Abstract
Background and aims
Dietary folate is believed to protect against colorectal cancer (CRC). However, few studies have addressed the role of circulating levels of folate. The aim of this study was to relate prediagnostic plasma folate and homocysteine concentrations and the methylenetetrahydrofolate reductase (MTHFR) 677C>T and 1298A>C polymorphisms to the risk of developing CRC.
Subjects
Subjects were 226 cases and 437 matched referents from the population based Northern Sweden Health and Disease Cohort.
Results
We observed a bell‐shaped association between plasma folate concentrations and CRC risk; multivariate odds ratio for middle versus lowest quintile 2.00 (95% confidence interval (CI) 1.13–3.56). In subjects with follow up times greater than the median of 4.2 years however, plasma folate concentrations were strongly positively related to CRC risk; multivariate odds ratio for highest versus lowest quintile 3.87 (95% CI 1.52–9.87; p trend = 0.007). Homocysteine was not associated with CRC risk. Multivariate odds ratios for the MTHFR polymorphisms were, for 677 TT versus CC, 0.41 (95% CI 0.19–0.85; p trend = 0.062), and for 1298 CC versus AA, 1.62 (95% CI 0.94–2.81; p trend = 0.028). Interaction analysis suggested that the result for 1298A>C may have been largely due to linkage disequilibrium with 677C>T. The reduced CRC risk in 677 TT homozygotes was independent of plasma folate status.
Conclusions
Our findings suggest a decreased CRC risk in subjects with low folate status. This possibility of a detrimental component to the role of folate in carcinogenesis could have implications in the ongoing debate in Europe concerning mandatory folate fortification of foods.
Keywords: colorectal neoplasm, folate, homocysteine, methylenetetrahydrofolate reductase, risk factors
Folate, a B vitamin found primarily in fruits and vegetables, particularly in leafy greens, is believed to have a protective role in colorectal cancer (CRC) development. This view is supported by several prospective studies of folate intake1,2,3,4,5,6 as well as human intervention studies of folate supplementation on surrogate endpoints or provisional markers of CRC,7,8,9 and studies using animal models.7,10
Although the evidence for a protective role for folate in CRC is convincing, epidemiological studies have focused overwhelmingly on dietary intake. In fact, to date only three prospective studies have assessed the effects of circulating folate levels on risk of CRC. The Physicians' Health Study of men in the United States reported an increased risk of borderline statistical significance in subjects with plasma folate concentrations indicative of deficiency11 whereas the Alpha‐Tocopherol Beta‐Carotene Study of male smokers in Finland found no association between serum folate concentrations and risk.12 A statistically significant reduced CRC risk for the highest versus lowest quartile of serum folate was noted in the New York Women's Health Study.13
The putative involvement of folate in CRC is based on its critical function as donor of one carbon groups for nucleotide synthesis and methylation reactions. The distribution of folate between these two pathways is determined in part by the activity of the methylenetetrahydrofolate reductase (MTHFR) enzyme. The substrate of the irreversible MTHFR reaction, 5,10‐methylenetetrahydrofolate, promotes nucleotide synthesis. Especially important is the synthesis of thymidylate from uracil, which minimises uracil misincorporation in DNA and thus also double strand breaks, and benefits mismatch repair. The product of the MTHFR reaction, 5‐methyltetrahydrofolate, provides one carbon groups for the methylation of the amino acid homocysteine to methionine, a reaction in which vitamin B12 acts as a coenzyme. Methionine is the precursor to S‐adenosylmethionine, the universal one carbon donor for methylation reactions, including DNA methylation.
A common polymorphism in the MTHFR gene, 677C>T, results in a thermolabile enzyme with a reduction in activity of up to 70% in TT homozygotes.14 A second polymorphism, 1298A>C, has also been found to affect enzyme function, although to a lesser extent than 677C>T.15,16 In CRC, evidence suggests a protective effect for the MTHFR 677C>T polymorphism,11,17,18,19,20,21,22 However, a few large studies have been less convincing.23,24,25,26 Some reports suggest that the putative protective role may be limited to individuals with high folate status,11,21,22 presumably because 5,10‐methylenetetrahydrofolate levels are sufficient to overcome the reduced enzyme activity, resulting in adequate methylation and enhanced DNA synthesis and repair. This effect may be counteracted by low folate status, since in this case the mutation reduces an already low availability of one carbon groups for methylation reactions. Studies of the 1298A>C polymorphism have been less frequent and less consistent in their findings17,18,20,21,23,27,28 but inverse associations with CRC risk have been reported somewhat more frequently than positive associations.
Global DNA hypomethylation, associated with genome instability, is a common trait in tumours.29 Hypermethylation of specific cytosine and guanine rich regions present in the promoters of approximately half of all human genes, called CpG islands, is also believed to be important in cancer development. CpG islands act as molecular switches, silencing gene expression when methylated. In the case of tumour suppressors, for example, promoter hypermethylation can thus aid tumorigenesis.29 Although dietary methyl group supplementation has been reported to increase both genomic and promoter DNA methylation,30,31 leading to gene silencing in the latter case, the extent to which folate status influences DNA methylation remains to be established.
The aim of this population based nested case referent study was to relate prediagnostic concentrations of plasma folate and homocysteine and the MTHFR 677C>T and 1298A>C polymorphisms to risk of developing CRC.
