Abstract
Background
Atrophy of the smooth muscle layers of the muscularis propria characterises oesophageal involvement in systemic sclerosis (scleroderma). The aetiology of this atrophy and of the resultant oesophageal dysfunction is unknown.
Objectives
To examine oesophageal tissue for evidence of fibrosis, vascular disease, inflammatory reactions and neural abnormalities to determine the possible causes of this disease process.
Methods
A case–control survey was conducted using oesophageal tissue from 74 scleroderma cases and 74 age, race and sex‐matched controls from our autopsy files. Histological evidence of oesophageal muscle atrophy was correlated with the degree of vascular changes, inflammatory infiltration, fibrosis, abnormalities of the myenteric plexus and reduction of interstitial cells of Cajal (ICC) using a predesigned semiquantitative descriptive method.
Results
Smooth‐muscle atrophy was found in 94% of scleroderma cases, and in 5% of controls (p<0.001). Atrophy was evident in the circular smooth muscle in 93% of cases, and in the longitudinal smooth muscle in 66% of cases. Intimal proliferation of arterioles was found in 38% of cases and in 5% of controls (p<0.001), but was not associated with smooth‐muscle atrophy (p = 0.29). Despite these vascular changes, there was no evidence of compromised perfusion, such as findings suggestive of acute ischaemic necroses. Minimal cellular infiltrates were seen in the myenteric plexus in 82% of cases and in 92% of controls (p = 0.091). ICC were found in fewer numbers in areas of atrophic smooth muscle compared with adjacent normal smooth muscle in selected scleroderma cases.
Conclusion
The pathological findings of oesophageal lesions in scleroderma seem inconsistent with either an ischaemic or an inflammatory process. The loss of circular and longitudinal smooth muscle in the distal scleroderma oesophagus may represent loss of normal neural function followed by secondary tissue atrophy, or may be a primary smooth muscle lesion.
The oesophagus is one of the most commonly affected regions of the gastrointestinal tract in systemic sclerosis (scleroderma)1; yet, the pathogenesis of the disease is not completely understood. Case reports note that oesophageal smooth muscle lesions of scleroderma are characterised by atrophy of the inner circular layer of the muscularis propria, with relative preservation of the outer longitudinal smooth muscle layer and with sparing of interdigitating skeletal muscle fibres.2,3,4 The tissue specificity of this lesion suggests that a unique injury is related to the function of this muscle layer. Although neurological, inflammatory and vascular injuries are postulated as pathogenetic mechanisms of scleroderma, no controlled studies exist that have investigated the association of these processes with the oesophageal smooth‐muscle lesion.
To investigate the potential cause of the unique oesophageal smooth‐muscle lesion in scleroderma, we compared oesophageal tissue from autopsied cases of scleroderma with age, race and sex‐matched controls to examine the relationship of smooth‐muscle atrophy and histological and inflammatory changes in the vasculature and nervous tissue. To our knowledge, this is the first study to specifically evaluate histological correlates of scleroderma oesophageal muscle atrophy. We focused on fibrosis, inflammation, the myenteric plexus and vasculature because of histological and functional evidence that each of these systems have pathogenic importance. Owing to their role in normal oesophageal motility, interstitial cells of Cajal (ICC) were investigated by immunohistochemical stains in a subgroup of scleroderma cases. This is the first published evaluation of ICC in scleroderma.
Materials and methods
Case selection
More than 50,000 autopsy records at The Johns Hopkins Hospital, Baltimore, Maryland, were searched using the keywords “scleroderma”, “systemic sclerosis” (scleroderma) and “Raynaud”. Cases were included if the review of the medical record identified clinical features that satisfied American College of Rheumatology (ACR) criteria for a diagnosis of scleroderma5 or identified the presence of three of five features of the calcinosis, Raynaud's phenomenon, oesophageal dysmobility, sclerodactyly and telangiectasia (CREST) syndrome and if oesophageal tissue was available for review. Controls were identified from the next non‐scleroderma autopsy conducted at our institution (John Hopkins Medical Institution, Baltimore, Maryland, USA) of the same sex, race and decade of age as each scleroderma case that was included in the series.
Clinical data
The autopsy record or the medical record of each scleroderma case was reviewed using a predesigned questionnaire to gather relevant clinical data. From these sources, the specific disease features that satisfied the ACR criteria for a diagnosis of scleroderma were collected. The classification of disease at the time of death was determined as limited or diffuse, by the presence of scleroderma skin disease distal to the elbows and knees (limited), or involving the proximal limbs or trunk (diffuse).6 Patients were classified as having the CREST syndrome if any three of the following aetiologies were present: subcutaneous calcinosis, Raynaud's phenomenon, oesophageal dysmotility, sclerodactyly or telangiectasia.7 This investigation was approved by our institutional review board.
