Abstract
Background
The risks and benefits of coxibs, non‐steroidal anti‐inflammatory drugs (NSAIDs), and aspirin treatment are under intense debate.
Objective
To determine the risk of peptic ulcer upper gastrointestinal bleeding (UGIB) associated with the use of coxibs, traditional NSAIDs, aspirin or combinations of these drugs in clinical practice.
Methods
A hospital‐based, case–control study in the general community of patients from the National Health System in Spain. The study included 2777 consecutive patients with endoscopy‐proved major UGIB because of the peptic lesions and 5532 controls matched by age, hospital and month of admission. Adjusted relative risk (adj RR) of UGIB determined by conditional logistic regression analysis is provided.
Results
Use of non‐aspirin‐NSAIDs increased the risk of UGIB (adj RR 5.3; 95% confidence interval (CI) 4.5 to 6.2). Among non‐aspirin‐NSAIDs, aceclofenac (adj RR 3.1; 95% CI 2.3 to 4.2) had the lowest RR, whereas ketorolac (adj RR 14.4; 95% CI 5.2 to 39.9) had the highest. Rofecoxib treatment increased the risk of UGIB (adj RR 2.1; 95% CI 1.1 to 4.0), whereas celecoxib, paracetamol or concomitant use of a proton pump inhibitor with an NSAID presented no increased risk. Non‐aspirin antiplatelet treatment (clopidogrel/ticlopidine) had a similar risk of UGIB (adj RR 2.8; 95% CI 1.9 to 4.2) to cardioprotective aspirin at a dose of 100 mg/day (adj RR 2.7; 95% CI 2.0 to 3.6) or anticoagulants (adj RR 2.8; 95% CI 2.1 to 3.7). An apparent interaction was found between low‐dose aspirin and use of non‐aspirin‐NSAIDs, coxibs or thienopyridines, which increased further the risk of UGIB in a similar way.
Conclusions
Coxib use presents a lower RR of UGIB than non‐selective NSAIDs. However, when combined with low‐dose aspirin, the differences between non‐selective NSAIDs and coxibs tend to disappear. Treatment with either non‐aspirin antiplatelet or cardioprotective aspirin has a similar risk of UGIB.
Treatment with non‐steroidal anti‐inflammatory drugs (NSAIDs) is associated with upper gastrointestinal bleeding (UGIB).1The primary reason for developing NSAIDs that selectively inhibit cyclo‐oxygenase (COX)‐2 was to reduce or eliminate adverse serious upper gastrointestinal disorders.1,2 Three outcome trials have been carried out to determine the incidence of UGIB complications with these compounds,3,4,5 but the relevance of these findings to clinical practice is unclear. As a non‐exposed control group was not included in those studies, it is not possible to directly estimate the excess risk of upper gastrointestinal complications associated with the use of coxibs. In addition, the doses of drugs used in the studies were higher than those commonly used in clinical practice. Finally, these compounds are used in “real life” in combination with other drugs (aspirin, other antiplatelet agents and anticoagulants), as well as in patients with multiple risk factors, a population inadequately represented in clinical trials.
More recently, the overall safety profile of selective COX‐2 inhibitors and traditional NSAIDs has come under intense debate, as in addition to the recently shown cardiovascular risk,6,7,8,9 the relative benefits of coxibs on the gastrointestinal tract are also being questioned.10 Therefore, it is essential to determine the actual risk of UGI complications with COX‐2 selective and traditional NSAIDs alone or combined with other compounds in a real‐life setting. Better information about the benefits and risks of the different treatments will help doctors make informed clinical decisions. The primary objective of our study was to quantify the risk of UGIB associated with coxib treatment as it is used in clinical practice. Secondary objectives were to quantify and compare the risk of UGIB associated with traditional NSAIDs, aspirin, other antiplatelet drugs, anticoagulants and various combinations of these compounds.
Patients and methods
Study design and population
A hospital based, case–control study with prospective case ascertainment and retrospective data collection was carried out between 2001 and 2004. Cases and controls were collected through a network of general hospitals integrated within the Spanish Association of Gastroenterology. Eligible participants were 20–85 years old and had been free of liver disease, coagulation disorders or malignancies for the previous 5 years.
Definitions
1. Case: A patient hospitalised because of gastrointestinal bleeding (haematemesis or melena), which was confirmed by hospital personnel through an endoscopic diagnosis as caused by peptic ulcer lesion. Cases with the following conditions were excluded: (a) any other cause of bleeding (gastro‐oesophageal varices, vascular lesions, tumours, Mallory–Weiss, associated coagulopathy and oesophagitis); (b) patients with unreliable sources of information; (c) patients refusing to participate; and (d) in‐hospital bleeding patients.
2. Control: Two controls matched by age (difference of 5 years), hospital and month of admission were selected. A patient was considered eligible as a control if he or she was admitted to hospital or was attending an outpatient visit for reasons considered to be unrelated to NSAIDs either as an indication or contraindication. Patients admitted to the hospital for any upper gastrointestinal disorder, lower gastrointestinal bleeding, osteoarticular condition or cardiovascular disorder were not eligible. When possible, unplanned admissions were selected (54%). When the identification of a control from a hospital was unsuccessful, people accompanying the case were selected as controls (12%).
3. Exposure: Drug use was considered to be current when the drug was taken up to 7 days before the index date. It was considered to be past when use ended earlier than 1 week before the index date. The index date for cases was the first day when the gastrointestinal bleeding episode was objectively noticed and for controls, the day of the outpatient visit or admission to the hospital. The index date was assigned a mean of 3 days in advance of the hospital admission date in 85% of the cases. Current use of NSAIDs was further subdivided into single, switching and multiple use. Current single use refers to receiving only the NSAID during the week before the index date. Current switching use refers to receiving only one NSAID in the week before the index date together with at least one other NSAID between 8 and 30 days before the index date. Current multiple use refers to receiving at least two different NSAIDs in the week before the index date. We studied the effect of duration, dose and plasma half life among current single users. We also analysed the effect of individual NSAIDs among current single users. A different exposure variable was created for coxibs (celecoxib and rofecoxib). Cardioprotective aspirin was defined as any dose up to 300 mg/day. Non‐aspirin antiplatelet drugs were clopidogrel, ticlopidine and trifusal.