Methods
Study population
This prospective study was based on the Northern Sweden Health and Disease Cohort, comprising three subcohorts, the Northern Sweden WHO Monitoring of Trends and Cardiovascular Disease (MONICA) Study, the Västerbotten Intervention Project (VIP), and the local Mammography Screening Project (MSP).32 In the MONICA cohort, 2000 or 2500 randomly selected 25–74 year olds living in Västerbotten and Norrbotten, the two northernmost counties in Sweden, were invited to participate in a health survey every four or five years from 1986 (mean participation rate 77%). In the VIP, founded in 1985, all residents of Västerbotten were invited to a similar health survey at their primary health care centre on turning 40, 50, and 60 years old (mean participation rate 59%). Comparisons of social characteristics between participants and non‐participants have shown little evidence of selection bias.33 As part of both programmes, participants were invited to complete an extensive lifestyles questionnaire, including a food frequency questionnaire, and to donate a fasting blood sample to the Northern Sweden Medical Biobank for use in future research. In the MSP, founded in 1995, all women in Västerbotten within the age range of approximately 50–70 years were invited to undergo mammography every two or three years. Age limits and frequency of examination have varied over the years, depending on the availability of public funds (participation in screening 85%, participation in screening and donation of blood sample 33%). By 2002, approximately 130 000 blood samples had been donated by 85 000 individuals to the Northern Sweden Health and Disease Cohort.
Selection of study subjects
CRC cases (ICD0 18.2–18.9 for colon, 19.9 and 20.9 for rectum) diagnosed between 1985 and 2002 were identified by linking the Northern Sweden Health and Disease Cohort with the National Cancer Registry, using Swedish personal numbers as the matching variable. Verification of diagnosis by histopathology was an inclusion criterion for the study, and questionable diagnoses were reviewed by a pathologist. Tumour site and clinical stage (Dukes') were extracted from pathology reports and patient charts. For each case, two referents were matched for sex, age (one year), subcohort, date of health survey (±6 months, generally to within one or two months), and fasting status at sample donation. Referents were alive and free from cancer at the time of CRC diagnosis of the matched case. Exclusion criteria for the study subjects were previous colorectal cancer diagnosis, prioritisation to other studies, insufficient plasma sample, and lack of at least one matching referent.
The study protocol was approved by the Research Ethics Committee of Umeå University, Umeå, Sweden, and the data handling procedures were approved by the National Computer Data Inspection Board.
Blood sampling and laboratory procedures
In the VIP and MONICA subcohorts, blood samples were obtained in the morning; 94.4% of subjects from these subcohorts had fasted for at least four hours (64.6% for at least eight hours) before blood samples were taken. For subjects from the MSP subcohort, information concerning fasting status was generally not obtained, and blood samples were collected throughout the day. All MSP subjects were thus considered to have fasted for 0–4 hours. The distribution of fasting durations for the full study group was: 0–4 hours, 30.8%; 4–8 hours, 22.5%; and at least 8 hours, 46.8%.
Venous blood samples were drawn into evacuated glass tubes. Heparinised plasma was obtained by centrifugation at 1500 g for 15 minutes, aliquoted, and stored at −80°C. Plasma folate was analysed by Quantaphase II radioassay (BioRad Diagnostic Group, California, USA), and total plasma homocysteine was measured by a fluorescence polarisation immunoassay on an IMx unit (Abbott Laboratories, Illinois, USA). Plasma specimens were analysed in matched triplets of one case and two referents, with the position of the cases varied at random within each triplet to avoid systemic bias and interassay variability. The investigators and laboratory staff were blinded to case and referent status. Intra‐assay coefficients of variation for folate were 3.5% and 5.4% for control samples with mean concentrations of 3.9 nmol/l and 21.0 nmol/l, respectively. Intra‐assay coefficients of variation for homocysteine were 2.2% and 1.6% for control samples with mean concentrations of 13.5 μmol/l and 27.1 μmol/l, respectively. Plasma analyses were performed at the Department of Medical Biosciences, Clinical Chemistry, Umeå University, Sweden.
MTHFR 677C>T and 1298A>C genotypes were generated using the TaqMan allelic discrimination method at the Centre for Genome Research, Department of Medical Biosciences, Umeå University, Sweden. TaqMan assays and reagents were from Applied Biosystems (Foster City, California, USA). PCR reactions were performed on GeneAmp PCR system 9700, and PCR programmes were according to the manufacturer (ABI). PCR products were analysed on an ABI PRISM 7900HT Sequence Detection System.
Statistical analysis
Baseline characteristics and study variables for cases and referents were compared by the Mann‐Whitney and χ2 tests. Plasma folate and homocysteine were divided into quintiles based on the variable distribution of the referent subjects. Quintile cut offs were determined separately for men and women. Linkage disequilibrium and haplotype frequencies for the MTHFR polymorphisms were calculated using the Haploview 3.2 software package.34 Odds ratios (OR) for disease and 95% confidence intervals (CI) were calculated by conditional logistic regression, and tests for trend were performed by treating the categories of plasma variables (labelled 1–5 for lowest to highest) and the MTHFR genotypes (labelled 1–3 for wild‐type, heterozygous, and homozygous mutant) as continuous linear variables in regression analyses. Missing values were treated as a separate category for each variable in multivariate analyses. Statistical tests and corresponding p values were two sided, and p values less than 0.05 were considered statistically significant. SPSS version 13.0 (Chicago, Illinois, USA) was used for all statistical analyses.