Histopathology review
CGPR and GMH reviewed all cases for which oesophageal tissue was available. The presence or absence of skeletal muscle was used to designate a tissue block as being from the upper or lower parts of the oesophagus, respectively; presence of a transition from squamous oesophageal to columnar gastric epithelium was used to identify the gastro‐oesophageal junction (GOJ). Cases were evaluated for histological evidence for the presence of Barrett's oesophagus. When multiple slides were available from a single region of the oesophagus, each slide was reviewed separately. Numerical data from multiple slides were handled by using the highest values in the range as representative of the region. Descriptive data from multiple slides were handled by considering the presence of an abnormality in any given slide to represent its presence in the region.
Mucosa
The mucosa was scored as normal, abnormal (inflammation, ulceration, inclusion bodies of herpes simplex virus infection or budding yeast of candida infection) or uninterpretable (mucosa not present).
Muscle
The inner and outer smooth‐muscle layers of the muscularis propria were examined separately for loss of muscle fibres using a semiquantitative scoring system. Smooth‐muscle fibre atrophy was scored as 0 (absent), 1 (affecting <50% of the muscle layer), 2 (affecting approximately 50% of the muscle layer), 3 (affecting >50%, but <100% of the muscle layer) or 4 (affecting 100% of the muscle layer). A maximum and mean atrophy score was used to represent a region including the distal, GOJ or proximal oesophagus for each case (when multiple slides were present) and then a mean of the individual scores was calculated for the cases and controls. Other features that were subjectively evaluated included evidence of collagen content (fibrosis), inflammatory infiltrates, and loss of or damage to skeletal muscle fibres.
Vasculature
The vasculature of the oesophagus was scored as normal, abnormal (varices, intimal proliferation, inflammation, thrombi, arteritis or necrosis) or uninterpretable (no blood vessels visible). When vascular changes were present, the location of the change was noted as being in the submucosa, within the muscularis propria, or in the adventitia of the oesophagus.
Nerves
The myenteric plexus was examined for the presence of the following inflammatory cells: lymphocytes, plasma cells, polymorphonuclear cells, eosinophils, macrophages and mast cells. When present, the inflammation was designated intraneural or perineural if the inflammatory cells appeared to be infiltrating or surrounding the nerve bundles, respectively. In the absence of inflammatory cells the myenteric plexus was considered to be normal, and if no neural elements were visible the myenteric plexus was considered to be uninterpretable.
Immunohistochemical analysis
Immunohistochemical stains with polyclonal antibody #C‐19 to the c‐kit receptor (Santa Cruz Biotechnology, Santa Cruz, California, USA) were carried out on three cases. Three cases were identified for immunohistochemical staining because of exceptional preservation of the tissue, the presence of relatively large regions of both atrophic and normal‐appearing smooth muscle on the same slide, the availability of paraffin wax tissue blocks and the orientation of the tissue. The oesophageal tissue was cut perpendicular to the long axis of the oesophagus, allowing easy identification of ICC in the circular muscle layer by their bipolar, elongated cell bodies.8 Regions of normal and atrophic circular smooth muscle were identified on Masson‐trichrome‐stained tissue and ICC were counted in the corresponding regions of #C‐19‐treated slides.
Analysis
The proportion of scleroderma cases versus controls with each pathological feature (smooth muscle atrophy, vascular intimal proliferation, etc) were compared using χ2 analysis or by Fisher's exact test where appropriate. Mean values of atrophy scores were compared using Student's t test and comparison of multiple groups with analysis of variance. All statistical analyses were carried out using Stata software, V.7. Significance was defined at an α‐level of 0.05, using a two‐tailed test.
Results
In all, 92 cases of scleroderma were identified by keyword search from The Johns Hopkins Hospital autopsy computer database. Nine cases did not meet the ACR criteria for scleroderma or study criteria for the CREST syndrome. Oesophageal tissue was not available for nine additional cases. Slides from the remaining 74 scleroderma cases and from 74 age, race and sex‐matched controls were reviewed. The year of autopsy ranged from 1929 to 2002.
Demographics and clinical disease information
Among the cases, 65% were female, with 61% Caucasian, 38% African‐American and 1% Hispanic. In all, 28% of cases died in their 40s, and 64% of patients were diagnosed with systemic sclerosis or scleroderma within 5 years of their death (table 1). The autopsy report documented that 48 of the 74 patients with scleroderma died of scleroderma‐related issues including heart failure,9 lung fibrosis,8 pulmonary arterial hypertension,8 gastrointestinal disease,10 kidney failure11 and multisystem deterioration.2 Raynaud's phenomenon was present in 84% of cases, whereas for 9% Raynaud's status could not be determined. Altogether 70% of cases met the major ACR criteria for scleroderma, 27% met the minor criteria and 3% met the study criteria for the CREST syndrome. Limited disease was more common than diffuse disease at death (64% and 36%, respectively).
