Abstract
To produce disease, viruses must enter the host, multiply locally in host tissues, spread from the site of entry, and overcome or evade host immune responses. At each stage in this infectious process, specific microbial and host genes determine the ultimate virulence of the virus. Genetic approaches have identified many viral genes that play critical roles in virulence and are presumed to target specific components of the host innate and acquired immune response. However, formal proof that a virulence gene targets a specific protein in a host pathway in vivo has not been obtained. Based on cell culture studies, it has been proposed that the herpes simplex virus type 1 gene ICP34.5 (ICP, infected cell protein) enhances neurovirulence by negating antiviral functions of the IFN-inducible double-stranded RNA-dependent protein kinase R or PKR [Chou, J., Chen, J.J., Gross, M. & Roizman, B. (1995) Proc. Natl. Acad. Sci. USA 92, 10516–10520]. Herein, we show that a virus that has been attenuated by deletion of ICP34.5 exhibits wild-type replication and virulence in a host from which the PKR gene has been deleted. We show that restoration of virulence is specific to ICP34.5 and PKR by using additional host and viral mutants. The use of recombinant viruses to infect animals with null mutations in host defense genes provides a formal genetic test for identifying in vivo mechanisms and targets of microbial virulence genes.
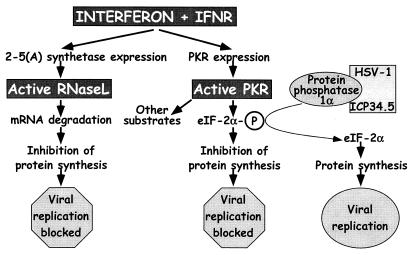
IFNs are key components of host innate immunity to virus infection that act through several pathways to block virus growth and virulence (refs. 1–3; Fig. 1). We considered the hypothesis that the ICP34.5 gene of herpes simplex virus type 1 (HSV-1) inactivates a specific component of IFN responses in vivo. This hypothesis is based on the observations that ICP34.5 inhibits the IFN-induced double-stranded RNA-dependent protein kinase R (PKR) system in cell culture (Fig. 1; refs. 4–6) and that viruses lacking ICP34.5 are profoundly attenuated in vivo (7, 8). Activation of PKR leads to phosphorylation of the α-subunit of eukaryotic initiation factor 2α (eIF-2α) and a subsequent shutdown of host and viral protein synthesis and replication (Fig. 1; refs. 9 and 10). In addition, PKR phosphorylates IκB and activates NF-κB, transcription factors that may contribute to IFN-dependent antiviral effects (11). The hypothesis that ICP34.5 targets PKR-dependent responses is attractive, because ICP34.5 binds to a protein phosphatase and causes it to dephosphorylate eIF-2α, thereby potentially negating the antiviral effects of PKR (Fig. 1; refs. 2 and 12).
Figure 1.
IFN-mediated induction of the antiviral state through independent pathways by RNase L and PKR. For RNase L, IFNs interacting with their receptors lead to expression of 2-5A synthetase, which, after double-stranded RNA binding, produces 2′,5′-oligoadenylates that lead to RNase L activation and blockage of viral replication. IFN also induces expression of PKR, which is also activated through double-stranded RNA binding. One of the activities of PKR is to phosphorylate eIF-2α leading to cessation of protein synthesis and blockage of viral replication. To evade this antiviral mechanism, HSV-1 ICP34.5 binds to protein phosphatase 1α and dephosphorylates eIF-2α, lifting the block on viral replication. Mice with null mutations in the IFN receptors, RNase L, and PKR (gray boxes) were used in this study to dissect the interaction of ICP34.5 with these pathways.