4. Gastrointestinal disorder history: We classified cases and controls as having a history of ulcer, dyspepsia or neither condition. A person was defined as having no history of ulcer if he or she reported no history of dyspepsia or ulcer (uncomplicated or complicated) before the index date. A person was defined as having a history of dyspepsia only if he or she did not report a history of peptic ulcer. Finally, a person was defined as having a history of peptic ulcer without or with complications (bleeding or perforation). All of these groups were mutually exclusive.
Data collection
Patients and controls were interviewed by the same person (a gastroenterologist or gastroenteroloy trainee) in each participating centre, in general, within 48 h of admission. To ensure reliable data collection, patients and controls were accompanied during the interview by a relative or someone who lived with them. A structured questionnaire with pictures of marketed drugs and a careful review of prescriptions was used. Interview data were completed with the patient's family and hospital clinical records. To avoid bias, interviewers were not involved in the design, were not the investigators at each centre of the study and were unaware of the actual objectives of the study. A study monitor periodically visited the centres to review the quality of the data collection process. Helicobacter pylori infection was determined in cases according to the standard clinical procedures in each hospital, but this was not included in the protocol, and therefore the presence or absence of this infection was not assessed in controls. A committee was designated to respond to any questions or doubts arising during the study and all data were introduced into a single database by one experienced data manager. Final data validation was carried out twice, first by MTA and then by LAG‐R, who rechecked drug names, doses and medical history.
Sample size calculation and analysis
On the basis of national prescription drug information, it was estimated that 0.25% of the general population uses selective COX‐2 inhibitors and that this percentage is higher in the elderly population. If coxibs were used by 1% of our control population and these drugs increased the risk of upper gastrointestinal bleeding by a factor of 2, the number of patients required to obtain a significant estimate of risk (α = 0.05, β = 0.80) would be around 2500 and the number of controls between 5000 and 6000. These numbers would also allow a precise estimation of the risk associated with widely used individual NSAIDs.
An initial exploratory analysis of all clinical and drug variables was carried out. Bivariate analysis was run before the construction of logistic regression models. Conditional and unconditional adjusted regression analyses were carried out to compute relative risks (RR) of UGIB and their 95% confidence intervals (CI) with STATA software. Results from conditional and unconditional models were similar. We present estimates from the conditional logistic regression analysis. The final multivariate model included age, sex, calendar semester, ulcer history, nitrates, oral anticoagulants, antiplatelets, acid‐suppressing drugs, NSAIDs, coxibs and aspirin. We assessed interactions between NSAIDs and aspirin, as well as other combinations, using one single term in the model for the joint effect. We calculated the synergy index (S) and 95% CI as a measure of departure from additive risks (S = 1 if there is no interaction).11
Results
Demographics and ulcer history
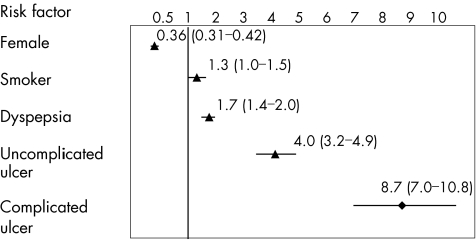
A total of 2800 cases of UGIB and 5600 controls were collected from the participating centres. In total, 2777 cases and 5532 controls meeting all eligibility criteria were included in the analysis. About 75% of the cases were aged >50 years. The mean age was 61 years in both cases and controls. Table 1 presents the crude estimate of RR associated with risk factors considered to be potential confounders that were included in the logistic regression models. Figure 1 shows the adjusted RR (adj RR) of UGIB associated with sex, smoking and history of peptic ulcer. In 53% of cases the bleeding lesion was located in the duodenum, in 41% in the gastric mucosa, in 5% lesions were present in both sites, and in 1% the exact site of the lesion was not reported. A duodenal ulcer was present in 52% of the cases, a gastric ulcer in 34%, ulcers in both sites in 4% and acute mucosal lesions in 10%. Stigmata of bleeding (Forrest I or II lesions) were found in 1118 patients (33% active bleeding, 33% visible vessel and 34% adherent clot); 1210 patients reported a Forrest III ulcer or an acute mucosal gastroduodenal lesion and no data on Forrest status were reported by the remaining 449 patients. Tests for H pylori infection at the time of the endoscopic procedure were noted in 62% of cases, with a positive result in 64%.
Table 1 Univariate estimates of relative risk and 95% confidence interval of upper gastrointestinal bleeding associated with several risk factors.
| Variable | Controls (n = 5532) n (%) | Cases (n = 2777) n (%) | Crude RR (95% CI) |
|---|---|---|---|
| Ulcer complication Hx | 269 (4.9) | 528 (19.0) | 4.94 (4.18 to 5.84) |
| Ulcer Hx | 336 (6.1) | 371 (13.4 | 2.47 (2.10 to 2.90) |
| Dyspepsia | 748 (13.5) | 365 (13.1) | 0.97 (0.84 to 1.11) |
| Male/female | 2897/2635 | 2010/767 | 0.34 (0.31 to 0.38) |
| Current smoker | 1194 (21.6) | 809 (29.1) | 2.04 (1.80 to 2.32) |
| Ex smoker | 1436 (26.0) | 874 (31.5) | 1.71 (1.53 to 1.92) |
| All ASA current use | 524 (9.5) | 746 (26.9) | 3.61 (3.16 to 4.12) |
| ASA past use | 334 (6.0) | 90 (3.2) | 0.63 (0.49 to 0.82) |
| NSAID current use | 511 (9.2) | 657 (23.7) | 3.04 (2.67 to 3.47) |
| NSAID past use | 328 (5.9) | 103 (3.7) | 0.71 (0.56 to 0.90) |
| Coxib current use | 67 (1.2) | 34 (1.2) | 1.00 (0.66 to 1.58) |
| Coxib past use | 25 (0.5) | 9 (0.3) | 0.70 (0.33 to 1.51) |
| Anticoagulant current use | 205 (3.7) | 179 (6.4) | 1.84 (1.49 to 2.28) |
| Anticoagulant past use | 10 (0.9) | 2 (0.1) | 0.40 (0.09 to 1.82) |
| Triflusal current use | 61 (1.1) | 43 (1.5) | 1.40 (0.95 to 2.08) |
| Triflusal past use | 5 (0.1) | 2 (0.1) | 0.80 (0.15 to 4.12) |
| Clopidogrel/Ticlop current use | 81 (1.5) | 107 (3.9) | 2.70 (2.01 to 3.62) |
| Clopidogrel/Ticlop past use | 4 (0.1) | 2 (0.1) | 1.10 (0.20 to 6.04) |
| PPI current use | 732 (13.2) | 239 (8.6) | 0.62 (0.53 to 0.73) |
| PPI past use | 100 (1.8) | 78 (2.8) | 1.53 (1.13 to 2.08) |
| H2‐RA current use | 192 (3.5) | 124 (4.5) | 1.31 (1.04 to 1.65) |
| H2‐RA past use | 50 (0.9) | 43 (1.5) | 1.74 (1.16 to 2.62) |
| Nitrate current use | 174 (3.1) | 102 (3.7) | 1.18 (0.91 to 1.52) |
| Nitrate past use | 8 (0.1) | 2 (0.1) | 0.50 (0.11 to 2.35) |
ASA, asprin; NSAID, non‐steroidal anti‐inflammatory drugs; PPI, proton pump inhibitor.