Results
A total of 260 CRC cases were identified and verified. Of these, 34 met one or more of the exclusion criteria, resulting in 226 CRC cases and 437 matched referents for inclusion in the study. DNA samples were unavailable for six cases and 22 referents. Baseline characteristics of the study subjects, as well as tumour site and stage, are presented in table 1. Due to the all female MSP subcohort, women were somewhat overrepresented in the Northern Sweden Health and Disease Cohort and thus also in this study (60% of cases). Median age (25th–75th percentiles) of cases at recruitment to the cohort was 59.8 (50.3–60.2) years for the full study group and 63.4 (57.7–68.0) years for the MSP. Median follow up time between recruitment to the cohort and diagnosis of CRC was 4.2 (2.4–6.7) years for the full study group and 2.9 (1.4–4.2) years for the MSP. No statistically significant differences in median concentrations of plasma folate or homocysteine were observed between cases and referents. Plasma folate concentrations did not vary by fasting duration but homocysteine concentrations were significantly higher in subjects who had fasted less than four hours (p = 0.600 for folate, p<0.001 for homocysteine; Kruskal‐Wallis test).
Table 1 Baseline characteristics of colorectal cancer cases and their matched referents, and tumour characteristics of cases.
| Cases | Referents | p Value* | |||
|---|---|---|---|---|---|
| No | Median or % | No | Median or % | ||
| Sex (M/F) (%) | 94/132 | 41.6/58.4 | 184/253 | 42.1/57.9 | 0.899 |
| Age (y)† | 226 | 59.8 (50.3–60.2) | 437 | 59.7 (50.2–60.2) | 0.661 |
| Follow up time (y)† | 226 | 4.2 (2.4–6.7) | |||
| Body mass index (kg/m2)† | 182 | 26.1 (23.5–29.0) | 350 | 25.9 (23.8–28.3) | 0.617 |
| P‐Folate (nmol/l)† | 221 | 8.1 (6.2–10.3) | 432 | 7.8 (5.8–11.1) | 0.704 |
| P‐Homocysteine (μmol/l)† | 226 | 11.7 (10.0–13.8) | 436 | 11.4 (9.6–13.8) | 0.312 |
| Current smoker (%) | 218 | 38 (17.4) | 424 | 90 (21.2) | 0.254 |
| Tumour site (%) | |||||
| Right colon | 72 | 31.9 | |||
| Left colon | 70 | 31.0 | |||
| Rectum | 84 | 37.2 | |||
| Dukes' stage‡ (%) | |||||
| A | 41 | 18.4 | |||
| B | 81 | 36.3 | |||
| C | 66 | 29.6 | |||
| D | 35 | 15.7 | |||
*Mann‐Whitney or χ2 test.
†Median (25th–75th percentile).
‡Dukes' stage could not be determined for three cases with only biopsy tissue available.
Plasma folate concentrations were significantly related to the risk of CRC in a bell‐shaped manner (table 2). Univariate ORs were, for the middle versus the lowest quintile, 2.26 (95% CI 1.29–3.95), and for the highest versus the lowest quintile, 1.55 (95% CI 0.85–2.85). Conditional logistic regression analysis was also performed using cut offs selected to explore the effects over a greater range of plasma folate concentrations. In this case, the OR for the highest versus the lowest category (⩾15.0 versus <5 nmol/l) was 1.10 (95% CI 0.52–2.32). Risk estimates were attenuated somewhat by adjustment for body mass index (BMI), current smoking, recreational and occupational physical activity levels, and alcohol consumption. In contrast with the findings for folate, plasma homocysteine concentrations were not significantly associated with CRC risk (table 2).
Table 2 Odds ratios for colorectal cancer by categories of plasma folate and homocysteine.
| Folate quintiles* | Q1 | Q2 | Q3 | Q4 | Q5 | p Trend |
| Cases/referents | 27/86 | 50/87 | 56/86 | 51/87 | 37/86 | |
| Unadjusted OR (CI) | 1.00 | 1.85 (1.07–3.19) | 2.26 (1.29–3.95) | 1.97 (1.12–3.47) | 1.55 (0.85–2.85) | 0.173 |
| Adjusted OR† (CI) | 1.00 | 1.72 (0.99–3.03) | 2.00 (1.13–3.56) | 1.87 (1.04–3.36) | 1.34 (0.72–2.50) | 0.325 |
| Folate categories‡ | <5 nmol/l | 5–8 nmol/l | 8–12 nmol/l | 12–15 nmol/l | ⩾15 nmol/l | p Trend |
| Cases/referents | 20/60 | 86/162 | 76/121 | 22/37 | 17/52 | |
| Unadjusted OR (CI) | 1.00 | 1.54 (0.88–2.70) | 1.86 (1.04–3.32) | 1.88 (0.89–3.96) | 1.10 (0.52–2.32) | 0.597 |
| Adjusted OR† (CI) | 1.00 | 1.52 (0.85–2.70) | 1.81 (0.99–3.29) | 1.71 (0.79–3.69) | 1.01 (0.47–2.19) | 0.734 |
| Hcys quintiles§ | Q1 | Q2 | Q3 | Q4 | Q5 | p Trend |
| Cases/referents | 39/86 | 37/88 | 54/88 | 52/88 | 44/86 | |
| Unadjusted OR (CI) | 1.00 | 0.93 (0.54–1.56) | 1.33 (0.80–2.21) | 1.30 (0.77–2.19) | 1.12 (0.65–1.95) | 0.376 |
| Adjusted OR† (CI) | 1.00 | 0.87 (0.50–1.49) | 1.26 (0.75–2.11) | 1.27 (0.74–2.17) | 1.12 (0.63–1.99) | 0.354 |
OR, odds ratio; CI, 95% confidence interval; Hcys, homocysteine.