Table 1 Demographics of patients with systemic sclerosis (scleroderma).
| Sex | n (%) |
|---|---|
| Female | 48 (65) |
| Male | 26 (35) |
| No of years from diagnosis to death | |
| <1 | 21 (28) |
| 1–5 | 26 (35) |
| 6–10 | 9 (12) |
| 11–15 | 8 (11) |
| >15 | 3 (4) |
| Unknown | 7 (9) |
| Race | |
| African‐American | 28 (38) |
| White | 45 (61) |
| Hispanic | 1 (1) |
| Age at death (years) | |
| 0–9 | 0 (0) |
| 10–19 | 2 (3) |
| 20–29 | 7 (9) |
| 30–39 | 11 (15) |
| 40–49 | 21 (28) |
| 50–59 | 14 (19) |
| 60–69 | 9 (12) |
| 70–79 | 8 (11) |
| 80–89 | 1 (1) |
| Unknown | 1 (1) |
Histological review
The number of slides available for review from each oesophageal region ranged from 0 to 13 for cases and from 0 to 3 for controls. At least one slide from one region had to be present for a case or control to be included in the study. Cases tended to have more slides than controls, probably because of interest in the histological findings during the original tissue preparation, which was carried out without anticipation of the current study, in most cases preceding the current study by decades.
Mucosa
Table 2 describes the mucosal findings in cases and controls. Inflammation was present in 44% of cases and 24% of controls at the GOJ (p = 0.06); in 35% of cases and 40% of controls in the distal oesophagus (p = 0.65); and in 10% of cases and 19% of controls in the proximal oesophagus (p = 0.39). Ulceration of the mucosa was more common in scleroderma cases than in controls in all regions of the oesophagus, although this difference was not significant in any single region (p = 0.39 for GOJ, p = 0.36 for distal oesophagus and p = 0.32 for proximal oesophagus). There were 23 of 49 (40%) patients with mucosal inflammation in the pre‐1980 group and 9 of 19 (47%) in the post‐1980 group. This was not significantly different by χ2 analysis. These data suggest that antisecretory agents that were in use for gastro‐oesophageal reflux disease after 1980 had no influence on the mucosal findings. Among the participants with scleroderma, there was one case of metastatic infiltrating lobular breast cancer, no cases of oesophageal cancer, one case of Barrett's oesophagus, no cytomegalovirus, two cases with evidence of herpes simplex virus and one case of candida.
Table 2 Findings on review of oesophageal tissue.
| Mucosal findings | GOJ | Distal oesophagus | Proximal oesophagus | All regions | ||||
|---|---|---|---|---|---|---|---|---|
| SSc | Control | SSc | Control | SSc | Control | SSc | Control | |
| n = 43 | n = 37 | n = 57 | n = 30 | n = 50 | n = 16 | n = 74 | n = 74 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Normal | 17 (40) | 26 (70)* | 29 (51) | 18 (60) | 39 (78) | 13 (81) | 34 (46) | 48 (65)** |
| Oesophagitis | 19 (44) | 9 (24) | 20 (35) | 12 (40) | 5 (10) | 3 (19) | 32 (47) | 24 (33) |
| Ulceration | 10 (23) | 5 (14) | 11 (19) | 3 (10) | 5 (10) | 0 (0) | 20 (27) | 8 (11)* |
| Herpes virus inclusions | 1 (2) | 1 (3) | 1 (2) | 1 (3) | 0 (0) | 0 (0) | 2 (3) | 2 (3) |
| Budding yeast | 1 (2) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| Uninterpretable | 5 (12) | 2 (5) | 6 (11) | 0 (0) | 4 (8) | 0 (0) | 4 (5) | 2 (3) |
| Vascular findings | ||||||||
| Normal | 25 (58) | 33 (89)* | 37 (65) | 24 (80) | 40 (80) | 16 (100) | 41 (55) | 65 (88)* |
| Intimal proliferation | 17 (40) | 2 (5)* | 16 (28) | 2 (7)** | 7 (14) | 0 (0) | 28 (38) | 4 (5)* |
| Varices | 0 (0) | 0 (0) | 2 (4) | 2 (7) | 0 (0) | 0 (0) | 2 (3) | 2 (3) |
| Thrombosis | 0 (0) | 1 (3) | 2 (4) | 1 (3) | 1 (2) | 0 (0) | 3 (4) | 2 (3) |
| Arteritis | 0 (0) | 0 (0) | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| Uninterpretable | 1 (2) | 1 (3) | 0 (0) | 1 (3) | 2 (4) | 0 (0) | 0 (0) | 2 (3) |
| Myenteric plexus findings | ||||||||
| Cellular infiltrate | 28 (65) | 32 (86)** | 43 (75) | 29 (97)** | 32 (64) | 15 (94)** | 61 (82) | 68 (92) |
| Lymphocyte | 27 (63) | 32 (86)** | 42 (74) | 29 (97)* | 32 (64) | 14 (88) | 59 (80) | 67 (91) |
| Plasma cell | 11 (26) | 3 (8) | 10 (18) | 2 (7) | 9 (18) | 1 (6) | 23 (31) | 6 (8)* |
| Polymorphonuclear cell | 2 (5) | 0 (0) | 5 (9) | 3 (10) | 0 (0) | 2 (13) | 6 (8) | 5 (7) |
| Mast cell | 1 (2) | 0 (0) | 6 (11) | 2 (7) | 0 (0) | 1 (6) | 7 (9) | 3 (4) |
| Macrophage | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Eosinophil | 1 (2) | 0 (0) | 4 (7) | 0 (0) | 1 (2) | 1 (6) | 4 (5) | 1 (1) |
| Perineural infiltrate | 27 (63) | 32 (86)** | 39 (68) | 29 (97)* | 30 (60) | 15 (94)** | 58 (78) | 68 (92)** |
| Intraneural infiltrate | 4 (9) | 1 (3) | 9 (16) | 1 (3) | 4 (8) | 0 (0) | 13 (18) | 2 (3)* |
| Uninterpretable | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
GEJ, gastro‐oesophageal junction; SSc, systemic sclerosis.