We previously demonstrated that an HSV-1 mutant in gene ICP34.5 grew to normal levels and showed wild-type virulence in mice lacking both type 1 (IFN-αβ) and type 2 (IFN-γ) IFN receptors (IFN-αβγR−/−). These results were obtained despite the fact that, in normal mice, the mutant had a 10,000-fold reduction in replication and neurovirulence (13). The restoration of a normal phenotype to the ICP34.5 mutant by deletion of IFN receptors was consistent with the hypothesis that some aspect of IFN action in vivo is the primary target of the ICP34.5 gene. Deletion of IFN receptors, however, also resulted in increased replication in mice of HSVs with null mutations in the ICP0, thymidine kinase (tk), ribonucleotide reductase, and virion host shutoff (vhs) genes. Increased viral replication in vivo resulting from loss of IFN receptors was therefore not a specific property of viruses lacking ICP34.5.
We determined that three criteria must be met to validate the hypothesis that ICP34.5 blocks PKR-dependent antiviral effects in vivo and that this interaction is pivotal for neurovirulence. First, an ICP34.5-deleted virus must show restored growth and virulence in mice lacking PKR (PKR−/− mice). Second, the growth and virulence of an ICP34.5-deleted virus must not be restored in mice lacking IFN-inducible antiviral pathways that are independent of PKR. Third, HSV strains with mutations in genes other than ICP34.5 must remain attenuated in PKR−/− mice. In this study, we test and meet these three criteria, showing that a virus that has been attenuated by deletion of ICP34.5 has wild-type replication and virulence in a host from which the PKR gene has been deleted. This specific phenotypic restoration both defines a key molecular target of ICP34.5 in vivo and provides the genetic criteria for determining mechanisms of microbial virulence in vivo.
Materials and Methods
Viruses and Cells Vero.
(African green monkey kidney) cells were propagated, and growth and assay of all virus strains were done as described (14). The KOS strain of HSV-1 was the background for mutant viruses UL41NHB (vhs nonsense mutation termed here Δvhs; ref. 15) and dl8.36tk (tk deletion, termed here Δtk; ref. 16). The ICP34.5 mutant 17TermA (termed here Δ34.5) and its marker-rescued virus 17TermAR (termed here control virus) as well as the tk deletion 17/tBTK(−) (termed here 17Δtk) and its marker-rescued virus 17/tBRTK(+) (termed here 17ΔtkR) were made in the background of strain 17 and were provided by Richard Thompson (University of Cincinnati, OH; refs. 8 and 17).
Mouse Strains.
Mice were housed and bred in the Washington University School of Medicine biosafety level 2 animal facility where sentinel mice were screened every 3–6 months for mouse pathogens and determined to be negative. Mice deleted for PKR and RNase L were in 129 Ev/Sv/C57BL/6J and C57BL/6J backgrounds, respectively (11, 18). Mice deleted for IFN receptors (IFN-αβR−/− and IFN-αβγR−/−) were in a 129 Ev/Sv background and were originally obtained from Michel Aguet (Institute of Molecular Biology, University of Zurich, Zurich; ref. 3). Strain 129 Ev/Sv (referred to as 129), C57BL/6 (Taconic, referred to as B6), and (129 Ev/Sv × C57BL/6)F1 hybrid (Taconic, referred to as 129/B6) mice were used as immunocompetent controls.
Animal Procedures.
Mice 6–8 weeks old were anesthetized with xylazine and ketamine. Corneas were scarified with 10 interlocking strokes with a 27-gauge needle, and 5 μl of medium containing 2 × 104 or 2 × 106 plaque-forming units (pfu) of virus was added per eye. Lids were massaged together briefly to promote adsorption of virus. Eye swab and trigeminal ganglion assays of acute infection were performed as described (15). Briefly, for eye swabs, eyes were proptosed, and moistened cotton swabs were used to sample the surface of the eye, going three times in a circular fashion around the eye and twice across the eye making an “X”, before placing the swab in 1 ml of tissue culture medium. Sampled trigeminal ganglia were homogenized in 1 ml of tissue culture medium by using 1-mm beads in a MiniBeadbeater-8 (Biospec Products, Bartlesville, OK). All samples were titered by standard plaque assay on Vero cells. All data presented are logarithmic means ± SEM, and the limit of detection was 10 pfu. Intracerebral inoculations were performed essentially as described (15). In brief, mice were inoculated intracerebrally with 20 μl of virus inoculum containing the appropriate virus, and time to death was recorded. All mouse procedures conformed to protocols approved by the Washington University Animal Studies Committee.