Figure 1 Adjusted relative risk and 95% confidence intervals of upper gastrointestinal peptic ulcer bleeding associated with different clinical risk factors. Relative risks were adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs, non‐steroidal anti‐inflammatory drugs, coxib and aspirin use.
NSAIDs, coxibs and aspirin
In all, 657 (24%) patients had taken at least one non‐aspirin NSAID in the week before hospital admission compared with 9% among controls. Current coxib use was present in 1.2% of both cases and controls. The corresponding percentages for aspirin (all doses) were 26.9% and 9.5%, respectively.
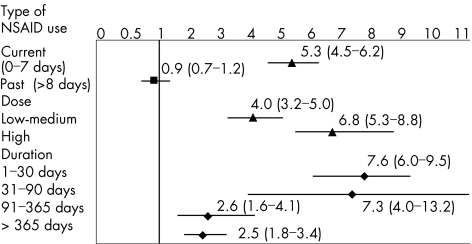
Figure 2 shows the frequency of use and estimates of RR of UGIB associated with non‐aspirin NSAIDs. Overall, the RR associated with current NSAID use was 5.3 (95% CI 4.5 to 6.2). Antecedents of peptic ulcer and concomitant use of proton pump inhibitors (PPIs) were the two most important confounders in the logistic regression model. When we removed these two variables from the full model, the estimate of RR for NSAID use decreased from 5.3 to 3.9, close to the crude estimate of 3. The RR of NSAIDs was dose‐dependent and was substantially greater in patients taking NSAIDs with a long plasma half life (table 2).
Figure 2 Adjusted relative risk and 95% confidence intervals of upper gastrointestinal peptic ulcer bleeding according to timing, dose, duration and indication of non‐steroidal anti‐inflammatory drugs (NSAIDs). Relative risks were adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs, NSAID, coxib and aspirin use. Cut‐off values for dose (in mg) were: aceclofenac 100, dexketoprofen 25, diclofenac 75, etodolac 400, fenbufen 900, fenoprofen 1200, flurbiprofen 100, ibuprofen 1200, indomethacin 75, ketoprofen 200, ketorolac 10, lornoxicam 8, meclofenamate 300, meloxicam 7.5, naproxen 750, niflumic acid 500, nimesulide 100, piroxicam 10 and tenoxicam 10. Duration of use was categorised only among NSAID current single users.
Table 2 Relative risk and 95% confidence interval of upper gastrointestinal bleeding according to plasma half life of non‐steroidal anti‐inflammatory drugs and stratified by daily dose.
| NSAID plasma half life | Patients | Controls | Age‐adjusted RR (95% CI) | Adjusted condition RR (95% CI)* |
|---|---|---|---|---|
| All NSAID dose | ||||
| <12 H | 406 | 388 | 2.4 (2.1 to 2.8) | 4.0 (3.3 to 4.9) |
| ⩾12 H | 207 | 95 | 5.1 (4.0 to 6.5) | 9.4 (6.9 to 12.7) |
| Low‐medium daily dose | ||||
| <12 H | 250 | 275 | 2.1 (1.8 to 2.5) | 3.5 (2.8 to 4.5) |
| ⩾12 H | 58 | 41 | 3.3 (2.2 to 4.9) | 6.4 (3.9 to 10.6) |
| High daily dose | ||||
| <12 H | 156 | 113 | 3.4 (2.5 to 4.1) | 5.0 (3.6 to 6.9) |
| ⩾12 H | 149 | 54 | 6.4 (4.7 to 8.8) | 12.4 (8.1 to 18.8) |
NSAIDs, non‐steroidal anti‐inflammatory drugs.
*Adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs, coxib and aspirin use.
Among non‐aspirin NSAIDs, diclofenac, aceclofenac and ibuprofen had the lowest risk of UGIB, whereas ketorolac and piroxicam presented the highest risk (table 3).
Table 3 Relative risk and 95% confidence interval of upper gastrointestinal bleeding associated with individual non‐steroidal anti‐inflammatory drugs.