*Folate quintile cut offs were for men: 5.1, 6.7, 8.1, and 11.3; and for women: 5.7, 7.4, 9.4, and 13.0 nmol/l.
†Adjusted for body mass index, current smoking, recreational and occupational physical activity, and alcohol intake (alcohol data were available for approximately two thirds of subjects).
‡Categories selected to explore a greater range of plasma folate concentrations.
§Homocysteine quintile cut offs were for men: 9.7, 10.9, 12.4, and 15.1; and for women: 8.8, 10.3, 12.0, and 14.1 μmol/l.
Subgroup analyses for various baseline and clinical characteristics are presented in table 3. In subjects with follow up times above the median of 4.2 years, plasma folate concentrations demonstrated a strong, positive, linear association with CRC risk (multivariate OR for highest versus lowest quintile 3.87 (95% CI 1.52–9.87); p trend = 0.007).
Table 3 Multivariate odds ratios (95% confidence intervals) for colorectal cancer by quintiles of folate after stratification by baseline and tumour characteristics.
| Plasma folate* | Q1 | Q2 | Q3 | Q4 | Q5 | p Trend |
|---|---|---|---|---|---|---|
| Sex: men | 1.00 | 2.71 (0.97–7.53) | 1.81 (0.62–5.26) | 2.16 (0.74–6.35) | 1.78 (0.59–5.39) | 0.726 |
| Sex: women | 1.00 | 1.17 (0.57–2.42) | 1.96 (0.94–4.09) | 1.50 (0.70–3.21) | 0.97 (0.42–2.17) | 0.698 |
| Age ⩽ 59 y† | 1.00 | 1.26 (0.50–3.15) | 1.66 (0.66–4.18) | 1.07 (0.37–3.09) | 1.51 (0.54–4.25) | 0.590 |
| Age >59 y† | 1.00 | 2.00 (0.95–4.23) | 2.41 (1.10–5.26) | 2.73 (1.27–5.87) | 1.24 (0.53–2.89) | 0.265 |
| Follow up ⩽4.2 y | 1.00 | 1.47 (0.66–3.26) | 1.63 (0.69–3.83) | 1.70 (0.74–3.94) | 0.55 (0.21–1.44) | 0.401 |
| Follow up >4.2 y | 1.00 | 2.15 (0.91–5.08) | 2.49 (1.04–6.00) | 2.64 (1.06–6.62) | 3.87 (1.52–9.87) | 0.007 |
| Site: right colon | 1.00 | 1.44 (0.53–3.93) | 1.78 (0.56–5.63) | 2.33 (0.78–6.93) | 2.03 (0.67–6.14) | 0.126 |
| Site: left colon | 1.00 | 1.87 (0.57–6.09) | 3.98 (1.19–13.26) | 2.31 (0.71–7.49) | 1.13 (0.27–4.77) | 0.586 |
| Site: rectum | 1.00 | 1.13 (0.38–3.37) | 0.78 (0.26–2.26) | 0.99 (0.31–3.07) | 0.50 (0.15–1.55) | 0.178 |
| Stage: Dukes' A/B | 1.00 | 2.07 (0.94–4.57) | 2.26 (0.99–5.14) | 2.27 (0.96–5.35) | 1.16 (0.44–3.02) | 0.576 |
| Stage: Dukes' C/D | 1.00 | 1.14 (0.47–2.77) | 1.68 (0.72–3.91) | 1.34 (0.56–3.25) | 1.27 (0.54–3.01) | 0.586 |
*Folate quintile cut offs were for men: 5.1, 6.7, 8.1, and 11.3; and for women: 5.7, 7.4, 9.4, and 13.0 nmol/l.
All odds ratios were adjusted for body mass index, current smoking, recreational and occupational physical activity, and alcohol intake.
Case/referent frequencies: men Q1–5, 7/36, 24/36, 20/36, 23/36, 19/36, women Q1–5, 20/50, 26/51, 36/50, 28/51, 18/50; age ⩽59 years Q1–5, 12/31, 19/41, 24/37, 12/33, 19/37, age >59 years Q1–5, 15/55, 31/46, 32/49, 39/54, 18/49; cases with follow up between recruitment to the cohort and CRC diagnosis ⩽ median 4.2 years Q1–5, 16/39, 25/44, 29/41, 28/42, 12/51, cases with follow up >median 4.2 years Q1–5, 11/47, 25/43, 27/45, 23/45, 25/35; right sided colon cancer cases Q1–5, 9/30, 17/31, 13/23, 17/32, 14/23, left sided colon cancer cases Q1–5, 6/29, 16/32, 25/29, 14/24, 8/22; rectal cancer cases Q1–5, 12/27, 17/24, 18/34, 20/31, 15/41; Dukes' stage A/B cases Q1–5, 14/48, 32/52, 31/47, 27/39, 15/46, Dukes' stage C/D cases Q1–5, 13/37, 16/34, 25/38, 24/46, 21/40.