Results
Specific Normalization of Growth of an ICP34.5− Virus in PKR−/− Mice.
We examined the replication of an ICP34.5 mutant in PKR−/− mice and used strain 129/B6 F1 hybrid mice as wild-type controls. All 129 and 129/B6 strain mice used in this study were bred in the 129 line, the same 129 line as was used to generate the PKR−/− and IFN-αβγR−/− mice. This manner of breeding was done to avoid any possible bias in the data caused by variation among 129 strains (19). As a positive control, we infected IFN-αβγR−/− mice, which lack activation of the entire IFN pathway, alongside strain 129 mice as wild-type controls (Fig. 1). As a control for host resistance-gene specificity, we also tested the hypothesis that RNase L is the target of ICP34.5 by concurrent infection of mice lacking RNase L (RNase L−/− mice; ref. 20) alongside B6 mice as wild-type controls. IFNs induce synthesis of 2-5(A) oligoadenylate synthetase whose product leads to the activation of latent RNase L (21). This pathway is important for controlling replication of both RNA and DNA viruses (22). We reasoned that RNase L−/− mice provide a valid control for analysis of an ICP34.5 mutant virus, because PKR and RNase L are both IFN-dependent antiviral pathways, but ICP34.5 is thought to target only PKR (ref. 4; Fig. 1).
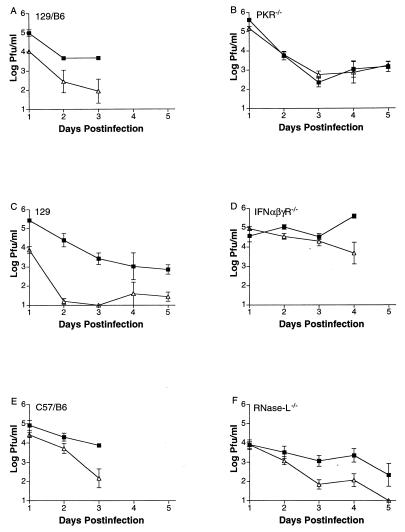
The various wild-type strains and PKR−/−, IFN-αβγR−/−, and RNase L−/− mice were infected via the cornea with the attenuated ICP34.5 mutant 17TermA (termed here Δ34.5) or its marker-rescued virus 17TermAR (termed here control virus), which has wild-type growth and virulence. Viral replication was measured in corneal epithelia and in trigeminal ganglia (Fig. 2). In all wild-type mouse strains, replication of Δ34.5 was significantly (P < 0.01) reduced in eyes by up to 1,000-fold relative to that of control virus (Fig. 2 A, C, and E). These results are consistent with those obtained with mice and rabbits, which have been published (23, 24). In PKR−/− and IFN-αβγR−/− mice, however, replication of Δ34.5 in corneas was statistically indistinguishable (P = 0.84 and P = 0.27) from that of control virus (Fig. 2 B and D). In contrast, in RNase L−/− mice, the attenuation of replication of Δ34.5 remained significant (P < 0.02) relative to control virus (Fig. 2F). A similar pattern was observed during acute infection in trigeminal ganglia (Fig. 3). Growth of Δ34.5 was almost undetectable in ganglia of RNase L−/− and all wild-type mouse strains but was restored to control virus levels in PKR−/− and IFN-αβγR−/− mice. Thus, null mutation in PKR, but not RNase L, restored normal growth of a virus lacking ICP34.5, thereby meeting the first and second criteria stated above.
Figure 2.
