Abstract
Background
A transjugular liver biopsy (TJLB) specimen is often smaller or more fragmented than a percutaneous liver biopsy (PLB) specimen. Recently, for PLB, the minimum requirements to evaluate chronic hepatitis have been set at 20–25 mm length and ⩾11 complete portal tracts.
Aim
To evaluate and compare length of TJLB and PLB specimens, portal tract number, fragmentation and adequacy for histopathological diagnosis and staging.
Patients and methods
326 consecutive TJLB specimens in 274 patients (109 who had undergone a transplantation), always using three passes (19‐G Tru‐cut) and 40 consecutive PLB specimens (15‐G Menghini).
Results
No technical failures occurred with the TJLB, and histological diagnosis was possible in 98.5%. The median (range) number of fragments was 5 (1–13) and the median total length was 22 (3–46) mm, with 65% of specimens ⩾20 mm and 36% ⩾25 mm; 60% of TJLB specimens were ⩾28 mm long had ⩾11 complete portal tracts. No difference in complete portal tract number or biopsy length was found between PLB and TJLB specimens.
Conclusion
A TJLB specimen with three passes is adequate for histological diagnosis, with 89% of specimens being either ⩾15 mm or having ⩾6 complete portal tracts. Although adequate sampling remains a limitation for staging and grading of chronic hepatitis, TJLB is comparable to PLB in this respect.
Liver biopsy is the gold standard for histological confirmation, diagnosis and severity of liver disease.1,2 Percutaneous liver biopsy (PLB) and transjugular liver biopsy (TJLB) are most often used.1,3,4,5,6,7 PLB is usually used first,8,9 and TJLB is used if there are contraindications such as major coagulopathy or moderate ascites.7,10,11,12 However, TJLB is considered to be less satisfactory than PLB because smaller, more fragmented and thinner samples are obtained more often.1,10
Liver biopsy specimens represent approximately 1/50 000 of the liver.10 Several studies have evaluated the minimum length and number of portal tracts necessary for optimal histological evaluation. Diagnostically, a PLB specimen of ⩾15 mm length has been considered to be necessary for accurate diagnosis in chronic liver disease13; a review10 also concluded that 6–8 complete portal tracts should be present for diagnosis, most histopathologists accepting six portal tracts. However, with the increasing need to assess fibrosis in chronic hepatitis C and non‐alcoholic fatty liver disease, specimens of 20–25 mm length or ⩾11 complete portal tracts have been considered to be necessary to reliably assess grading and staging, and to reduce sampling errors.14,15
However, TJLB has received less attention, with the evaluation of only small cohorts.16,17,18,19,20 Our aim was to evaluate length, fragmentation and number of portal tracts in a large TJLB series and to audit sample adequacy in relation to recently proposed standards for diagnosis, and grading and staging.14,15
Material and methods
We evaluated 326 consecutive TJLB specimens in 274 patients between January 2003 and May 2004. The main indications for TJLB were (a) prothrombin time ⩾5 s from control or platelet count <50 000/mm3; (b) gross ascites, small cirrhotic liver or severe obesity; and (c) for patients with a liver transplant, a protocol liver biopsy or differential diagnosis of abnormal liver function tests, when either coagulopathy, cardiovascular compromise or patient cooperation might jeopardise the safe undertaking of a PLB.
We collected routine demographic, clinical and laboratory data. TJLB specimens were taken in the interventional radiology suite, after written consent, with continuous monitoring by electrocardiography and of oxygen saturation. The right internal jugular vein was punctured under ultrasound guidance after local anaesthesia. A guide wire (Cordis Corporation, Miami, Florida, USA) followed by a sheath was introduced into the inferior vena cava and, then, using a Cobra catheter (Cordis Corporation), to the hepatic veins, under fluoroscopic control. Hepatic phlebography was carried out to evaluate hepatic vein anatomy. The needle was inserted towards the liver via a 5.0‐F catheter and a sample obtained by passing the needle through the hepatic vein wall. All procedures were carried out by an experienced radiologist or hepatologist using a 19‐G (Cook (Sandet 6, DK‐4632 Bjaeverskov) or Kimal (Middlesex, UB8 2SA, UK)) Tru‐cut type biopsy needle (maximum core length 15–20 mm and external diameter 1 mm). Three passes were performed, our standard procedure since January 2003, regardless of size and adequacy of each core from each pass. After this, a small amount of contrast media was injected to check for capsular puncture. TJLB was considered technically unsuccessful if it was impossible to obtain a liver sample for any reason. Major complications (ie, a supraventricular tachycardia, capsular perforation or intraperitoneal haemorrhage) were always recorded. Day cases were followed up in hospital for up to 6 h, and inpatients for 24 h. Formal reporting by patients of any subsequent problems was evaluated from their discharge information sheet. Patients were seen within 2 weeks to discuss biopsy results.
