [PubMed] [Google Scholar]
- 7.Mantyh P W, Catton M, Maggio J E.et al Alterations in receptors for sensory neuropeptides in human inflammatory bowel disease. Adv Exp Med Biol 1991298253–283. [DOI] [PubMed] [Google Scholar]
- 8.Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann Allerg Asthma Im 20039034–40. [DOI] [PubMed] [Google Scholar]
- 9.Richardson J D, Vasko M R. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 2002302839–845. [DOI] [PubMed] [Google Scholar]
- 10.De Felipe C, Herrero J F, O'Brien J A.et al Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998392394–397. [DOI] [PubMed] [Google Scholar]
- 11.Mantyh P W, Rogers S D, Honore P.et al Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 1997278275–279. [DOI] [PubMed] [Google Scholar]
- 12.Sandler A D, Maher J W, Weinstock J V.et al Functional and morphological characteristics of neuronal substance P in the canine gastroesophageal junction. J Surg Res 199355372–381. [DOI] [PubMed] [Google Scholar]
- 13.Smid S D, Blackshaw L A. Neuromuscular function of the human lower esophageal sphincter in reflux disease and Barrett's esophagus. Gut 200046756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartho L, Lenard L, Patacchini R.et al Tachykinin receptors are involved in the “local efferent” motor response to capsaicin in the guinea‐pig small intestine and esophagus. Neuroscience 199990221–228. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay A. Effect of substance P on the lower esophageal sphincter of the opossum. Gastroenterology 197875278–281. [PubMed] [Google Scholar]
- 16.Sandler A, Maher J W, Weinstock J.et al Tachykinins in the canine gastroesophageal junction. Am J Surg 1991161165–170. [DOI] [PubMed] [Google Scholar]
- 17.Huang C H, Kuo I C, Xu H.et al Mite allergen induces allergic dermatitis with concomitant neurogenic inflammation in mouse. J Invest Dermatol 200312289–293. [DOI] [PubMed] [Google Scholar]
- 18.Zygmunt P M, Petersson J, Andersson D A.et al Vanilloid receptor on sensory nerves mediate the vasodilator action of anandamide. Nature 1999400452–457. [DOI] [PubMed] [Google Scholar]
- 19.Hwang S W, Cho H, Kwak J.et al Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin‐like substances. Proc Natl Acad Sci U S A 2000976155–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennings E A, Vaughan C W, Roberts L A.et al The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro. J Physiol 2003548121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McVey D C, Schmid P C, Schmid H H.et al Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR‐1). J Pharmacol Exp Ther 2003304713–722. [DOI] [PubMed] [Google Scholar]
- 22.Stein B E, Schwartzman M L, Carroll M A.et al Rabbit esophagus metabolizes arachidonic acid predominantly via a lipoxygenase pathway. Prostaglandins Leukot Essent Fatty Acids 19883475–80. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Ozawa Y, Furuta Y.et al Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol 198232445–456. [DOI] [PubMed] [Google Scholar]
- 24.Caterina M J, Schumacher M A, Tominaga M.et al The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 1997389816–824. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Jennings T A, Eaton J W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A 1998958841–8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zavrous Y, Rieder G, Ferguson A.et al Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am J Physiol Gastrointest Liver Physiol 2002282G175–G183. [DOI] [PubMed] [Google Scholar]
- 27.Mathews P, Asís Q, Facer P.et al Increased expresion of the capsaicin receptor TRPV1 in the inflamed human esophagus. Gastroenterology. 2004;126;A427. [DOI] [PubMed]
- 28.Bradley P P, Priebat D A, Christensen R D.et al Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol 198278206–209. [DOI] [PubMed] [Google Scholar]
- 29.Yiangou Y, Facer P, Dyer N H.et al Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 20013571338–1339. [DOI] [PubMed] [Google Scholar]
- 30.Ward S M, Bayguinov J, Won K J.et al Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol 2003465121–135. [DOI] [PubMed] [Google Scholar]
- 31.Trevisani M, Smart D, Gunthorpe M J.et al Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor‐1. Nat Neurosci 20025546–551. [DOI] [PubMed] [Google Scholar]
- 32.Szallasi A, Conte B, Goso C.et al Characterization of a peripheral vanilloid (capsaicin) receptor in the urinary bladder of the rat. Life Sci 199352221–226. [DOI] [PubMed] [Google Scholar]
- 33.Lui L, Simon S A. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neuroscience Lett 199122829–32. [DOI] [PubMed] [Google Scholar]
- 34.Docherty R J, Yeats J C, Piper A S. Capsazepine block of voltage‐activated calcium channels in rat dorsal root ganglion neurons in culture. Br J Pharmacol 19971211461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding S M. Gastroesophageal reflux and asthma: insight into the association. J Allergy Clin Immun 1999104251–259. [DOI] [PubMed] [Google Scholar]
- 36.Hamamoto J, Kohrogi H, Kawano O.et al Esophageal stimulation by hydrochloric acid causes neurogenic inflammation in the airways in guinea pigs. J Appl Physiol 199782738–745. [DOI] [PubMed] [Google Scholar]
- 37.Baker A P, Hillegass L M, Holden D A.et al Effect of kallidin, substance P, and other basic polypeptides on the production of respiratory macromolecules. Am Rev Respir Dis 1977115811–817. [DOI] [PubMed] [Google Scholar]
- 38.Kohrogi H, Graf P D, Sekizawa K.et al Neutral endopeptidase inhibitors potentiate substance P‐ and capsaicin‐induced cough in awake guinea pigs. J Clin Invest 1988822063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daoui S, D'Agostino B, Gallelli L.et al Tachykinins and airway microvascular leakage induced by HCl intra‐oesophageal instillation. Eur Respir J 200220268–273. [DOI] [PubMed] [Google Scholar]
- 40.Honda I, Kohrogi H, Yamaguchi T.et al Tachykinin antagonist FK224 inhibits neurokinin A‐, substance P‐ and capsaicin‐induced human bronchial contraction. Fund Clin Pharm 199711260–266. [DOI] [PubMed] [Google Scholar]
- 41.Nault M A, Vincent S G, Fisher J T. Mechanisms of capsaicin‐ and lactic acid‐induced bronchoconstriction in the newborn dog. J Physiol 1999515567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joos G F, De Swert K O, Schelfhout V.et al The role of neural inflammation in asthma and chronic obstructive pulmonary disease. Ann N Y Acad Sci 2003992218–230. [DOI] [PubMed] [Google Scholar]