Replication of control virus (closed squares) and Δ34.5 (open triangles) in corneas. (A) Growth in wild-type 129/B6 mice (one experiment with four to six samples per time point; P < 0.01 by two-tailed t test). (B) PKR-deficient mice (three experiments with four samples per time point in each; P > 0.8). (C) Wild-type 129 mice (two experiments with four samples per time point in each; P < 0.01). (D) IFN-αβγ receptor-deficient mice (two experiments with four samples per time point in each; P > 0.27). (E) Wild-type C57BL/6 mice (one experiment with four to six samples per time point; P < 0.01). (F) RNase L-deficient mice (three experiments with four samples per time point in each; P < 0.02). All data were analyzed by two-tailed t tests, and values shown are logarithmic means (± SEM). Control virus and Δ34.5 are both in the background of strain 17. Limit of detection is 10 pfu.
Figure 3.
Replication of control virus (solid bars) and Δ34.5 (shaded bars) in trigeminal ganglia 3 days after infection of wild-type 129/B6 mice (one experiment; four samples per time point; P < 0.001), PKR-deficient mice (three experiments; four samples per time point in each; P > 0.9), wild-type 129 mice (two experiments with four samples per time point in each; P < 0.001), IFN-αβγ receptor-deficient mice (two experiments with four samples per time point in each; P > 0.3), wild-type C57BL/6 mice (one experiment with six samples per time point; P < 0.001), and RNase L-deficient mice (three experiments with four samples per time point in each; P < 0.01). Values shown are logarithmic means (± SEM). Control virus and Δ34.5 are both in the background of strain 17.
Normalization of Growth by Deletion of PKR Is Specific to Viruses Lacking ICP34.5.
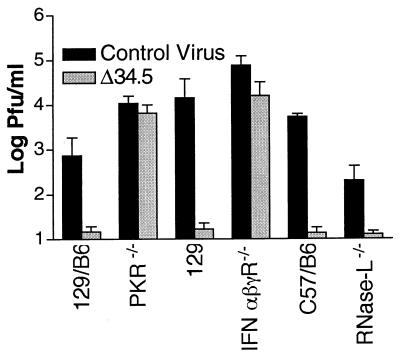
We considered the possibility that PKR is key for antiviral effects of IFN against all attenuated viruses whose replication can be enhanced significantly in mice lacking the IFN receptors. Two other attenuated viruses, the thymidine kinase (tk) null mutant dl8.36tk (termed Δtk) and the virion host shutoff (vhs) null mutant UL41NH (termed here Δvhs), were therefore used as controls for the specificity of the ICP34.5-PKR relationship. Replication of Δtk and Δvhs in corneas and ganglia of wild-type mice was reduced relative to KOS (Fig. 4 A and D). In corneas and trigeminal ganglia of IFN-αβγR−/− mice, replication of Δtk and Δvhs was enhanced, although not restored completely (Fig. 4 B and D). In contrast, deletion of PKR did not (P > 0.05) enhance the growth of either virus significantly (Fig. 4 C and D), in striking contrast to results obtained with Δ34.5 (Figs. 1 and 2). Both of these mutants (Δtk and Δvhs), however, are in the background of HSV-1 KOS, whereas Δ34.5 and its control virus are in the background of strain 17 syn+. We therefore considered the hypothesis that the lack of phenotypic restoration of Δtk and Δvhs was due to these viruses being in the background of a different, less virulent strain. To address this hypothesis, PKR−/− and 129/B6 mice were infected with a tk mutant (termed 17Δtk) or marker-rescued virus (termed Δtk17R) made in the background of strain 17 syn+, the same background as Δ34.5 and its control virus. In corneas and trigeminal ganglia (Fig. 4 E–G), the replication of 17Δtk was not enhanced in PKR−/− mice compared with that in 129/B6 control mice. This result demonstrated that deletion of PKR reverses the growth-deficient phenotype of the ICP34.5 mutant and does not result in a generalized increase in growth of all attenuated viruses, thereby meeting the third of the criteria stated above.
Figure 4.