*p⩽0.01 for SSc v controls.
**p⩽0.05 for SSc v controls.
Smooth muscle
Although nearly the entire scleroderma group (94%) had some degree of atrophy either in the circular or in the longitudinal layer of oesophageal smooth muscle, the controls rarely showed smooth muscle atrophy (5%; p<0.001). Moreover, when atrophy was observed in controls, it was mild, involving <50% of the smooth muscle. In all, 93% of the cases had atrophic areas in the circular layer, whereas 66% of cases had them in the longitudinal smooth‐muscle layer. The circular muscle layer was more likely to be atrophic in the cases at the GOJ and in the distal oesophagus than those at the proximal oesophagus (86%, 86% and 72%, respectively), whereas the opposite was true of the longitudinal fibres (47% and 49% atrophy at both the GOJ and the distal oesophagus and 60% in the proximal oesophagus; fig 1). The circular layer had dramatically more severe atrophy than the longitudinal layer, especially in the distal oesophagus and at the GOJ. The mean atrophy score (scale 0, no atrophy and 4, 100%) for scleroderma cases (using maximum values for each individual) was 2.1 for the circular layer and 0.7 for the longitudinal layer at the GOJ, 2.3 and 0.7 at the distal oesophagus, and 1.7 and 1.2 in the proximal oesophagus. To assess for detection bias, we also examined the mean atrophy scores for the participants and there were no significant differences in the observation of the difference between atrophy in circular versus longitudinal muscle layers at each level of the oesophagus. The atrophy score for the circular layer corresponds to about 50% of the circular smooth muscle, with much less atrophy in the longitudinal smooth muscle. No association was seen between disease duration and degree of smooth muscle atrophy (p = 0.921 using maximum atrophy scores, p = 0.55 using mean atrophy scores).
Figure 1 Smooth‐muscle atrophy is more common in (systemic sclerosis (SSc)) cases than in controls, and is most common in the circular layers of the distal oesophagus and the gastro‐oesophageal junction (GOJ).
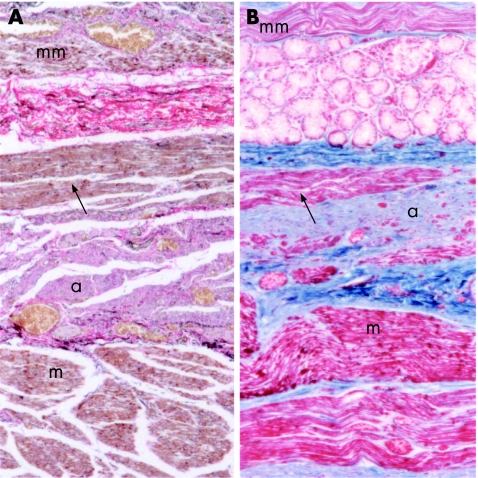
There was a striking tendency for central regions of smooth muscle bundles to be atrophic, whereas normal muscle remained at the periphery (fig 2A). Of note, muscular atrophy did not involve the interdigitating skeletal muscle, even when the associated smooth muscle was atrophic (fig 2B). Several cases had a non‐atrophic anomalous muscle strip that ran parallel to the longitudinal layer. This strip was located on the luminal side of the atrophic circular layer, such that the atrophic circular muscle lay between two normal strips of longitudinal muscle (fig 3), a finding that suggests selective loss of circular smooth muscle. Cases of severely atrophic smooth muscle showed thinning of the muscle layer, with the interstitial fibres appearing to have collapsed. In no cases was there convincing evidence of increased collagen deposition and, therefore, tissue fibrosis was not a significant finding. Occasionally, smooth muscle was infiltrated with inflammatory cells when diffuse, severe oesophagitis was present. However, in the absence of severe oesophagitis, there was no tendency for inflammatory cells to be present in smooth muscle.