†Age 59 years was selected to approximate the median 59.8 years without dividing the study group in the middle of a large sampling cluster at 60 years.
The MTHFR 677C>T and 1298A>C polymorphisms were in Hardy‐Weinberg equilibrium in both referents and cases (p = 0.399 for 677C>T and p = 0.377 for 1298A>C for the full study group). Strong linkage disequilibrium between the two polymorphisms was also observed (D′ 1.0 (95% CI 0.96–1.0); r2 0.204; LOD 44.38). The estimated haplotype frequencies were 677C–1298A, 37.5%; 677C–1298C, 34.8%; 677T–1298A, 27.7%; and 677T–1298C, 0%. The 677C>T polymorphism, particularly TT homozygosity, was associated with lower plasma folate and higher homocysteine concentrations (Kruskal‐Wallis test, p<0.001 for both). For 1298A>C, no statistically significant association with either plasma variable was observed.
The 677C>T polymorphism was inversely related to CRC risk (OR for TT versus CC 0.44 (95% CI 0.21–0.88); p trend = 0.048), a finding that was essentially unchanged in magnitude but attenuated in significance after adjustment for BMI, smoking, recreational and occupational physical activity, and alcohol consumption (OR 0.41 (95% CI 0.19–0.85); p trend = 0.062) (table 4). In contrast with MTHFR 677C>T, a positive association with CRC risk was found for the 1298A>C mutation (OR for CC versus AA 1.52 (95% CI 0.90–2.57); p trend = 0.063), the p trend for which became statistically significant after adjustment for BMI, smoking, recreational and occupational physical activity, and alcohol consumption (p trend = 0.028) (table 4). However, in a multivariate interaction analysis of genotype combinations, with 677CC‐1298AA subjects as the reference group, ORs for the 1298A>C polymorphism were considerably lower (table 4). When MTHFR 677C>T and 1298A>C were included in the same multivariate model, risk estimates were not materially affected, but none was statistically significant (data not shown).
Table 4 Frequencies of the methylenetetrahydrofolate reductase (MTHFR) 677C>T and 1298A>C polymorphisms, and odds ratios for colorectal cancer by genotype and genotype combination.
| MTHFR 677C>T | CC | CT | TT | p Trend |
| Cases/referents (total %) | 123/212 (52.8) | 85/161 (38.7) | 12/42 (8.5) | |
| Unadjusted OR (CI) | 1.00 | 0.90 (0.63–1.27) | 0.44 (0.21–0.88) | 0.048 |
| Adjusted OR† (CI) | 1.00 | 0.91 (0.62–1.30) | 0.41 (0.19–0.85) | 0.062 |
| MTHFR 1298A>C | AA | AC | CC | p Trend |
| Frequency (total %) | 85/189 (43.3) | 103/173 (43.7) | 32/50 (13.0) | |
| Unadjusted OR (CI) | 1.00 | 1.36 (0.94–1.96) | 1.52 (0.90–2.57) | 0.063 |
| Adjusted OR† (CI) | 1.00 | 1.41 (0.96–2.06) | 1.62 (0.94–2.81) | 0.028 |
| Genotype combinations*† | 677CC | 677CT | 677TT | |
| 1298AA, OR (CI) | 1.00 | 0.76 (0.41–1.37) | 0.46 (0.20–1.04) | |
| 1298AC, OR (CI) | 1.00 (0.57–1.76) | 1.20 (0.67–2.15) | – | |
| 1298CC, OR (CI) | 1.23 (0.64–2.36) | – | – |
OR, odds ratio; CI, 95% confidence interval.
*Adjusted for body mass index, current smoking, recreational and occupational physical activity, and alcohol intake
†Case/referent frequencies: MTHFR 677CC–1298AA, 34/61; 677CT‐1298AA, 39/87; 677TT‐1298AA, 12/41; 677CC‐1298AC, 57/101; 677CT‐1298AC, 46/72; 677CC‐1298CC, 32/50.
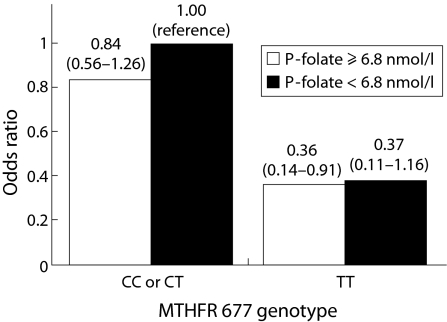
A multivariate interaction analysis of CRC risk by combinations of MTHFR 677C>T genotype and plasma folate status is presented in fig 1. Subjects were classified as having adequate or inadequate plasma folate status using a cut off of 6.8 nmol/l. For both folate categories, the CC and CT genotypes yielded very similar ORs and were therefore combined. Our observation of a reduced CRC risk in subjects homozygous for the T allele is shown here to be essentially independent of folate status.