Replication of KOS, Δtk, Δvhs, 17Δtk, and 17ΔtkR in corneas and trigeminal ganglia. Replication in corneas is shown for KOS (closed squares), Δtk (open triangles), and Δvhs (open circles) in wild-type 129 mice (A), IFN-αβγR−/− mice (B), and PKR-deficient mice (C). (D) Growth in trigeminal ganglia of wild-type 129, PKR-deficient, and IFN-αβγ receptor-deficient mice of KOS (solid bars), Δtk (shaded bars), and Δvhs (open bars). Replication in corneas is also shown for 17Δtk (open triangles) and 17ΔtkR (closed squares) in wild-type 129/B6 mice (E) and PKR−/− mice (F). (G) Growth in trigeminal ganglia of wild-type 129/B6 and PKR-deficient mice of 17ΔtkR (shaded bars) and 17Δtk (solid bars). Values shown are logarithmic means from four to six samples (± SEM). Δtk and Δvhs are in the background of strain KOS. 17Δtk and 17ΔtkR are in the background of strain 17.
Specific Normalization of Virulence of an ICP34.5− Virus in PKR−/− Mice.
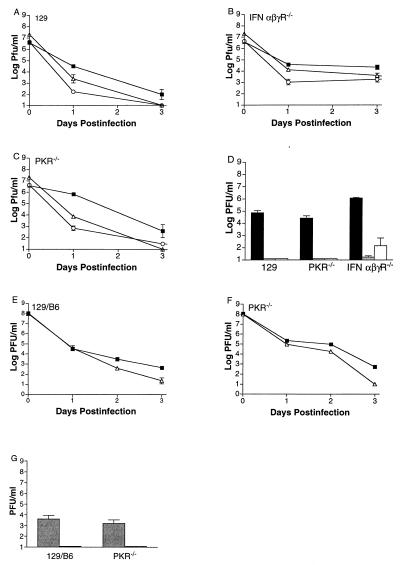
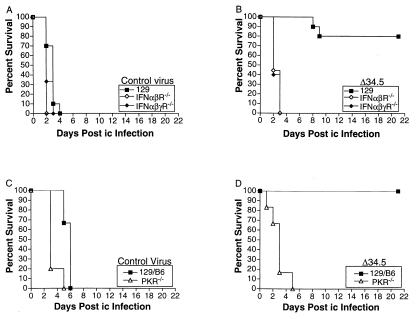
In addition to reduced growth, HSV strains lacking ICP34.5 are 104- to 106-fold less neurovirulent than control viruses as measured by lethality to mice after corneal or intracerebral (i.c.) infection (7, 8). We reasoned, therefore, that if ICP34.5 negates PKR in vivo, the removal of PKR from mice should restore lethality completely to the Δ34.5 virus. The virulence of Δ34.5 and the control virus was therefore assessed by measuring survival of mice after i.c. or corneal infection. Given that the activation and antiviral activity of PKR depend on IFNs and their receptors, we first assessed the lethality of Δ34.5 and the control virus after i.c. infection of IFNR−/− mice. After i.c. injection with the control virus, 100% of 129 wild-type, IFN-αβR−/−, and IFN-αβγR−/− mice died within 4 days of infection (Fig. 5A). Infection of IFN-αβR−/− and IFN-αβγR−/− mice with Δ34.5 resulted in 100% death within 3 days of infection but only 20% death of 129 wild-type mice (Fig. 5B). Similar data were obtained after infection of PKR−/− mice. After i.c. injection with the control virus, 100% of 129/B6 wild-type and PKR−/− mice died within 6 days of infection (Fig. 5C). After i.c. injection with the Δ34.5 virus, 100% of PKR−/− mice died within 5 days of infection, but none of the 129/B6 wild-type mice died (Fig. 5D). All of the aforementioned experiments were performed in duplicate with an input dose of 2 × 104 pfu. Identical results were obtained in multiple experiments performed at a dose of 2 × 105 pfu (data not shown). These data show that deletion of IFN receptors or PKR can restore virulence to an ICP34.5 null mutant.
Figure 5.