| Individual NSAID* | Cases (n = 2777) | Controls (n = 5532) | Age‐adjusted RR (95% CI) | Adjusted condition RR (95% CI)† |
|---|---|---|---|---|
| Non‐use | 2017 | 4693 | Reference | Reference |
| Ibuprofen | 174 | 162 | 2.5 (2.0 to 3.1) | 4.1 (3.1 to 5.3) |
| Diclofenac | 126 | 140 | 2.1 (1.6 to 2.7) | 3.1 (2.3 to 4.2) |
| Aceclofenac | 31 | 52 | 1.4 (0.9 to 2.2) | 2.6 (1.5 to 4.6) |
| Naproxen | 80 | 46 | 4.0 (2.8 to 5.8) | 7.3 (4.7 to 11.4) |
| Piroxicam | 98 | 32 | 7.2 (4.8 to 10.7) | 12.6 (7.8 to –20.3) |
| Indomethacin | 20 | 14 | 3.3 (1.7 to 6.6) | 9.0 (3.9 to 20.7) |
| Meloxicam | 20 | 13 | 3.6 (1.8 to 7.2) | 9.8 (4.0 to 23.8) |
| Ketorolac | 24 | 7 | 8.0 (3.4 to 18.5) | 14.4 (5.2 to 39.9) |
| Lornoxicam | 9 | 6 | 3.5 (1.2 to 9.8) | 7.7 (2.4 to 24.4) |
| Ketoprofen | 14 | 5 | 6.5 (2.3 to 18.2) | 8.6 (2.5 to 29.2) |
| Other NSAIDs | 17 | 6 | 6.7 (2.6 to 16.9) | 13.8 (4.2 to 44.8) |
*Estimates of relative risk were calculated for individual NSAID drugs with five or more exposed controls. Other NSAIDs include flurbiprofin, meclofenamate, morniflumateniflumic acid, nimesulide and tenoxicam.
†Adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs, coxib and aspirin use.
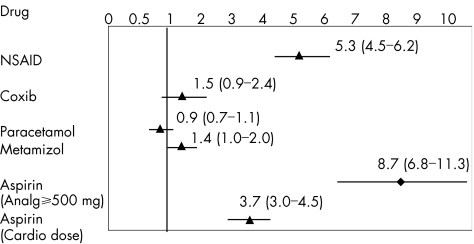
Use of coxibs had a modest increased relative risk of UGIB. Rofecoxib was associated with an increased risk of UGIB (RR 2.1; 95% CI 1.1 to 4.0), whereas the corresponding estimate for celecoxib was 1.0 (95% CI 0.4 to 2.1; table 4). Comparing current users of NSAIDs with current users of coxibs, the estimate of RR was 4.4 (95% CI 2.4 to 8.1). Estimates of RR for drugs used as analgesics were 1.4 (95% CI 1 to 2) for metamizol (dipyrone) and 0.9 (95% CI 0.7 to 1.1) for paracetamol (fig 3).
Table 4 Relative risk and 95% confidence interval of upper gastrointestinal bleeding according to timing, dose and duration of coxibs.
| Cases (n = 2777) | Controls (n = 5532) | Age‐adjusted RR (95% CI) | Adjusted condition RR (95% CI)* | |
|---|---|---|---|---|
| Coxib use | ||||
| Non‐use | 2734 | 5440 | Reference | Reference |
| Current (0–7 days) | 34 | 67 | 1.0 (0.7 to 1.5) | 1.5 (0.9 to 2.4) |
| Celecoxib | 13 | 31 | 0.8 (0.4 to 1.6) | 1.0 (0.4 to 2.1) |
| Rofecoxib | 23 | 36 | 1.3 (0.8 to 2.2) | 2.1 (1.1 to 4.0) |
| Past (⩾8 days) | 9 | 25 | 0.7 (0.3 to 1.5) | 1.0 (0.4 to 2.3) |
| Rofecoxib dose† | ||||
| Non‐use | 2749 | 5481 | Reference | Reference |
| Low medium | 5 | 12 | 0.8 (0.3 to 2.4) | 2.2 (0.6 to 7.8) |
| High | 18 | 24 | 1.5 (0.8 to 2.8) | 2.0 (0.9 to 4.4) |
| Coxib duration | ||||
| Non‐use | 2734 | 5440 | Reference | Reference |
| 1–30 days | 10 | 11 | 1.8 (0.8 to 4.3) | 1.7 (0.6 to 4.7) |
| 31–90 days | 5 | 12 | 0.8 (0.3 to 2.4) | 2.0 (0.6 to 6.5) |
| >90 days | 19 | 44 | 0.9 (0.5 to 1.5) | 1.2 (0.6 to 2.4) |
*Adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs, NSAID and aspirin use.
†Low‐medium doses were up to 12.5 for rofecoxib. There was insufficient variability with celecoxib (in most instance, a low‐medium dose of 200 mg/day was used) to analyse the dose response.
Figure 3 Comparative adjusted relative risk and 95% confidence intervals of upper gastrointestinal peptic ulcer bleeding according to the type of drugs used (non‐steroidal anti‐inflammatory drugs (NSAIDs), coxibs, paracetamol and aspirin). Relative risks were adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs and drug use (NSAID, paracetamol, coxib or aspirin).
Overall, use of aspirin (all doses) had an increased risk of UGIB similar to that observed with non‐aspirin NSAIDs. Table 5 shows a clear dose dependency, and the use of the smallest cardioprophylactic dose of aspirin (100 mg/day) had the lowest RR. Figure 3 shows the differences in association between the relative risk of upper gastrointestinal bleeding and different types of NSAIDs.
Table 5 Relative risk and 95% confidence interval of UGIB according to timing, dose and duration of aspirin.
| Cases (n = 2777) | Controls (n = 5532) | Age‐adjusted RR (95% CI) | Adjusted condition RR (95% CI)* | |
|---|---|---|---|---|
| Aspirin use | ||||
| Non‐use | 1941 | 4674 | Reference | Reference |
| Current (0–7 days) | 746 | 524 | 3.5 (3.1 to 4.0) | 5.3 (4.5 to 6.3) |
| Past (8 days and more) | 90 | 334 | 0.6 (0.5 to 0.8) | 0.7 (0.6 to 1.0) |
| Aspirin dose | ||||
| Non‐use | 1941 | 4674 | Reference | Reference |
| 100 mg | 132 | 185 | 1.8 (1.4 to 2.2) | 2.7 (2.0 to 3.6) |
| 200 mg | 126 | 122 | 2.5 (2.0 to 3.3) | 3.8 (2.7 to 5.2) |
| 300 mg | 114 | 74 | 3.8 (2.8 to 5.1) | 6.1 (4.3 to 8.7) |
| 500 mg | 259 | 112 | 5.6 (4.4 to 7.0) | 7.5 (5.7 to 9.9) |
| 1 g | 76 | 24 | 7.6 (4.8 to 12.4) | 10.4 (6.1 to 17.8) |
| >1 g | 39 | 7 | 13.3 (5.9 to 29.8) | 21.2 (8.7 to 51.9) |
| Aspirin duration | ||||
| Non‐use | 1941 | 4674 | Reference | Reference |
| 1–30 days | 300 | 88 | 8.2 (6.4 to 10.4) | 10.2 (7.7 to 13.5) |
| 31–90 days | 32 | 10 | 7.9 (3.9 to 16.1) | 15.8 (6.8 to 36.8) |
| 91–365 days | 103 | 67 | 3.8 (2.8 to 5.2) | 7.4 (5.0 to 11.1) |
| >1 year | 311 | 359 | 2.1 (1.8 to 2.5) | 3.1 (2.5 to 3.8) |
*Adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets, acid‐suppressing drugs, NSAID and coxib use.