Figure 1 Odds ratios (95% confidence interval) for colorectal cancer for combinations of plasma (P) folate status (cut off 6.8 nmol/l selected as a lower limit for adequate plasma folate status) and MTHFR 677 genotype (CC and CT yielded very similar odds ratios and were therefore combined), adjusted for body mass index, current smoking, recreational and occupational physical activity, and alcohol intake. Number of cases/referents: plasma folate ⩾6.8 nmol/l and MTHFR 677 CC or CT, 145/244; plasma folate ⩾6.8 nmol/l and MTHFR 677 TT, 4/18; plasma folate <6.8 nmol/l and MTHFR 677 CC or CT, 59/124; plasma folate <6.8 nmol/l and MTHFR 677 TT, 7/24.
Discussion
In this prospective, population based study, we report a bell‐shaped association between plasma folate concentrations and CRC risk. This observation is in contrast with considerable evidence supporting a protective effect for folate in CRC development.1,7 Results also indicated a reduced CRC risk associated with the MTHFR 677C>T polymorphism that was present even in subjects with an inadequate folate status.
This is only the second prospective CRC study to incorporate data on plasma folate levels as well as both the MTHFR 677C>T and 1298A>C polymorphisms.27 It is also the largest prospective study of circulating levels of folate and risk of CRC. Greater power would, however, have been desirable for the subgroup analyses. Although we were unable to control for a number of covariates, including other dietary intake variables, aspirin or non‐steroidal anti‐inflammatory drugs, and hereditary CRC, factors such as folate degradation during storage and seasonal fluctuations in folate status were accounted for by the matching process. Levels of red blood cell folate provide a more stable measure of folate status than do plasma concentrations but in large epidemiological studies a single measurement of plasma folate per subject appears to be adequate.35
The main strength of this study was its prospective design, which minimised the problems of selection bias and reverse causation. Given the slow growth of CRC, undiagnosed tumours in the case group at baseline almost certainly existed. However, excluding subjects with follow up times less than one year did not materially affect risk estimates (data not shown).
Our results seem to suggest a reduced CRC risk for subjects with the lowest folate levels (table 2). Although studies have provided some evidence that supraphysiological folate levels may enhance colorectal tumorigenesis,36,37,38 a cancer preventative effect for folate deficiency seems less likely. However, the MTHFR 677C>T polymorphism, which limits the accessibility of folate for methylation reactions, has been reported to decrease the risk of C→T transitions in the p53 gene.39 These mutations occur particularly often at methylated CpG island “hotspots” and are a common event in colorectal tumorigenesis. A decrease in the number and size of induced colorectal tumours in folate deficient rats has also been observed40 but a review by Kim suggested that the quality of the test and control diets rather provides further evidence for a carcinogenic effect of supraphysiological folate intakes.7
Recently, the idea that folate might protect against CRC development but aid the progression of an established neoplasm has gained attention.7,41,42 In the present study, however, a strong positive association between folate and CRC risk was apparent in subjects with follow up times over the median of 4.2 years, who would likely have had a lower prevalence of undiagnosed CRC at baseline (table 3). Folate might be speculated to promote the transition from adenoma to carcinoma, a serious consideration given the high prevalence of adenoma in western populations, including Sweden.43 However, this should also have resulted in a positive risk association in the subgroup with shorter follow up times. Of course, the observation may simply be a chance finding. It is none the less interesting and may warrant further study.
Plasma folate concentrations were considerably lower in the present study than in the two previous prospective reports of an inverse association between serum or plasma folate status and CRC risk, despite blood samples in those studies having been collected before the implementation of mandatory folic acid fortification in the USA.11,13 For example, in the New York University Women's Health Study,13 the lowest quartile cut off was comparable with our highest quintile cut off. Although our study included a greater proportion of fasting blood samples, we observed no variation in plasma folate concentrations by fasting duration. However, in northern Sweden, consumption of fruits and vegetables, major sources of folate in populations without fortification of foods, has been reported to be among the lowest in Europe.44 Our study population may thus be better suited to investigating the effects of low plasma folate concentrations on CRC risk. The low folate status of the study group may also have precluded detection of a protective effect for high plasma folate concentrations. The observed bell‐shaped, rather than linear, relationship between plasma folate concentrations and CRC risk supports this interpretation.
In the prospective Alpha‐Tocopherol Beta‐Carotene Study of male smokers in Finland,12 both plasma folate concentrations and results were comparable with those of the present study. Thus an ethnic explanation for the discrepancy between our findings and the two previous American studies cannot be excluded.11,13
In contrast with previous reports suggesting a possible positive association between homocysteine and CRC risk,13,45 no clear relationship was observed in the present study. This may have been due to the relatively high homocysteine levels of the study subjects or possibly to confounding by folate, although including both plasma folate and homocysteine concentrations in the same multivariate model did not materially affect risk estimates (data not shown).
The MTHFR 677C>T polymorphisms were associated with a decreased CRC risk in this study, which is in line with several previous reports,11,17,18,19,20,21,22 However, as demonstrated in fig 1, we found no evidence that this risk reduction might be eliminated or reversed when folate status is inadequate. This contradicts some findings,11,21,22 including those of Ma et al, in which a similar figure was presented, but not other large dietary studies.23,28 Although ours may be a chance finding due to the low number of TT homozygous subjects, it underscores the need for further investigation of the gene‐nutrient interaction.