Survival of wild-type, IFN receptor-deficient, and PKR-deficient mice after intracerebral infection. After intracerebral infection, survival of wild-type 129 (closed squares), IFN-αβ receptor-deficient (open diamonds), and IFN-αβγ receptor-deficient (closed diamonds) mice is shown for control virus (A) and Δ34.5 (B). Survival of wild-type 129/B6 (closed squares) and PKR-deficient (open triangles) mice is shown for control virus (C) and Δ34.5 virus (D). At least 10 mice were used over two experiments to generate the data shown. Control virus and Δ34.5 are both in the background of strain 17.
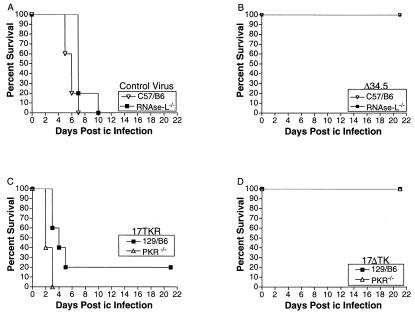
We considered the hypothesis that deletion of other IFN-dependent antiviral pathways, independent of PKR, could restore virulence to Δ34.5. We therefore examined the virulence of Δ34.5 and control virus in RNase L−/− mice (Fig. 6). Control virus killed all mice (RNase L−/− or C57BL/6J wild type) within 10 days (Fig. 6A). In contrast, all mice survived after infection with Δ34.5 (Fig. 6B). This result showed that ICP34.5-dependent neurovirulence could not be restored via deletion of RNase L. We considered a further caveat that deletion of PKR could restore virulence to attenuated viruses lacking genes other than ICP34.5. To test this caveat, PKR−/− and wild-type mice were i.c. infected with an avirulent recombinant virus lacking tk (17Δtk) and a marker-rescued control virus (17ΔtkR). Both viruses were in the background of HSV-1 strain 17 syn+ and therefore isogenic with Δ34.5. After injection of 17ΔtkR, 100% of PKR−/− and 80% 129/B6 mice died, whereas no mice died after injection of 17Δtk (Fig. 6 C and D). These data show that deletion of PKR does not restore virulence to a virus lacking a gene unrelated to ICP34.5.
Figure 6.
Survival of wild-type, RNase L-deficient, and PKR-deficient mice after intracerebral infection. After intracerebral infection, survival of wild-type C57BL/6 (open triangles) and RNase L-deficient (closed squared) mice is shown for control virus (A) and Δ34.5 (B). Survival of wild-type 129/B6 (closed squares) and PKR-deficient (open triangles) mice is shown for 17Δtk (C) and 17ΔtkR virus (D). At least 10 mice were used over two experiments to generate each curve. Control virus, Δ34.5, 17Δtk, and 17ΔtkR are all in the background of strain 17.
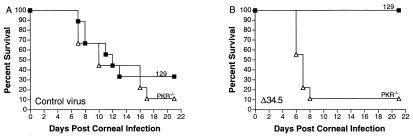
Finally, we wished to examine whether the restoration of virulence to Δ34.5 by deletion of PKR could be demonstrated by using a more physiological route of infection, via the periphery. After corneal infection with the control virus, survival kinetics of wild-type or PKR−/− mice were indistinguishable with 70–90% of mice dying within 21 days (Fig. 7A). In contrast, none of the wild-type mice died after Δ34.5 infection, but 90% of the PKR−/− mice infected with Δ34.5 died within 21 days (Fig. 7B). The restoration of virulence is therefore independent of the route of infection. In addition, the similarity of the survival curves for the wild-type and PKR−/− mice infected with the control virus indicates that ICP34.5 is a highly efficient inactivator of the antiviral effects of PKR. Taken together, these data show that deletion of PKR or IFN receptors, but not RNase L, reverses the attenuated virulence phenotype of the Δ34.5 mutant and that phenotypic restoration is specific to viruses lacking ICP34.5.
Figure 7.
Survival of wild-type 129 and PKR-deficient mice after corneal infection. Survival of wild-type 129 (closed squares) and PKR-deficient (open triangles) mice is shown for control virus (A) and Δ34.5 (B). At least 10 mice were used over two experiments to generate each curve. Control virus and Δ34.5 are both in the background of strain 17.