UGIB, upper gastrointestinal bleeding.
Combination of NSAIDs, coxibs and cardioprotective aspirin
Concomitant use of NSAID or coxib with cardioprotective aspirin had a relative risk of UGIB that was in excess of what would be expected by a simple additive effect of the drugs (table 6). The synergy index of interaction was 1.6 (95% CI 0.9 to 3.1) for NSAID/aspirin and 5.2 (95% CI 1.0 to 26.3) for coxib/aspirin. Data on the interaction between cardioprotective aspirin and individual NSAIDs or coxibs were limited; yet, the magnitude of the interaction varied between different individual NSAIDs. Concurrent use of diclofenac and cardioprotective aspirin had the lowest RR of UGIB among people taking aspirin and one of the five most widely used individual NSAIDs (RR 5.7; 95% CI 2.6 to 12.5).
Table 6 Relative risk and 95% confidence interval of upper gastrointestinal bleeding associated with concurrent use of non‐steroidal anti‐inflammatory drugs and low‐dose aspirin, as well as coxibs and low‐dose aspirin.
| Cases (n = 2777) | Controls (n = 5532) | Age adjusted RR (95% CI) | Adjusted condition RR (95% CI)* | |
|---|---|---|---|---|
| NSAID and low‐dose aspirin interaction† | ||||
| Non‐use | 1706 | 4321 | Reference | Reference |
| NSAID only | 558 | 456 | 3.3 (2.8 to 3.7) | 5.3 (4.4 to 6.3) |
| Low‐dose aspirin only | 317 | 356 | 2.6 (2.2 to 3.1) | 3.9 (3.1 to 4.9) |
| NSAID and low‐dose aspirin | 55 | 27 | 6.1 (3.8 to 9.7) | 12.7 (7.0 to 23.0) |
| Coxib and low‐dose aspirin interaction | ||||
| Non‐use | 2356 | 5020 | Reference | Reference |
| Coxib only | 28 | 63 | 1.0 (0.6 to 1.5) | 1.0 (0.5 to 1.8) |
| Low‐dose aspirin only | 366 | 379 | 2.3 (2.0 to 2.7) | 3.6 (2.9 to 4.5) |
| Coxib and low‐dose aspirin | 6 | 4 | 3.7 (1.0 to 13.1) | 14.5 (3.3 to 63.9) |
NSAIDs, non‐steroidal anti‐inflammatory drugs.
*Adjusted for age, sex, calendar semester, ulcer history, nitrates, anticoagulants, antiplatelets and acid‐suppressing drugs.
†Categorised only among NSAID current single users.
Use of other drugs
Other antiplatelet agents (clopidogrel or ticlopidine) were used in 3.9% of cases and 1.5% of controls. The RR of UGIB associated with thienopyridines was 2.8 (95% CI 1.9 to 4.2) and was similar between clopidogrel and ticlopidine (data not shown). The magnitude of the interaction between NSAIDs and clopidogrel or ticlopidine (RR 15.2; 95% CI 4.1 to 56.5) was similar to that observed between low‐dose aspirin and thienopyridines (RR 16.4; 95% CI 5.4 to 49.7).
The use of triflusal, a weak non‐aspirin NSAID used as an antiplatelet agent in Spain, was reported in 1.5% of cases and 1.1% of controls, and had an RR of 1.9 (95% CI 1.1 to 3.3). The use of oral warfarin‐like anticoagulants (dicumarinics) had a dose‐dependent increased risk of UGIB (table 7). Concomitant use of anticoagulants and NSAIDs had an RR of 19.3 (95% CI 8.2 to 45.3). Use of subcutaneous low‐molecular‐weight heparin had a small increased risk of UGIB (RR 1.3; 95% CI 0.4 to 4.3). Use of oral steroids was not associated with an increased risk of UGIB, and we found no interaction with NSAIDs (data not shown). Overall, PPI use was associated with a significant reduction of the risk of UGIB (RR 0.3; 95% CI 0.3 to 0.4). Concomitant use of PPI with NSAID was not associated with an increased RR of UGIB (RR 0.9; 95% CI 0.7 to 1.3).
Table 7 Relative risk and 95% confidence interval of UGIB associated with anticoagulants according to daily dose as well as the interaction with NSAIDs.
| Cases (n = 2777) | Controls (n = 5532) | Age‐adjusted RR (95% CI) | Adjusted condition RR (95% CI)* | |
|---|---|---|---|---|
| Anticoagulants | ||||
| Non‐use | 2598 | 5327 | Reference | Reference |
| Dicumarinics 1 mg | 55 | 73 | 1.6 (1.1 to 2.2) | 2.6 (1.6 to 4.0) |
| Dicumarinics 2–3 mg | 47 | 70 | 1.4 (1.0 to 2.0) | 2.4 (1.5 to 3.8) |
| Dicumarinics ⩾4 mg | 33 | 19 | 3.6 (2.0 to 6.4) | 6.6 (3.2 to 13.4) |
| Dicumarinics dose unknown | 44 | 43 | 2.1 (1.4 to 3.3) | 2.8 (1.7 to 4.8) |
| Anticoagulants and NSAIDs† | ||||
| Non‐use | 1784 | 4500 | Reference | Reference |
| NSAID use only | 622 | 504 | 3.0 (2.6 to 3.4) | 5.0 (4.2 to 5.9) |
| Dicumarinics only | 144 | 196 | 1.8 (1.5 to 2.3) | 2.8 (2.1 to 3.7) |
| Combination | 35 | 9 | 9.7 (4.6 to 20.2) | 19.3 (8.2 to 45.3) |
NSAIDs, non‐steroidal anti‐inflammatory drugs; UGIB, upper gastrointestinal bleeding.
