The emergence of autoimmunity, including Crohn's disease (CD) where the immune relationship with commensal bacteria is corrupted, has been linked to hygiene.1,2 A gradual decline in endoparasites is but one argument that might explain this phenomenon.3 Weinstock and colleagues have successfully tested the pig whipworm, Trichuris suis, in patients with inflammatory bowel disease (IBD).4,5 However, repeated inoculation was required and concern has been raised that aberrant migration could occur.6 The haematophagous hookworm, Necator americanus (NA), is proposed as an alternative. We have tested if CD patients tolerate hookworm infection, and the practical issues associated with establishing reservoir donors (RDs).
Over 700 million people remain infected with hookworms. Infective larvae (L3i) are acquired through skin contact with contaminated soil.7 Auto‐reinfection, direct person to person infection, aberrant migration, and hypobiosis do not occur. Adult worms live in the host small intestine for an average of five years. Infection can be easily terminated with an anthelminthic. Anaemia is the only disease of consequence but is an unusual outcome in properly nourished individuals. Using L3i originally obtained from Madang, Papua New Guinea, but maintained in a healthy researcher in the UK, five CD subjects with longstanding but mostly inactive disease and three RDs each received a carefully measured inoculum (table 1). Subsequently, four additional CD subjects with chronic and mostly active disease were inoculated with L3i cultured from faeces provided by an RD, and the original CD cohort were reinoculated from week 27 to week 30. Ethics approval was granted by the Townsville Health Service District Institutional Ethics Committee. Haematological and clinical measurements are expressed as mean (95% confidence interval).
Table 1 Crohn's disease activity index (CDAI) in CD subjects inoculated with infective larvae (L3i). Subsequently, the five CD subjects first inoculated were reinoculated from week 27 to week 30 and four CD subjects with chronic and mostly active disease were inoculated with larvae sourced from one of the authors.
| ID, age (y), sex | Initial inoculation trial | Reinoculation trial | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (weeks) | Time (weeks) | |||||||||
| 0–4 | 5–8 | 9–12 | 13–16 | 17–20 | 27 | 30 | 35 | 39–41 | 45 | |
| CD1 55 M | ||||||||||
| Inoculum therapy | 25 L3i | 25 L3i | ||||||||
| CDAI | 79 | 60 | 89 | 77 | 68 | 96 | 93 | 62 | ||
| CD2 46 M | ||||||||||
| Inoculum therapy | 25 L3i | 25 L3i | ||||||||
| CDAI | 38 | 114 | 20 | 68 | 48 | 30 | 36 | |||
| CD3 41 F | ||||||||||
| Inoculum therapy | 25 L3i P5 M15 | P5 M15 | P5 M7.5 | 25 L3i P5 M10 | P5 M10 | P5 M10 | M10 | M10 | ||
| CDAI | 46 | 71 | 85 | 83 | 90 | 36 | 30 | 92 | 95 | |
| CD4 34 M | ||||||||||
| Inoculum therapy | 50 L3i P38 M30 | P38 M30 | P50 M30 | P25 M30 | P5 M30 | 50 L3i P5 M20 | P2.5 M20 | M20 | P25 M30 | P20 M30 |
| CDAI | 260 | 230 | 232 | 264 | 118 | 60 | 442 | 60 | 122 | |
| CD5 21 F | ||||||||||
| Inoculum therapy | 50 L3i P10 M20 | P10 M20 | P13 M20 | P10 | P7.5 | P5 | 25 L3i P5 | P25 M20 | P15 M20 | P10 M20 |
| CDAI | 144 | 151 | 103 | 79 | 73 | 410 | 180 | 44 | 61 | |
| CD6 33 F | ||||||||||
| Inoculum therapy | 50 L3i P15 M20 | P10 M20 | P5 M20 | P5 M20 | P7.5 M20 | |||||
| CDAI | 49 | 32 | 6 | 29 | 100 | |||||
| CD7 33 M | ||||||||||
| Inoculum therapy | 50 L3i A150 | P25 A150 | P5 A150 | P5 A150 | P8 A150 | |||||
| CDAI | 260 | 114 | 96 | 125 | 118 | |||||
| CD8 46 M | ||||||||||
| Inoculum therapy | 50 L3i M20 | M20 | M20 | M20 | M20 | |||||
| CDAI | 145 | 159 | 171 | 152 | 186 | |||||
| CD9 44 F | ||||||||||
| Inoculum therapy | 100 L3i P5 M20 | P10 M20 | P5 M20 | P5 M20 | P10 M20 | |||||
| CDAI | 173 | 127 | 106 | 76 | 125 | |||||
L3i, n 3rd stage N americanus larvae inoculated percutaneously; P, prednisone n mg/day; A, azathioprine n mg/day; M, methotrexate n mg/week.