Discussion
Our results show the feasibility of a dual genetic approach involving mutagenesis of both pathogen and host for the definition of pathways and precise molecular targets in disease. We therefore propose three general criteria for such future definitions. First, the attenuation of the specifically mutated virus must be restored in terms of growth and virulence in a host lacking the target of the mutated viral gene. Second, the growth and virulence of the specifically mutated virus must not be restored in a host lacking other viral resistance pathways that are independent of the pathway in question. Third, attenuated viral strains with mutations in genes irrelevant to the one in question must remain attenuated in a host deleted of the resistance factor being analyzed. The second and third criteria are especially important for proof of specificity. They ensure that the particular deficient host does not restore every attenuated virus to full virulence and that the restoration of virulence in that host is specific to a particular virulence determinant. It should be noted that three criteria for proof that a gene has immunoregulatory function have been suggested previously (25). These were, first, that mutation of the viral gene should not affect growth in vitro. Second, a targeted revertant of the recombinant virus should display no phenotypic difference from wild type. This particular criterion is the accepted standard for studies of viral genetics in which marker rescue of the mutated virus is performed to ensure that its phenotype is due only to the intended mutation and not due to some secondary unanticipated mutation. This criterion was met for all mutant viruses used in this study. Third, attenuation caused by the lack of the immunomodulatory function should be phenotypically complemented in a host that is unable to exert the resistance that is targeted by the mutated viral gene. Our studies reported herein therefore complement and significantly extend these previous criteria in providing important genetic criteria for determining mechanisms of microbial virulence in vivo.
We included controls to address two additional important issues, the possible contribution of virus strain and mouse strain to our observed results. By including the attenuated mutant virus 17Δtk, constructed in the same background (strain 17syn+) as the Δ34.5 mutant under study, we ruled out the possibility that deletion of PKR restores virulence to all strain 17-derived viruses. We also considered the hypothesis that the restoration of growth and virulence to Δ34.5 in PKR−/− mice was due to a secondary host gene differing between 129 and B6 mouse strains. We observed, however, that Δ34.5 is attenuated in 129, B6, and 129/B6 F1 hybrids, indicating that there is no gene or combination of genes that can normalize the growth or virulence of the Δ34.5 virus. This finding allows us to conclude that deletion of PKR is specifically responsible for restoration of the attenuated phenotype of the ICP34.5 mutant virus.
A number of previous studies have been performed in which the reversal of attenuation of viruses lacking certain virulence genes has been attempted or accomplished by removal or depletion of the presumed host targets of such virulence genes (25–29). In one of these studies (26), it was shown that an attenuated murine cytomegalovirus strain lacking the Fc receptor gene did not show the expected restoration of virulence in antibody-deficient mice, indicating the necessity for careful in vivo genetic analysis of host–pathogen interactions. Other studies have successfully shown restoration of virulence of murine cytomegalovirus and HSV-1 mutants but did not fulfill the second and third criteria listed above, thereby lacking absolute evidence of the specificity of viral–host factor interactions in question (25, 27, 29). In contrast, all criteria were met in a study in which the virulence of an HSV-1 ICP47-deficient virus, but not a glycoprotein E-deficient virus, was restored by depletion of CD8+, but not CD4+ T cells (28), although the specific pathways presumably targeted by ICP47, the TAP transporters, were not mutated in the animals used. In this study, we have shown that deletion of PKR, but not RNase L, reversed the attenuated phenotype of an HSV-1 ICP34.5 null mutant with restoration of both growth and neurovirulence. The growth and virulence of null mutants in unrelated viral virulence genes were not restored in PKR-deficient mice. This result is in contrast to previous results with IFN receptor-deficient mice in which the growth of several mutants was enhanced significantly (13). Loss of PKR therefore does not nonspecifically restore virulence to all attenuated viruses. Furthermore, we demonstrated that the lack of PKR pathway in the host did not result in enhanced replication or virulence of the control virus. This demonstration implies that ICP34.5 is a highly efficient inactivator of the antiviral effects of PKR. These data formally show that the PKR pathway is the specific target for ICP34.5 in vivo and verify the mechanism by which ICP34.5 mutants are attenuated.