*Adjusted for age, sex, calendar semester, ulcer history, nitrates, antiplatelets, acid‐suppressing drugs, coxib and aspirin use.
†Categorised only among NSAID current single users.
Effect modification of NSAIDs with age, sex and ulcer history
The relative risk of UGIB associated with NSAIDs was rather constant across all age categories. The RR was 5.1 among current male users of NSAIDs (95% CI 3.9 to 6.8), whereas the corresponding RR among females was 5.7 (95% CI 3.9 to 8.2). The relative risk associated with NSAID use varied according to antecedents of peptic ulcer. NSAID users with a previous episode of ulcer (uncomplicated or bleeding/perforated peptic ulcer) had an RR of 4.6 (95% CI 1.9 to 11.0), whereas patients without peptic ulcer history had an RR of 5.6 (95% CI 4.6 to 7.0).
Discussion
This case–control study has shown that use of selective COX‐2 inhibitors has a modest increase in the risk of UGIB due to peptic lesions, which was entirely accounted for by a twofold increase with rofecoxib, whereas no increase was observed with celecoxib. The relative risk of UGIB observed with celecoxib was similar to that observed with paracetamol or the combination of PPIs with NSAIDs. The overall relative risk of UGIB observed with coxib use was lower than estimates of relative risk associated with traditional NSAIDs and lower than that observed with cardioprotective aspirin in clinical practice. It is also important to note that NSAIDs with a long plasma half life had a greater relative risk of UGIB compared with NSAIDs with a short plasma half life, independent of daily dose.
Among traditional NSAIDs, diclofenac, aceclofenac and ibuprofen were associated with the lowest RR of UGIB. Other individual NSAIDs had a higher RR, and our results are consistent with previous reports12,13 showing that piroxicam and ketorolac were the two indivdual NSAIDs associated with the greatest risk of UGIB. It is also worth noting that meloxicam (15 mg/day was the dose used among 70% of controls) had a similar relative risk of UGIB as other common traditional NSAIDs. These observations are of interest as diclofenac and aceclofenac have either little use or are not available in some countries (eg, USA), whereas other NSAIDs such as meloxicam have been increasingly prescribed around the world since the withdrawal of rofecoxib, probably owing to the “false” perception that this drug is safer than other compounds based on a scarcity of data comparing this compound with other NSAIDs in observational studies.
Aspirin was the individual NSAID most commonly used in our study population. This is consistent with a general trend found in other recent studies14,15 and points to several important aspects in clinical practice. The first aspect is that use of “cardioprotective” aspirin is now an important gastrointestinal public health issue accounting for about 15% of all patients with UGIB in our country. The second aspect is that “analgesic” aspirin at doses of ⩾500 mg/day (most of it being over‐the‐counter use), although representing less than one third of all aspirin use, was associated with a similar burden of UGIB as “cardioprotective” aspirin, as use of aspirin at such daily doses resulted in a very high excess risk of UGIB.16,17 This last point is an important finding not sufficiently documented in previous observational studies, suggesting that free availability of analgesic aspirin needs to be re‐evaluated from a clinical standpoint.
Outcome clinical trials have previously shown contradictory results. In Vioxx Gastrointestinal Outcome Research, rofecoxib (50 mg/day) had a reduced incidence of upper gastrointestinal complications when compared with naproxen (500 mg twice daily)3; however, in the Celecoxib Long‐term Arthritis Safety Study4 the incidence of these events was not statistically different from that observed with standard NSAIDs. Subsequently, a few observational studies have shown that celecoxib use had a reduced risk of gastrointestinal bleeding when compared with non‐aspirin traditional NSAIDs, but there was no agreement regarding the risk of UGIB associated with rofecoxib use.18,19,20 Unlike some of these studies that used computerised databases,18,19 our study evaluated the risk of UGIB associated with the use of coxib, NSAIDs, aspirin and combinations of these drugs as reported by the patient.
Strengths of our study include the large sample size, the use of the organised national healthcare system in Spain, the well‐defined logistics of case ascertainment, the validation of all patients with the original clinical records, and the structured data collection methods of collecting data on prescription and non‐prescription drug consumption. A weakness of our study was the low prevalence of use of some drugs, which precluded a more precise evaluation of the risk of UGIB in several instances, and thus reduces the robustness of some of our findings. Information bias may also be present, especially when analysing information such as duration of treatment or past use. Indeed, our control group may have different drug use from the general population that they aim to represent, but overall our data agree with previous reports from the same country.20,21 We carried out sensitivity analyses using different subgroups of controls and found that the results and conclusions were robust between them. Finally, most previous hospital‐based studies used the day of admission as the index date among cases to ascertain the exposure status. We assigned the index date as the date of the first objective sign of UGIB among cases. As expected, the use of the hospitalisation date would have overestimated the risk associated with drugs used commonly as short‐term analgesic treatment (data not shown).
Randomised controlled studies4,5 have shown that high‐dose coxib treatment combined with low‐dose aspirin had a similar incidence of upper gastrointestinal complications as that observed with a high dose of traditional NSAIDs and low‐dose aspirin. We have now documented that combining cardioprotective aspirin, either with traditional standard doses of NSAIDs or with coxibs, potentiates the risk of UGIB beyond that expected from a simple additive effect of these agents. This observation supports the hypothesis that some of the benefits of coxibs in the gastrointestinal tract are due to their lack of any clinical antiplatelet effect, and that when combined with antiplatelet agents, coxibs lose their safety advantage over traditional NSAIDs. However, the magnitude of interaction between cardioprotective aspirin and NSAIDs may be different with different NSAIDs.22 Here, we reported that concurrent use of diclofenac with low‐dose aspirin presented the lowest RR of UGIB among patients taking low‐dose aspirin and traditional NSAIDs. Therefore, it is possible that a similar finding could be observed with individual COX‐2 selective inhibitors (celecoxib v rofecoxib), but the limited exposure of our study population to concurrent cardioprotective aspirin and individual coxibs prevented this analysis.