Figure 2 (A) Representative picture from the distal oesophagus of a scleroderma case showing the tendency of smooth‐muscle bundles to have central atrophy (a) with histologically normal smooth muscle at the periphery (m; Masson trichrome). (B) In the proximal oesophagus, when smooth and skeletal muscle fibres are present in the same tissue specimen, skeletal muscle fibres (sk) are free of visible abnormalities, whereas interdigitating and closely apposed histologically smooth muscle (m) is atrophic (a) (haematoxylin and eosin).
Figure 3 Reproduction of two systemic sclerosis tissue specimens that have an aberrant strip of longitudinal smooth muscle (black arrow) on the lumenal surface of the circular muscle that is not atrophic despite severe atrophy of the circular muscle (a). The muscularis mucosa is labelled mm and the longitudinal layer is labelled m. The mucosa and lumen are out of the frame of the photo, but would be found towards the top of the page (A, Verhoef‐van Gieson elastic; B, Masson trichrome).
Vasculature
Vascular intimal proliferation was found in 38% of cases and in 5% of controls (p<0.001). Table 2 shows the vascular lesions in the oesophageal region. Vessels in the GOJ and the distal oesophagus were more likely to show intimal proliferation than the proximal oesophagus (40%, 28% and 14%, respectively). Vessels with intimal proliferation were found in a patchy distribution in the submucosa, adventitia or in the muscularis of the oesophagus, in no discernable pattern. The presence of vascular intimal proliferation was not associated with smooth‐muscle atrophy (p = 0.29); thus smooth‐muscle atrophy occurred in areas with normal‐appearing vasculature. No evidence of ischaemic necrosis of the muscularis or any other layers of the oesophagus was found. An analysis for the proportion of patients with intimal proliferation in each of three disease duration categories (<1 year, 1–5 years and >5 years) showed no difference between the groups for the presence of vascular disease (p = 0.9) by analysis of variance.
Myenteric plexus
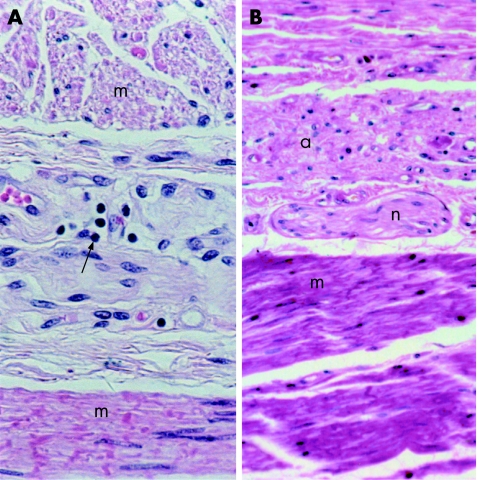
Cellular infiltrates were identified in the myenteric plexus in more controls than cases (92% of controls and 82% of scleroderma cases; p = 0.091). Table 2 shows the cell types of the infiltrates found at the myenteric plexus in the oesophageal region. Although a few specimens in each group showed robust infiltrates, most were trivial, involving few lymphocytes (fig 4). Intraneural infiltrates were more likely to be found in cases than in controls (18% and 3%, respectively; p = 0.005), but within the cases, intraneural infiltrates were not associated with smooth‐muscle atrophy (p = 0.55). Excellent histological preservation in a few specimens showed that lymphatic vessels surrounded the myenteric plexus. Lymphatic vessels in this area may account for the trivial cellular infiltrates seen in such a large proportion of scleroderma and control tissue. In both groups, lymphocytes comprised the vast majority of inflammatory cells associated with the myenteric plexus; however, cases were significantly more likely to have plasma cells associated with the myenteric plexus than controls (31% and 8%, respectively; p = 0.001). The presence of a lymphocytic infiltrate, plasma cell infiltrate or infiltrate of any cell type was not significantly associated with smooth‐muscle atrophy (p = 0.57, 0.16, 1.00, respectively). The myenteric plexus of cases and controls was otherwise normal, with no abnormalities visible by light microscopy.
Figure 4 Both controls and cases have a high prevalence of trivial cellular infiltrates (most often lymphocytic, black arrow) associated with the myenteric plexus. Two extremes are included to show the lack of association between cellular infiltrates and smooth muscle atrophy: (A) a control with a cellular infiltrate and histologically normal circular and longitudinal smooth muscle (m), and (B) case with normal nerve elements (n) associated with atrophic circular smooth muscle (a) and normal longitudinal smooth muscle (m). (A,B) Haematoxylin and eosin staining.