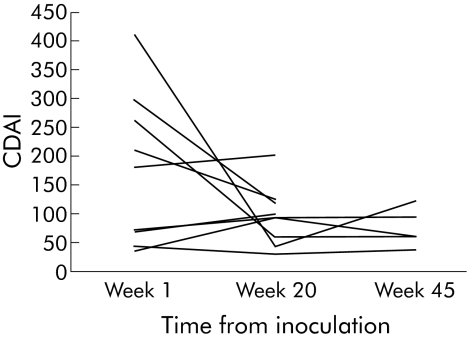
The inoculation caused a mild itch within five minutes that disappeared after a few days in eight CD subjects and a pruritic rash that lasted two weeks in the RDs, who also developed a painful transient enteropathy. Neither respiratory symptoms nor detectable aberrant migration occurred. In the CD cohort, blood eosinophilia developed from week 5 (mean 2.60×109/l (1.89) v week 1 0.18×109/l (0.10) v week 20 0.59 (0.20)). Patent infection had established by week 20 in all cases. CD activity index (CDAI) remained unchanged until week 17, possibly in part due to a hookworm related enteropathy recognisable because of blood eosinophilia and faecal Charcot‐Leydon crystals.8 After 20 weeks, the IBD questionnaire was improved (mean 151 (14) v 179 (20)) and the four week cumulated CDAI scores was decreased (mean 141 (31) v 87 (15)).9 Haemoglobin fell marginally (week 1 mean 135.6 (7.8) g/l v week 20 129.3 (4.1) g/l). Reinoculation of the five CD subjects first exposed caused no apparent adverse effect. Disease reactivation, as defined by a CDAI >150, occurred in two (CD4, CD5; table 1) after the doses of long term immune suppressive drugs had been reduced. The subject (CD3–7) driven trend was to reduce immune suppression as health improved, a strategy often associated with worsening of symptoms. The five CD subjects first inoculated were in remission at week 45 (fig 1).
Figure 1 Initial Crohn's disease activity index (CDAI) score for each CD patient versus score at week 20 and at week 45 for the first five inoculated cases (mean 165 (95% confidence interval 145) v 64 (25), p = 0.132; mean 165 v 75 (29), p = 0.246).
Our pilot study has established a potential for NA, already a fact of life for many millions, as a candidate parasite to inoculate those with autoimmune disease. The natural advantages are lifecycle and migration predictability, ability to control the size of and eliminate a colony, and the parasite's longevity. Inoculation proved safe, even in immune suppressed patients. Our hope that NA would suppress autoreactivity sufficiently to allow immune suppressive therapy to be stopped was unrealistic. Recent and compelling evidence has shown that IBD is self sustaining.10 It may be that after remission is achieved, endoparasites will offer an alternative or adjunct to immune suppressive therapy, a priority for some people with CD.
Footnotes
Conflict of interest: None declared.
References
- 1.Bach J F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002347911–920. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky D K. Inflammatory bowel disease. N Engl J Med 2002347417–429. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock J V, Summers R W, Elliott D E.et al The possible link between de‐worming and the emergence of immunological disease. J Lab Clin Med 2002139334–338. [DOI] [PubMed] [Google Scholar]
- 4.Summers R W, Elliott D E, Urban J F.et al Trichuris therapy for active ulcerative colitis: a randomised trial. Gastroenterology 2005128825–832. [DOI] [PubMed] [Google Scholar]
- 5.Summers R W, Elliott D E, Urban J F., Jret al Trichuris suis therapy in Crohn's disease. Gut 20055487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Kruiningen H J, West A B. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory disease. Inflamm Bowel Dis 200511515. [DOI] [PubMed] [Google Scholar]
- 7.Hotez P J, Brooker S, Bethony J M.et al Hookworm infection. N Engl J Med 2004351799–807. [DOI] [PubMed] [Google Scholar]
- 8.Best W R, Becktel J M, Singleton J W.et al Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenteroogy 197670439–444. [PubMed] [Google Scholar]
- 9.Guyatt G, Mitchell A, Irvine E J.et al A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 198996804–810. [PubMed] [Google Scholar]
- 10.Faubion W A, de Jong Y P, Molina A A.et al Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in the periphery. Gastroenterology 20041261759–1771. [DOI] [PubMed] [Google Scholar]