We also found that use of non‐aspirin antiplatelet agents did not have a lower risk of UGIB than low‐dose cardioprotective aspirin. Furthermore, concurrent use of non‐aspirin antiplatelet agents with traditional NSAIDs also potentiated the risk of UGIB, supporting the hypothesis that the antiplatelet effect of low‐dose aspirin is the main mechanism associated with its increased risk of UGIB. It also agrees with our finding that there were more patients with bleeding duodenal than gastric ulcers, suggesting that H pylori‐induced duodenal ulcers may be pre‐existing lesions in many patients who bled with the addition of antiplatelet agents.23 These data complement recent reports from a small study that found that in high‐risk patients, clopidogrel treatment had a higher incidence of UGIB than the combination of low‐dose aspirin with esomeprazole.24 It is also important to note that the increasingly used combination of clopidogrel and low‐dose aspirin25 presented a greatly increased risk of UGIB in our study. Finally, we have found that the use of oral anticoagulants was an independent risk factor for UGIB and that in combination with NSAIDs the risk is further potentiated.
Finally, our study found that the RR associated with NSAID use varied according to whether peptic ulcer history was present or not, which was the main independent risk factor of UGIB. The presence of this major risk factor reduces the effect of other risk factors (eg, negative interaction with NSAID use) and explains why the estimate of RR of UGIB for NSAID use in patients with ulcer history was lower than that observed in patients without ulcer history as already found in previous studies.26 Notwithstanding, it should be remembered that the baseline incidence of ulcer bleeding is much higher in patients with ulcer history than among patients without peptic ulcer antecedents1,24 and consequently patients with ulcer history have a greater excess risk due to NSAIDs despite a relatively smaller RR associated with its use.
In summary, our data provide an additional basis to assess the relative gastrointestinal safety of various anti‐inflammatory drugs. Now that use of coxibs and some traditional NSAIDs has been shown to have an increased risk of serious cardiovascular events,6,7,8,9 all risks and benefits with the use of traditional NSAIDs and coxibs need to be thoroughly re‐evaluated. The small increased risk of developing UGIB either with coxib use or with a combination of PPI with traditional NSAIDs found in this study suggests that they may be used preferentially when the main objective is to reduce the risk of gastrointestinal complications. On the other hand, a large proportion of patients with cardiovascular risk factors will be taking low‐dose aspirin and our study indicates that the gastrointestinal advantage of coxibs tend to disappear in these patients. Together with the modest increase in cardiovascular events6,7,8,9 observed with coxibs and some traditional NSAIDs, special caution would be desirable when using these agents in such a population until more data are available. Our data also suggest that non‐aspirin antiplatelet drug use alone or combined with traditional NSAIDs should not be considered a safer alternative to low‐dose cardioprotective aspirin alone or in combination with NSAIDs, respectively.
APPENDIX A
Table AI lists the names of the investigators and the respective hospitals that constitute the Asociación Española de Gastroenterología.
TableAI Asociación Española de Gastroenterología.
| Investigators | Hospital |
|---|---|
| Angel Lanas | Hospital Clínico Universitario |
| Maite Arroyo | Zaragoza |
| Juan Arenas | |
| Juan Arenas Mirave | Hospital Donostia |
| Eva Zapata | San Sebastian |
| Fernando Gomollón | Hospital Universitario Miguel Server, Zaragoza |
| Roberto Araméndiz | |
| Jose Maria Pique | Hospital Clínic y Provincial |
| Faust Feu | Barcelona |
| Julio Ponce | Hospital La Fe |
| Guillermo Bastida | Valencia |
| Luis Rodrigo | Hospital General de Asturias |
| Lola Fuentes | Oviedo |
| Santos Santolaria Piedrafita | Hospital General San Jorge |
| Miguel Montoro Huguet | Huesca |
| Rebeca Conde | |
| Xavier Calvet | Institut Universitari Parc Tauli |
| Mercedes Güell | Hospital de Sabadell |
| Carlos Martin de Argila | Hospital Ramon y Cajal |
| Sofía Aleman | Madrid |
| Enrique Quintero | Hospital Universitario de la Laguna |
| David Nicolas | Tenerife |
| Fernando Borda | Hospital de Navarra. |
| Ana Borda | Pamplona |
| Enrique Dominguez | Complejo Hospitalario de Santiago |
| Santiago de Compostela | |
| Pedro Almela | Hospital Clínico |
| Valencia | |
| Manuel Castro | Hospital de Valme. |
| Enrique García | Sevilla |
| Miguel Perez Mateo | Hospita General Universitario |
| Juan Penalva | Alicante |
| José Póveda | |
| Monserrat Andreu | Hospital del Mar |
| Barcelona | |
| Jose Maria Navarro | Hospital Costa del Sol |
| Marbella. Málaga | |
| Montse Forné | Mutua Terrassa |
| Barcelona | |
| Luis Bujanda | Hospital San Eloy |
| Baracaldo | |
| Angel Gonzalez | Hospital Reina Sofía |
| Córdoba | |
| Joaquim Balanzo | Hospital de San Pau |
| Barcelona | |
| Eduardo Moreno | Hospital Dr Pesset |
| Valencia | |
| Miguel Bixquert | Hospital Arnau de Vilanova |
| Valencia | |
| Mercedes Barenys | Hospital Viladecans |
| Barcelona | |
| Jose Luis Martín | Hospital San Cecilio |
| Granada | |
| Paquilu Sousa | Hospital Santa Ana |
| Motril | |
| Llucia Tito | Hospital de l'Espirit Sant |
| Santa Coloma | |
| Enrique Medina | Hospital General |
| Valencia | |
| Esteban Saperas | Hospital Vall d́Hebron |
| Barcelona | |
| Agustín Balboa | Hospital De la Selva |
| Blanes | |
| Jesús Espinel | Complejo Universitario |
| León | |
| Joaquin Yangüela | Hospital San Millan de Logroño |
| Logroño | |
| Marta Piñol | German Trias y Pujol |
| Badalona |
Supplementary Material
Acknowledgements
We thank Verónica Bryant for data recording of the questionnaires, and Drs Steven Lanes, Sonia Hernandez Diaz and James Scheiman for critically reviewing earlier versions of this article.