Immunohistochemical studies
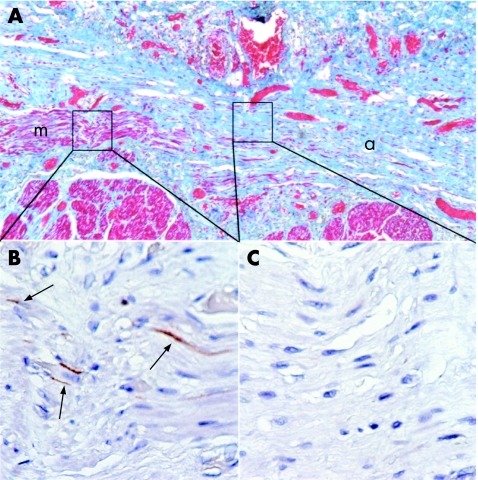
Immunohistochemical stains for c‐kit in three scleroderma cases allowed ICC to be identified in smooth‐muscle fibres parallel to the plane of section. ICC appeared as thin, elongated fibres (fig 5). Four regions of atrophic and four regions of histologically normal smooth muscle were examined for the presence of ICC. Although ICC were present in regions of normal‐appearing smooth muscle (mean number of ICC 17.5, range 13–25), they were less densely distributed in regions of atrophic smooth muscle of comparable size (mean number of ICC = 3.5, range 0–8; fig 5).
Figure 5 Interstitial cells of Cajal (ICC) are less dense in areas of smooth‐muscle atrophy than in areas of normal smooth muscle in systemic sclerosis (SSc). (A) Masson trichrome stain of the oesophagus of an SSc case with the lumenal surface towards the top of the page, showing regions of histologically normal circular smooth muscle (m), and atrophic circular smooth muscle (a). (B) C‐kit stain of a region of normal smooth muscle showing ICC (black arrows). (C) C‐kit stain of an atrophic region of smooth muscle showing no ICC. (B,C) C‐kit immunohistochemistry.
Discussion
Our study is the first case–control investigation of smooth‐muscle pathology and associated lesions of the oesophagus in scleroderma. Our review showed that oesophageal smooth‐muscle atrophy, especially in the circular layer, is common in scleroderma, but is not associated with vascular disease, tissue fibrosis or an inflammatory process in the myenteric plexus or with any neural abnormality visible by histological examination. In this first investigation of ICC in scleroderma, we found that the numbers of ICC were fewer in areas of atrophic circular smooth muscle than in areas of normal‐appearing circular smooth muscle.
Evidence of ischaemic disease in the oesophagus is suggested by functional studies that show impaired oesophageal blood flow as measured by oesophageal rewarming.9 Non‐inflammatory intimal cell proliferation in arterioles of scleroderma cases has been described in an autopsy record review.10 In addition, ultrastructural studies found endothelial damage in dermal microvasculature,11 and capillary endothelial cell swelling, basement membrane thickening and lamination in vessels of the muscularis propria of the oesophagus.12 In our study, normal‐appearing skeletal muscle interdigitated with atrophic smooth muscle, a distribution of atrophic lesions that seem inconsistent with a vascular aetiology. Also, there was no evidence of ischaemic necrosis in any of the 74 cases reviewed. Although vascular intimal proliferation was seen, it was not associated with areas of smooth‐muscle atrophy. The findings in our study are not consistent with a lesion primarily caused by abnormal microcirculation.
Inflammatory infiltrates were not present in the smooth muscle of the scleroderma cases unless there was severe, transmural oesophagitis. This suggests that inflammatory infiltration of the tissue is not a cardinal feature of the pathogenesis of oesophageal smooth‐muscle lesions of scleroderma. Tissue fibrosis is often evoked as the cause of the oesophageal disease in scleroderma. However, we did not find that fibrosis was a major contributor to the smooth‐muscle lesions in our series. The thickened tissue that we would expect to find in fibrotic smooth muscle was not present—in fact, the severely atrophic smooth‐muscle layers in the oesophagus in scleroderma were thinner than normal smooth‐muscle layers, appearing to have collapsed without fibrosis. This observation coincides with those of other authors of case reports and uncontrolled series who have noted that atrophy is more prominent than fibrosis.3,10,13 Scleroderma oesophagus is characterised by a dilated rather than stenotic oesophagus, supporting our histological finding.
