There is growing interest in the potential contributions of distortions in intestinal microbiota to human intestinal disease.1,2,3,4,5 Our understanding of intestinal microbiota complexity and dynamics is evolving, but is still in its infancy.6,7,8 We have previously shown that microflora profiles are (1) unique to an individual; (2) stable over a period of at least 8 weeks; and (3) not affected by minor short‐term changes in diet.9
Little is known about (1) changes in microbiota in patients undergoing a screening colonoscopy; (2) if and when microbiota returns to its normal pre‐colonoscopy composition; and (3) whether short‐term distortions increase disease risks.
We investigated microbiota changes in five patients undergoing screening colonoscopy. Colon preparation was adequate and polyps were detected in two patients (A and E); congested mucosa with chronic inflammation and lymphatic infiltrate was detected in patient B, and the remaining two patients (C and D) had no abnormalities.
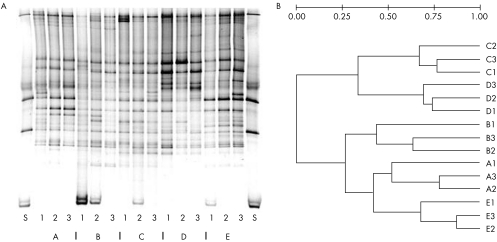
Analysis of the faecal microbiota by denaturing gradient gel electrophoresis (DGGE) using primers against both the V3 and the V6–V8 regions showed unique profiles for each patient (fig 1). In three of the five patients, the two profiles detected after the colonoscopy were more similar to each other and clearly different from the precolonoscopy profile; in the two other patients, we found less of a difference between the precolonoscopy and postcolonoscopy profiles.
Figure 1 (A) Denaturing gradient gel electrophoresis (DGGE) profiles of five patients before and after colonoscopy. Each lane on the gel represents the faecal microbiota profile from a patient at one time point. Each band represents a molecular species characterised by unique denaturation characteristics. S, standard lane; A–E, patients at time points 1, 2 and 3. (B) Dendogram of the DGGE profiles from five patients (A–E) before (A–E1) and after (A–E2 and A–E3) colonoscopy (Ward's algorithm, Dice coefficient). Branch length indicates differences in the correlation coefficients in the distance matrix, generated using the Diversity Database software (Bio‐Rad, Hercules, CA, USA).
Archaeal DNA was detected by PCR in one patient and was not detected in three patients at all time points; in the remaining patient, it was detected only in the sample collected following the colonoscopy. 7α‐Dehydroxylase was detected in four of five patients: in two patients at all three time points and in the other two patients only after the colonoscopy. Dissimilatory sulphite reductase was detected in three patients: in patient D before and after 6–8 weeks, in patient E at both time points after the colonoscopy and in patient C only once after the colonoscopy.
Fluorescent in situ hybridisation analysis indicated considerable variation in the proportions of bacteria hybridising to the group‐specific and species‐specific probes, with no trends detected in precolonoscopy and postcolonoscopy samples.
Table 1 Proportion of bacterial groups (number of cells hybridising to probe/total number of 4′,6‐diamidino‐2‐phenylindole‐2HCl‐stained cells) as determined by fluorescent in situ hybridisation.
| Sample | FISH | PCR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Erec482 | Bac303 | Bif164 | LAB158 | EC1531 | Archaea | 7αDH | DSR | ||
| A | 1 | 0.11 | 0.31 | 0.04 | 0.012 | ND | – | – | – |
| 2 | 0.24 | 0.42 | 0.06 | 0.019 | ND | + | + | – | |
| 3 | 0.21 | 0.97 | 0.06 | 0.013 | ND | – | – | – | |
| B | 1 | 0.06 | 0.09 | 0.02 | 0.009 | D | – | – | – |
| 2 | 0.15 | 0.27 | 0.05 | 0.041 | D | – | – | – | |
| 3 | 0.33 | 0.21 | 0.01 | 0.013 | D | – | + | – | |
| C | 1 | 0.15 | 0.07 | 0.04 | 0.008 | D | + | – | – |
| 2 | 0.11 | 0.05 | 0.04 | 0.007 | ND | + | – | + | |
| 3 | 0.28 | 0.13 | 0.05 | 0.049 | D | + | – | – | |
| D | 1 | 0.17 | 0.33 | 0.05 | 0.016 | ND | – | + | + |
| 2 | 0.16 | 0.22 | 0.02 | 0.022 | D | – | + | – | |
| 3 | 0.67 | 0.32 | 0.04 | 0.034 | D | – | + | + | |
| E | 1 | 0.28 | 0.09 | 0.04 | 0.020 | ND | – | + | – |
| 2 | 0.15 | 0.14 | 0.02 | 0.005 | ND | – | + | + | |
| 3 | 0.16 | 0.23 | 0.03 | 0.005 | ND | – | + | + | |
Bac303, Bacteroides‐Prevotella group; Bif64, Bifidobacterium; D, detected; 7αDH, 7α dehydroxylases; DSR, dissimilatory sulphite reductases; EC1531, Escherichia coli; Erec482, Eubacterium rectale‐Clostridium coccoides group; FISH, fluorescent in situ hybridisation; LAB158, Lactobacillus and Enterococcus group; ND, not detected; PCR, polymerase chain reaction with primers against archaea; +, detected; −, not detected.