We also evaluated 40 consecutive PLB specimens in patients without cirrhosis with abnormal liver function tests, considering them to be a comparison group. The PLB was carried out using a 15‐G Menghini needle (Unomedical Ltd, Redditch, UK). All TJLB and PLB specimens were formalin fixed and embedded in a paraffin wax block. Serial sections, 5 μm thick, were cut and stained with haematoxylin and eosin (H&E), reticulin, Victoria blue, Masson Trichrome and Perls' method for iron. Pathologists (APD and AQ) initially reviewed biopsy sections without the clinical information. The number and length of each fragment (cores that were completely separated) were recorded. The length (mm) was measured with a ruler. The width (mm) was evaluated in a random cohort of 40 TJLB specimens using measurement under the microscope, recording the maximum diameter found. The total length was given by adding the fragment lengths. A sample was “too fragmented” when the histopathologists decided that the fragment number and size did not permit histological interpretation.
The portal tracts in each fragment on H&E‐stained sections were counted both as complete portal tracts and as partial portal tracts (PPTs) as defined by Crawford et al21—that is, “focus of connective tissue containing at least two luminal structures”. A portal tract was considered to be complete when its full circumference was visible, or when at least three quarters of the circumference and three luminal structures (portal vein, hepatic artery and bile duct) were visible. A portal tract was considered to be partial when its circumference was incomplete and contained any two luminal structures. Foci with only one luminal structure were not counted as portal tracts. A single portal tract and its branches cut on a tangential plane can appear as multiple adjacent portal tracts. In this instance, we regarded these structures as part of a single portal tract, when in the projection trajectory of the ramification of a dominant portal tract with little interposed parenchyma. When relatively large portal tracts (ie, containing septal bile ducts (>100 μm) or medium sized (40–100 μm) interlobular bile ducts22) were seen, complete portal tracts were defined only if all three portal structures were visible, and partial portal tracts if only two structures were present. Connective tissue cut longitudinally, along side the biopsy edges, or large areas of connective tissue attached to the end of the biopsy core and containing only one vein, artery or bile duct were not considered to be portal tracts. Portal tracts were not counted in TJLB specimens that had severe architectural distortion (ie, cirrhosis, advanced fibrosis and massive necrosis), because it was not possible to reliably recognise, separate and count individual portal tracts, in keeping with a previous publication evaluating portal tract number.23 This is because with severe fibrosis and cirrhosis, fibrous bridging is accompanied by loss of the portal tract boundaries, abnormal vascularisation, bile duct loss and ductular reaction, resulting in complete effacement of the lobular architecture. The total number of complete portal tracts or PPTs was the summation of complete portal tracts or PPTs, respectively, in each fragment. The total portal tract number was the summation of total complete portal tracts and PPTs.
To evaluate fragmentation in reducing the number of complete portal tracts, we evaluated the PPTs at each end of each fragment (and not at its sides), considering them to be contiguous when there were >3 fragments (each representing one pass of the three passes performed at each biopsy), and thus representing the maximum theoretical number of complete portal tracts.
Statistical analysis
All data were analysed using the statistical package SPSS V.10.0. The χ2 test was used for comparing qualitative variables and the t test and Mann–Whitney U test for comparing quantitative variables. Quantitative variables normally distributed were expressed as mean (standard deviation (SD)) and non‐normally distributed as median values (range). Significance testing was two sided and set at p<0.05. The correlation between total length and total number of complete portal tracts in each TJLB specimen and in each fragment (ie, as a separate liver biopsy) was evaluated by Spearman's correlation. Comparisons were made between cirrhotic and non‐cirrhotic biopsies, non‐transplant versus transplant biopsies and between biopsies carried out for grading and staging (known chronic hepatitis C, alcoholic liver disease/non‐alcoholic steatohepatitis before transplant) and others carried out for diagnostic reasons, and those for both diagnosis and staging or grading.
Results
Patient characteristics
The 274 patients had a median age of 51 (13–87) years and 174 (63.5%) were men; 165 were patients who did not have a liver transplant, each underwent only one TJLB. In the 109 patients with a liver transplant, 16 had two TJLBs, 10 had 3, 4 had 4 and 1 had 5 TJLBs (table 1).
Table 1 Demographic and clinical characteristics of the 274 patients who underwent transjugular liver biopsy.
| Patients characteristics | Without liver transplant (n = 165) | With liver transplant (n = 109) |
|---|---|---|
| Age (years), mean (SD) | 48 (15) | 52 (9)* |
| Sex (male/female) | 92:73 | 82:27† |
| Cause of underlying disease, n (%)‡ | ||
| Primary liver disease | 126 (77) | 109 (100) |
| HCV | 21 (13) | 42 (39) |
| Alcoholic liver disease/non‐alcoholic steatohepatitis | 48 (29) | 24 (22) |
| PBC/PSC | – | 15 (14) |
| Autoimmune | 13 (8) | 4 (3) |
| Cryptogenic | 31 (19) | 16 (15) |
| Other§ | 13 (8) | 8 (7) |
| Haematological disease, n (%)¶ | 25 (15) | – |
| Other/unknown | 14 (8) | – |
HCV, hepatitis C virus; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
*p = 0.01.