Studies of gastrointestinal motility involving patients with scleroderma suggest that neurological dysfunction is common, and that it may precede myopathic dysfunction and histological changes of smooth muscle.14 Manometric evaluation of four cases showed oesophageal hypomotility in areas that later proved to have histologically normal smooth muscle.13 Pharmacological studies of oesophageal motility in patients with scleroderma using manometry showed a response to direct stimulation of oesophageal smooth muscle early in the disease that was not present in patients with more advanced disease.15 Another manometric study showed that patients with scleroderma and recent‐onset dysphagia had uncoordinated myoelectric hyperactivity, whereas patients with disease of longer duration showed diminished myoelectric activity.16 Manometric studies of other portions of the gastrointestinal tract have shown similar findings.17,18,19 The aetiology of these findings is unknown but is presumed to be due to neural dysfunction that precedes muscle disease.
Our review found no histological abnormalities of the myenteric plexus, similar to case reports and series that noted histologically normal myenteric plexus in the oesophagus and other regions of the gastrointestinal tract of patients with scleroderma.13,20,21 Other investigators describe degranulating mast cells and eosinophils associated with myenteric plexus of the intestine in scleroderma.22,23 Our review found lymphocytes, not mast cells, to be the predominant inflammatory cell associated with the myenteric plexus in the oesophagus, consistent with the reported observation of round‐cell infiltrates in the oesophageal myenteric plexus of scleroderma.13 In our study, inflammatory infiltrates were found with comparable frequency in cases and controls, suggesting that the presence of inflammatory cells in the myenteric plexus is not specific to scleroderma.
Although our study found that the myenteric plexus appears normal by light microscopy and an ultrastructural examination of the nerve endings in the oesophagus in scleroderma by other groups showed no abnormalities,12 this does not exclude the possibility that abnormal neural function is involved in the selective loss of smooth muscle. Neural lesions proximal to the tissue studied could cause downstream dysfunction without altering the histological appearance of the myenteric plexus. Antimyenteric neuronal antibodies are found in the serum from patients with scleroderma,24 and passive transfer of human scleroderma sera containing antimyenteric neuronal antibody produces disruption of the migrating motor complex activity in the small intestine of the rat.25 Functional abnormalities caused by or associated with antimyenteric neuronal antibodies could affect oesophageal motility and smooth muscle integrity without altering the histological appearance of the myenteric plexus.
ICC are thought to have a key role in normal oesophageal motility, and therefore injury or dysfunction of ICC may be involved in the oesophageal dysfunction of scleroderma. In this first investigation of ICC in scleroderma, in a limited number of specimens, ICC were fewer in number in atrophic smooth muscle compared with areas of non‐atrophic smooth muscle in the scleroderma cases. Oesophageal ICC are associated with smooth, not skeletal, muscle, and are found in both the longitudinal and the circular layers of smooth muscle.26 The role of ICC in scleroderma pathology is a topic that needs further study.
One important limitation of our case–control autopsy review is that we could not distinguish between actively evolving disease and static lesions. However, cases with longer duration between diagnosis and death were not more likely to have greater degrees of atrophy than cases with shorter duration of disease. This finding may reflect that the insult is a one‐time event, which takes place early in disease. To maximise the number of scleroderma cases, we used tissue from autopsies spanning many decades, introducing variability in tissue preparation. The number of slides available for review was greater for cases than for controls, potentially introducing a bias towards finding more histological abnormalities in the cases. However, the degree of abnormality (eg, muscle atrophy) in cases was so striking, we do not believe that detection bias played a significant role in our evaluation. The lack of difference in the presence of neural inflammatory infiltrate in cases and controls supports this criteria.
This review of a large number of scleroderma cases with contemporaneous controls shows that smooth muscle atrophy, especially of the circular layer, of the oesophagus is characteristic of scleroderma and appears unrelated to vascular lesions, fibrosis, inflammation or to myenteric plexus abnormalities. We also report that the ICC are found in reduced numbers in regions of smooth muscle atrophy. The pattern of preferential, severe atrophy of the circular smooth muscle in the absence of another definable lesion suggests that the dysfunction of the oesophagus in scleroderma is related to the innervation of this layer at a level not observable by light microscopy, or that the lesion is due to a primary smooth‐muscle disease process.
Acknowledgements
We thank Jolene Patey and Pam Hill for their assistance in the preparation of this manuscript and the Scleroderma Research Foundation for their support of the Johns Hopkins Scleroderma Center.
Abbreviations
ACR - American College of Rheumatology
CREST - calcinosis, Raynaud's phenomenon, oesophageal dysmobility, sclerodactyly and telangiectasia
GOJ - gastro‐oesophageal junction
ICC - interstitial cells of Cajal
SSc - systemic sclerosis
Footnotes
Competing interests: None.