Our observation of an increased CRC risk in subjects with the MTHFR 1298A>C polymorphism contradicts a number of previous reports of a decreased, albeit seldom statistically significant, CRC risk associated with 1298CC homozygosity,17,18,21,23,27,28 but supports one large study.20 Based on the interaction analysis in table 4, it seems the association between MTHFR 1298A>C and CRC risk may have been largely due to the strong linkage disequilibrium with the MTHFR 677C>T polymorphism.
In light of accumulating evidence that dosage and timing may be critical to the effect of folate in carcinogenesis, concerns have been raised about the consequences of folic acid fortification on cancer incidence.46 Supporting this are the results of the recent Aspirin‐Folate Polyp Prevention Trial, in which folic acid supplementation was associated with a statistically significant 44% increase in the number of recurrent adenomas.36 Our observations, which provide additional evidence for a detrimental component to the role of folate in colorectal tumorigenesis, may thus have implications in the ongoing debate in Europe concerning mandatory folic acid fortification of foods.
In conclusion, in this nested case referent study, plasma folate concentrations were associated with CRC risk in a bell‐shaped manner. In subjects with longer follow up times, plasma folate concentrations were strongly positively related to CRC risk. The MTHFR 677C>T polymorphism was associated with a reduced risk of CRC that was independent of folate status. We speculate that inadequate folate status in the background population allowed us to investigate associations at the lower end of the biological folate spectrum. Without negating the possibility of a protective effect for high folate levels, our findings suggest that low folate status may reduce the risk of CRC.
Acknowledgements
This study was financially supported by grants from the Swedish Cancer Society and the Cancer Research Foundation in Northern Sweden. We thank all participants in the Northern Sweden Health and Disease Cohort, as well as Åsa Ågren, Veronica Hellström, John Hutilainen, and Hubert Sjödin of the Northern Sweden Medical Biobank, Umeå University. Thanks also to Kerstin Näslund and Le Thu Trinh, Medical Biosciences (Pathology and Clinical Chemistry, respectively), Umeå University, for excellent technical assistance, and Hans Stenlund, Department of Public Health and Clinical Medicine, Epidemiology, Umeå University, Umeå, Sweden, for advice on statistical procedures.
Abbreviations
CRC - colorectal cancer
MTHFR - methylenetetrahydrofolate reductase
MONICA - Northern Sweden WHO Monitoring of Trends and Cardiovascular Disease Study
VIP - Västerbotten Intervention Project
MSP - Mammography Screening Project
OR - odds ratio
BMI - body mass index
Footnotes
Conflict of interest: None declared.
References
- 1.Sanjoaquin M A, Allen N, Couto E.et al Folate intake and colorectal cancer risk: a meta‐analytical approach. Int J Cancer 2005113825–828. [DOI] [PubMed] [Google Scholar]
- 2.Larsson S C, Giovannucci E, Wolk A. A prospective study of dietary folate intake and risk of colorectal cancer: modification by caffeine intake and cigarette smoking. Cancer Epidemiol Biomarkers Prev 200514740–743. [DOI] [PubMed] [Google Scholar]
- 3.Wei E K, Giovannucci E, Wu K.et al Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004108433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konings E J, Goldbohm R A, Brants H A.et al Intake of dietary folate vitamers and risk of colorectal carcinoma: results from the Netherlands Cohort Study. Cancer 2002951421–1433. [DOI] [PubMed] [Google Scholar]
- 5.Su L J, Arab L. Nutritional status of folate and colon cancer risk: evidence from NHANES I epidemiologic follow‐up study. Ann Epidemiol 20011165–72. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Stampfer M J, Colditz G A.et al Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med 1998129517–524. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y I. Role of folate in colon cancer development and progression. J Nutr 20031333731–9S. [DOI] [PubMed] [Google Scholar]
- 8.Khosraviani K, Weir H P, Hamilton P.et al Effect of folate supplementation on mucosal cell proliferation in high risk patients for colon cancer. Gut 200251195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paspatis G A, Karamanolis D G. Folate supplementation and adenomatous colonic polyps. Dis Colon Rectum 1994371340–1341. [DOI] [PubMed] [Google Scholar]
- 10.Cravo M L, Mason J B, Dayal Y.et al Folate deficiency enhances the development of colonic neoplasia in dimethylhydrazine‐treated rats. Cancer Res 1992525002–5006. [PubMed] [Google Scholar]
- 11.Ma J, Stampfer M J, Giovannucci E.et al Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res 1997571098–1102. [PubMed] [Google Scholar]
- 12.Glynn S A, Albanes D, Pietinen P.et al Colorectal cancer and folate status: a nested case‐control study among male smokers. Cancer Epidemiol Biomarkers Prev 19965487–494. [PubMed] [Google Scholar]
- 13.Kato I, Dnistrian A M, Schwartz M.et al Serum folate, homocysteine and colorectal cancer risk in women: a nested case‐control study. Br J Cancer 1999791917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frosst P, Blom H J, Milos R.et al A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 199510111–113. [DOI] [PubMed] [Google Scholar]
- 15.van der Put N M, Gabreels F, Stevens E M.et al A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural‐tube defects? Am J Hum Genet 1998621044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg I S, Jacques P F, Selhub J.et al The 1298A‐‐>C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis 2001156409–415. [DOI] [PubMed] [Google Scholar]