We analysed 96‐clone libraries from all three stools per patient (GenBank DQ904637–DQ905931, DQ905933–DQ905956). By using the ∫–Libshuff programme,10 we determined which of the libraries originated from the same source, and whether they were subsets or were derived from different sources. For none of the patients did all three libraries derive from the same source, indicating variation in microflora composition. For patient A, the libraries before and 6–8 weeks afterwards originated from the same source (ΔCX1,Y3 and ΔCY1,X3 >0.009), and the library before was a subset of the library detected 2–4 weeks afterwards. For patient B, all libraries originated from different sources (all ΔCX,Y and ΔCY,X <0.009). For patient C, the libraries at 2–4 and 6–8 weeks after the procedure originated from the same source (ΔCX2,Y3 and ΔCY2,X3 >0.009). For patient D, the library generated after 6–8 weeks was a subset of both other libraries (ΔCY1,X3 and ΔCY2,X3 >0.009). For patient E, the libraries after the procedure were a subset of the precolonoscopy library (ΔCY1,X2 and ΔCY1,X3 >0.009) and the library 2–4 weeks afterwards was a subset of that after 6–8 weeks (ΔCY3,X2 >0.009).
Our observations with different methods did not agree in many details, probably due to different biases inherent in each method. However, all methods indicated that microbiota composition is disturbed in patients undergoing screening colonoscopy, which might have implications for potential health effects that we do not yet understand. Simple DGGE profiling, with universal and group‐specific primer sets, might currently be most efficient for monitoring the complex human microbiota composition over time. There is a clear need for improving our understanding of the dynamics in microbiota composition.
Footnotes
Funding: Work in the lab of VM is supported by ACS grant MRSGT CCE‐107301. This work was supported by the University of Maryland General Clinical Research Center, Grant M01 RR 16500, General Clinical Research Centers Program, National Center for Research Resources (NCRR), NIH.
Competing interests: None.
References
- 1.Tannock G W. Analysis of the intestinal microflora: a renaissance. Antonie Van Leeuwenhoek 199976265–278. [PubMed] [Google Scholar]
- 2.McCracken V J, Simpson J M, Mackie R I.et al Molecular ecological analysis of dietary and antibiotic‐induced alterations of the mouse intestinal microbiota. J Nutr 20011311862–1870. [DOI] [PubMed] [Google Scholar]
- 3.Rafter J. Lactic acid bacteria and cancer: mechanistic perspective. Br J Nutr 200288(Suppl 1)S89–S94. [DOI] [PubMed] [Google Scholar]
- 4.Manichanh C, Rigottier‐Gois L, Bonnaud E.et al Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 200655205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol H, Seksik P, Rigottier‐Gois L.et al Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis 200612106–111. [DOI] [PubMed] [Google Scholar]
- 6.Ley R E, Backhed F, Turnbaugh P.et al Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 200510211070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckburg P B, Bik E M, Bernstein C N.et al Diversity of the human intestinal microbial flora. Science 20053081635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S R, Pop M, Deboy R T.et al Metagenomic analysis of the human distal gut microbiome. Science 20063121355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mai V, Katki H A, Harmsen H.et al Effects of a controlled diet and black tea drinking on the fecal microflora composition and the fecal bile acid profile of human volunteers in a double‐blinded randomized feeding study. J Nutr 2004134473–478. [DOI] [PubMed] [Google Scholar]
- 10.Schloss P D, Larget B R, Handelsman J. Integration of microbial ecology and statistics: a test to compare gene libraries. Appl Environ Microbiol 2004705485–5492. [DOI] [PMC free article] [PubMed] [Google Scholar]