References
- 1.Poirier T J, Rankin G B. Gastrointestinal manifestations of progressive systemic scleroderma based on a review of 364 cases. Am J Gastroenterol 19725830–44. [PubMed] [Google Scholar]
- 2.Lindsay J R. Esophageal lesions in diffuse scleroderma. Laryngoscope 19495983–112. [DOI] [PubMed] [Google Scholar]
- 3.Abrams H L, Carnes W H, Eaton J. Alimentary tract in disseminated scleroderma with emphasis on small bowel. Arch Intern Med 19549461–81. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz S, Skinsnes O K. Generalized progressive scleroderma: report of an instance of esophagoscopic perforation of the esophagus with description of roentgenological and necropsy findings. Am J Roentgenol 194962359–367. [PubMed] [Google Scholar]
- 5.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 198023581–590. [DOI] [PubMed] [Google Scholar]
- 6.LeRoy E C, Black C, Fleischmajer R.et al Scleroderma (systemic sclerosis): classification subsets and pathogenesis. J Rheumatol 198815203–205. [PubMed] [Google Scholar]
- 7.Velayos E E, Mas A T, Stevens M D.et al The “CREST” syndrome. Comparison with systemic sclerosis (scleroderma). Arch Intern Med 19791391240–1244. [DOI] [PubMed] [Google Scholar]
- 8.Torihashi S, Horisawa M, Watanabe Y. c‐Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst 19997538–50. [DOI] [PubMed] [Google Scholar]
- 9.Belch J J, Land D, Park R H.et al Decreased oesophageal blood flow in patients with Raynaud's phenomenon. Br J Rheumatol 198827426–430. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo W A, Fries J F, Masi A T.et al Pathologic observations in systemic sclerosis (scleroderma). A study of fifty‐eight autopsy cases and fifty‐eight matched controls. Am J Med 196946428–440. [DOI] [PubMed] [Google Scholar]
- 11.Prescott R J, Freemont A J, Jones C J.et al Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol 1992166255–263. [DOI] [PubMed] [Google Scholar]
- 12.Russell M L, Friesen D, Henderson R D.et al Ultrastructure of the esophagus in scleroderma. Arthritis Rheum 1982251117–1123. [DOI] [PubMed] [Google Scholar]
- 13.Treacy W L, Baggenstoss A H, Slocumb C H.et al Scleroderma of the esophagus: a correlation of histologic and physiologic findings. Ann Intern Med 196359351–356. [DOI] [PubMed] [Google Scholar]
- 14.Sjogren R W. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum 1994371265–1282. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S, Fisher R, Lipshutz W.et al The pathogenesis of esophageal dysfunction in scleroderma and Raynaud's disease. J Clin Invest 1972512663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bortolotti M, Pinotti R, Sarti P.et al Esophageal electromyography in scleroderma patients with functional dysphagia. Am J Gastroenterol 1989841497–1502. [PubMed] [Google Scholar]
- 17.DiMarino A J, Carlson G, Myers A.et al Duodenal myoelectric activity in scleroderma. Abnormal responses to mechanical and hormonal stimuli. N Engl J Med 19732891220–1223. [DOI] [PubMed] [Google Scholar]
- 18.Bassotti G, Battaglia E, Debernardi V.et al Esophageal dysfunction in scleroderma: relationship with disease subsets. Arthritis Rheum 1997402252–2259. [DOI] [PubMed] [Google Scholar]
- 19.Greydanus M P, Camilleri M. Abnormal postcibal antral and small bowel motility due to neuropathy or myopathy in systemic sclerosis. Gastroenterology 198996110–115. [DOI] [PubMed] [Google Scholar]
- 20.Castleman B, McNeely B U. Case records of the Massachusetts General Hospital, case 28‐1965. N Engl J Med 19652721340–1348. [DOI] [PubMed] [Google Scholar]
- 21.Stafford‐Brady F J, Kahn H J, Ross T M.et al Advanced scleroderma bowel: complications and management. J Rheumatol 198815869–874. [PubMed] [Google Scholar]
- 22.Malandrini A, Selvi E, Villanova M.et al Autonomic nervous system and smooth muscle cell involvement in systemic sclerosis: ultrastructural study of 3 cases. J Rheumatol 2000271203–1206. [PubMed] [Google Scholar]
- 23.DeSchryver‐Kecskemeti K, Clouse R E. Perineural and intraneural inflammatory infiltrates in the intestines of patients with systemic connective‐tissue disease. Arch Pathol Lab Med 1989113394–398. [PubMed] [Google Scholar]
- 24.Howe S, Eaker E Y, Sallustio J E.et al Antimyenteric neuronal antibodies in scleroderma. J Clin Invest 199494761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaker E Y, Kuldau J G, Verne G N.et al Myenteric neuronal antibodies in scleroderma: passive transfer evokes alterations in intestinal myoelectric activity in a rat model. J Lab Clin Med 1999133551–556. [DOI] [PubMed] [Google Scholar]
- 26.Faussone‐Pellegrini M S, Cortesini C. Ultrastructural features and localization of the interstitial cells of Cajal in the smooth muscle coat of human esophagus. J Submicrosc Cytol Pathol 198517187–197. [PubMed] [Google Scholar]