Abbreviations
COX - cyclo‐oxygenase
NSAIDs - non‐steroidal anti‐inflammatory drugs
PPI - proton pump inhibitor
UGIB - upper gastrointestinal bleeding
Footnotes
Funding: This study was made possible by funds from grant C03/02 from the National Institute of Research Carlos III and by unrestricted grants provided by Pfizer Spain, Merck Spain, AstraZeneca Spain, Uriach Laboratories and Rottapharm to the Spanish Association of Gastroenterology (AEG). These contributors have not had a role in the conduction of the study, the interpretation of data or writing of the manuscript.
Dr Angel Lanas coordinated the study and contributed to the analysis and interpretation of data. Dr Lanas and Dr Luis Alberto García Rodríguez designed the study and drafted the manuscript. Dr Luis Alberto García Rodríguez carried out the statistical analysis of data and contributed to the analysis and interpretation of data. Dr Maria T Arroyo coordinated the collection of data between collaborating hospitals. All investigators made major contributions to the collection of data, revised data interpretation and had the opportunity to revise the manuscript. This study was presented at the plenary session of the last American Gastroenterological Association meeting.
References
- 1.Wolfe M M, Lichtenstein D R, Singh G. Gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs. N Engl J Med 19993401888–1899. [DOI] [PubMed] [Google Scholar]
- 2.Brooks P, Emery P, Evans J F.et al Interpreting the clinical significance of the differential inhibition of cyclooxygenase‐1 and cyclooxygenase‐2. Rheumatology (Oxford) 19998779–788. [DOI] [PubMed] [Google Scholar]
- 3.Bombardier C, Laine L, Reicin A.et al Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 20003431520–1528. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein F E, Faich G, Goldstein J L.et al Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study. JAMA 20002841247–1255. [DOI] [PubMed] [Google Scholar]
- 5.Schnitzer T J, Burmester G R, Mysler E.et al Comparison of lumiracoxib with naproxen and ibuporfen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications:randomised controlled trial. Lancet 2004364665–674. [DOI] [PubMed] [Google Scholar]
- 6.Bresalier R S, Sandler R S, Quan H.et al Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 20053521092–1102. [DOI] [PubMed] [Google Scholar]
- 7.Solomon S D, McMurray J J V, Pfeffer M A.et al Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 20053521071–1080. [DOI] [PubMed] [Google Scholar]
- 8.Nussmeier N A, Whelton A A, Brown M T.et al Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 20053521081–1091. [DOI] [PubMed] [Google Scholar]
- 9.Hernández‐Díaz S, Varas‐Lorenzo C, García Rodríguez L A. Non‐steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol 200698266–274. [DOI] [PubMed] [Google Scholar]
- 10.Becker J C, Domschke W, Pohle T. Current approaches to prevent NSAID‐induced gastropathy—COX selectivity and beyond. Br J Clin Pharmacol 200458587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman K J, Greeland S.Modern epidemiology. 2nd edn. Philadelphia: Lippincott‐Raven Publishers, 1998
- 12.García Rodríguez L A, Cattaruzzi C, Troncon M G.et al Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti‐inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med 199815833–39. [DOI] [PubMed] [Google Scholar]
- 13.Henry D, Lim L L, Garcia Rodriguez L A.et al Variability in risk of gastrointestinal complications with individual non‐steroidal anti‐inflammatory drugs: results of a collaborative meta‐analysis. BMJ 19963121563–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Abajo F J, García Rodríguez L A. Risk of upper gastrointestinal bleeding and perforation associated with low‐dose aspirin as plain and enteric‐coated formulations. BMC Clin Pharmacol 200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stack W A, Atherton J C, Hawkey G M.et al Interactions between Helicobacter pylori and other risk factors for peptic ulcer bleeding. Aliment Pharmacol Ther 200216497–506. [DOI] [PubMed] [Google Scholar]
- 16.Lanas A, Sekar M C, Hirschowitz B I. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992103862–869. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox C M, Shalek K A, Cotsonis G. Striking prevalence of over‐the‐counter nonsteroidal anti‐inflammatory drug use in patients with upper gastrointestinal hemorrhage. Arch Intern Med 199415442–46. [PubMed] [Google Scholar]
- 18.Mamdani M, Rochon P A, Juurlink D N.et al Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo‐oxygenase‐2 inhibitors or conventional non‐steroidal anti‐inflammatory drugs. BMJ 2002325624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norgard B, Pedersen L, Johnsen S P.et al COX‐2‐selective inhibitors and the risk of upper gastrointestinal bleeding in high‐risk patients with previous gastrointestinal diseases: a population‐based case‐control study. Aliment Pharmacol Ther 200419817–825. [DOI] [PubMed] [Google Scholar]
- 20.Laporte J R, Ibanez L, Vidal X.et al Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf 200427411–420. [DOI] [PubMed] [Google Scholar]
- 21.Lanas A, Bajador E, Serrano P.et al Nitrovasodilators, low‐dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N Engl J Med 2000343834–839. [DOI] [PubMed] [Google Scholar]
- 22.Weideman R A, Kelly K C, Kazi S.et al Risks of clinically significant upper gastrointestinal events with etodolac and naproxen: a historical cohort analysis. Gastroenterology 20041271322–1328. [DOI] [PubMed] [Google Scholar]
- 23.Huang J Q, Sridhar S, Hunt R H. Role of Helicobacter pylori infection and non‐steroidal anti‐inflammatory drugs in peptic‐ulcer disease: a meta‐analysis. Lancet 200235914–22. [DOI] [PubMed] [Google Scholar]
- 24.Chan F K, Ching J Y, Hung L C.et al Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 2005352238–244. [DOI] [PubMed] [Google Scholar]
- 25.Sabatine M S, Cannon C P, Gibson M C.et al Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N Engl J Med 20053521179–1189. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez‐Diaz S, Garcia Rodriguez L A. Association between between non‐steroidal anti‐inflammatory drugs and upper gastrointestinal bleed/perforation: an overview of epidemiological studies published in the 1990s. Arch Intern Med 20001602093–2099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.