Acknowledgments
This work was supported by grants from the National Institutes of Health to D.A.L. (EY 10707 and EY 09083), B.R.G.W. (AI34039), R.H.S. (NEI 44059), and H.W.V. (AI39616). Core grant support from the National Eye Institute and Research to Prevent Blindness to the Department of Ophthalmology is acknowledged. We acknowledge Margaret Yu and Andy O'Guin for technical assistance and Rick Thompson for generously providing recombinant viruses. We also thank Sam Speck, Peggy MacDonald, Lynda Morrison, and their labs for their input throughout this project.
Abbreviations
- HSV
herpes simplex virus
- PKR
double-stranded RNA-dependent protein kinase R
- pfu
plaque-forming unit
- i.c.
intracerebral
- ICP
infected cell protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100415697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100415697
References
- 1.Stark G, Kerr L, Williams B R G, Silverman R H, Schreiber R. Annu Rev Biochem. 1998;67:27–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Vilcek J, Sen G C. In: Virology. Fields B N, editor. Philadelphia: Lippencott-Raven; 1996. pp. 375–399. [Google Scholar]
- 3.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 4.Chou J, Chen J J, Gross M, Roizman B. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He B, Gross M, Roizman B. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J, Roizman B. Proc Natl Acad Sci USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou J, Kern E R, Whitley R J, Roizman B. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 8.Bolovan C A, Sawtell N M, Thompson R L. J Virol. 1994;68:48–55. doi: 10.1128/jvi.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts W K, Hovanessian A, Brown R E, Clemens M J, Kerr I M. Nature (London) 1976;264:477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- 10.Gale M, Katze M G. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Yang Y-L, Flati V, Der S, Kadereit S, Haque J, Deb A, Reis L, Weismann C, Williams B R G. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He B, Gross M, Roizman B. J Biol Chem. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- 13.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman M J, Virgin H W. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader K A, Ackland-Berglund C, Miller J, Pepose J, Leib D A. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 15.Strelow L I, Leib D A. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplitt M G, Tjuvajev J G, Leib D A, Berk J, Pettigrew K D, Posner J B, Pfaff D W, Rabkin S D, Blasberg R G. J Neuro-Oncol. 1994;19:137–147. doi: 10.1007/BF01306455. [DOI] [PubMed] [Google Scholar]
- 17.Thompson R L, Sawtell N M. J Virol. 2000;74:965–974. doi: 10.1128/jvi.74.2.965-974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y L, Reis L F, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Nat Genet. 1997;6:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 20.Zhou A, Paranjape J, Brown T L, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, et al. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemens M, Williams B R G. Cell. 1978;13:565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou A, Paranjape J M, Der S D, Williams B R, Silverman R H. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- 23.Whitley R J, Kern E R, Chatterjee S, Chou J, Roizman B. J Clin Invest. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perng G C, Ghiasi H, Slanina S M, Nesburn A B, Wechsler S L. J Virol. 1996;70:2883–2893. doi: 10.1128/jvi.70.5.2883-2893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krmpotic A, Messerle M, Crnkovic-Mertens I, Polic B, Jonjic S, Koszinowski U H. J Exp Med. 1999;190:1285–1296. doi: 10.1084/jem.190.9.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crnkovic-Mertens I, Messerle M, Milotic I, Szepan U, Kucic N, Krmpotic A, Jonjic S, Koszinowski U H. J Virol. 1998;72:1377–1382. doi: 10.1128/jvi.72.2.1377-1382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell H E, Vally H, Lynch D M, Fleming P, Shellam G R, Scalzo A A, Davis-Poynter N J. Nature (London) 1997;386:510–514. doi: 10.1038/386510a0. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith K, Chen W, Johnson D C, Hendricks R L. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris J D, Friedman H M. J Exp Med. 1999;190:1637–1646. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]