- 17.Kono S, Chen K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci 200596535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: A HUGE review. Am J Epidemiol 2004159423–443. [DOI] [PubMed] [Google Scholar]
- 19.Ulvik A, Vollset S E, Hansen S.et al Colorectal cancer and the methylenetetrahydrofolate reductase 677C‐‐> T and methionine synthase 2756A‐‐> G polymorphisms: a study of 2,168 case‐control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev 2004132175–2180. [PubMed] [Google Scholar]
- 20.Yin G, Kono S, Toyomura K.et al Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci 200495908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Marchand L, Donlon T, Hankin J H.et al B‐vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control 200213239–248. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Giovannucci E, Kelsey K.et al A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res 1996564862–4864. [PubMed] [Google Scholar]
- 23.Keku T, Millikan R, Worley K.et al 5,10‐methylenetetrahydrofolate reductase codon 677 and 1298 polymorphisms and colon cancer in African Americans and whites. Cancer Epidemiol Biomarkers Prev 2002111611–1621. [PubMed] [Google Scholar]
- 24.Sachse C, Smith G, Wilkie M J.et al A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis 2002231839–1849. [DOI] [PubMed] [Google Scholar]
- 25.Shannon B, Gnanasampanthan S, Beilby J.et al A polymorphism in the methylenetetrahydrofolate reductase gene predisposes to colorectal cancers with microsatellite instability. Gut 200250520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slattery M L, Potter J D, Samowitz W.et al Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 19998513–518. [PubMed] [Google Scholar]
- 27.Chen J, Ma J, Stampfer M J.et al Linkage disequilibrium between the 677C>T and 1298A>C polymorphisms in human methylenetetrahydrofolate reductase gene and their contributions to risk of colorectal cancer. Pharmacogenetics 200212339–342. [DOI] [PubMed] [Google Scholar]
- 28.Curtin K, Bigler J, Slattery M L.et al MTHFR C677T and A1298C polymorphisms: diet, estrogen, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 200413285–292. [DOI] [PubMed] [Google Scholar]
- 29.Jones P A, Baylin S B. The fundamental role of epigenetic events in cancer. Nat Rev Genet 20023415–428. [DOI] [PubMed] [Google Scholar]
- 30.Pufulete M, Al‐Ghnaniem R, Khushal A.et al Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut 200554648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterland R A, Jirtle R L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 2003235293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallmans G, Agren A, Johansson G.et al Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort‐evaluation of risk factors and their interactions. Scand J Public Health 20036118–24. [DOI] [PubMed] [Google Scholar]
- 33.Weinehall L, Hallgren C G, Westman G.et al Reduction of selection bias in primary prevention of cardiovascular disease through involvement of primary health care. Scand J Prim Health Care 199816171–176. [DOI] [PubMed] [Google Scholar]
- 34.Barrett J C, Fry B, Maller J.et al Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 200521263–265. [DOI] [PubMed] [Google Scholar]
- 35.Drogan D, Klipstein‐Grobusch K, Wans S.et al Plasma folate as marker of folate status in epidemiological studies: the European Investigation into Cancer and Nutrition (EPIC)‐Potsdam study. Br J Nutr 200492489–496. [DOI] [PubMed] [Google Scholar]
- 36.Cole B F, Baron J A, Sandler R S.et al A randomized trial of folic acid to prevent colorectal adenomas. Proc Am Assoc Cancer Res 2005464399 [Google Scholar]
- 37.Kim Y I, Salomon R N, Graeme‐Cook F.et al Dietary folate protects against the development of macroscopic colonic neoplasia in a dose responsive manner in rats. Gut 199639732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wargovich M J, Chen C D, Jimenez A.et al Aberrant crypts as a biomarker for colon cancer: evaluation of potential chemopreventive agents in the rat. Cancer Epidemiol Biomarkers Prev 19965355–360. [PubMed] [Google Scholar]
- 39.Ulrich C M, Curtin K, Samowitz W.et al MTHFR variants reduce the risk of G:C‐>A:T transition mutations within the p53 tumor suppressor gene in colon tumors. J Nutr 20051352462–2467. [DOI] [PubMed] [Google Scholar]
- 40.Le Leu R K, Young G P, McIntosh G H. Folate deficiency reduces the development of colorectal cancer in rats. Carcinogenesis 2000212261–2265. [DOI] [PubMed] [Google Scholar]
- 41.Song J, Medline A, Mason J B.et al Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res 2000605434–5440. [PubMed] [Google Scholar]
- 42.Song J, Sohn K J, Medline A.et al Chemopreventive effects of dietary folate on intestinal polyps in Apc+/Msh2−/− mice. Cancer Res 2000603191–3199. [PubMed] [Google Scholar]
- 43.Blom J, Liden A, Jeppsson B.et al Compliance and findings in a Swedish population screened for colorectal cancer with sigmoidoscopy. Eur J Surg Oncol 200228827–831. [DOI] [PubMed] [Google Scholar]
- 44.Agudo A, Slimani N, Ocke M C.et al Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr 200251179–1196. [DOI] [PubMed] [Google Scholar]
- 45.Martinez M E, Henning S M, Alberts D S. Folate and colorectal neoplasia: relation between plasma and dietary markers of folate and adenoma recurrence. Am J Clin Nutr 200479691–697. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y I. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004801123–1128. [DOI] [PubMed] [Google Scholar]