†p = 0.001.
‡For patients with a liver transplant is the indication for liver transplantation.
§Other underlying diseases include drug‐induced hepatitis, haemochromatosis, Wilson's disease.
¶Haematological disease includes lymphoma, leukaemia, graft versus host disease.
In patients who did not have a liver transplant, 126 (77%) had suspected primary liver disease and 25 (15%) had underlying haematological disease. There were 70 (42%) patients with cirrhosis and 95 (58%) without cirrhosis. In all, 48 of 126 (29%) had alcoholic liver disease/non‐alcoholic steatohepatitis. Table 1 shows detailed diagnoses. Hepatitis C virus‐related cirrhosis was the major indication for liver transplant in 42 (38.5%) patients, followed by alcoholic liver disease/non‐alcoholic steatohepatitis in 24 (22%) and primary biliary cirrhosis or primary sclerosing cholangitis in 15 (14%; table 1).
Characteristics of TJLB specimens
No TJLB failed to obtain liver samples using three passes. The specimen was adequate for histological diagnosis in all but 5 (1.5%) samples: three were too small and two were too fragmented. We recorded no major complications.
Evaluation of fragmentation, length, width and portal tracts, and comparison with PLB specimens
The median total length was 22 (range 3–46) mm, with a median of 5 (range 1–13) fragments; 290 (89%) TJLB specimens were ⩾15 mm long, 213 (65%) were ⩾20 mm and 116 (36%) were ⩾25 mm. We found no significant difference in total length between specimens from patients with and without cirrhosis (23 v 22 mm, p = 0.07), and also in the median number of fragments (4 v 5, p = 0.08). We also found no significant difference in length between biopsy specimens from patients with or without liver transplant (23 v 22 mm, p = 0.45). Biopsy specimens of patients without a transplant had less fragments than those of patients with a transplant (4 v 5, p = 0.035; table 2). The maximum width was a median of 0.6 (range 0.5–0.8) mm.
Table 2 Characteristics of the transjugular liver biopsy specimens (n = 326).
| Characteristics | Without liver transplant (n = 165) | With liver transplant (n = 161) | p Value |
|---|---|---|---|
| Inadequate sample, n | 2 | 3 | NS |
| Total length, mm | 23 (4–44) | 22 (3–46) | NS |
| Number of fragments | 4 (1–11) | 5 (1–13) | 0.035 |
| Number of complete portal tracts* | 8.5 (1–26) | 8 (0–24) | NS |
| Number of partial portal tracts* | 6 (0–16) | 5 (0–14) | NS |
| No portal tract evaluation | 97 (59) | 35 (22) | <0.001 |
| Cirrhosis | 70 (42) | 15 (9) | <0.001 |
| Total length >15 mm | 146 (88) | 144 (89) | NS |
NS, not significant.
Values are n (%) or median (range).
*In 194 transjugular liver biopsy specimens in which portal tracts were counted.
In 132 TJLB specimens (58% in patients without a liver transplant/22% in those with a liver transplant, p<0.001), 40% of the total, portal tracts were not counted because of cirrhosis (n = 85, 64.4%), severe fibrosis (n = 20, 15.1%), severe infarction or necrosis (n = 18, 13.6%), ductopenia (n = 7, 5.2%) and excessive fragmentation (n = 2, 1.5%) (table 2). Thus, portal tracts were counted in 194 TJLB specimens: median number of total portal tracts (sum of complete portal tracts and PPTs) was 13 (range 2–38); median total number of complete portal tracts was 8 (range 0–26) and median number of PPTs was 5 (range 0–16). The median ratio of complete portal tracts to PPTs was 1.6 (range 0–12).
The median length and complete portal tracts in the biopsy speciemens taken for diagnostic purposes (n = 202) or solely for staging or grading (n = 124) or for both (n = 137) were as follows: length, 22 (3–46), 22 (9–44) and 22 (9–44) mm, and the number of complete portal tracts, 8 (0–26), 8 (3–25) and 8 (3–25), respectively. The proportions of TJLB specimens ⩾15 mm were 89%, 88% and 87%, and those ⩾25 mm were 34%, 38% and 38% in the three groups, respectively. The proportion of TJLB specimens with ⩾6 complete portal tracts were 73%, 82% and 80%, and those with ⩾11 complete portal tracts were 26%, 24% and 24% in the three groups, respectively. We found no significant differences (all p>0.89). We also found no differences when solely considering TJLB for chronic hepatitis C, in which the median length was 22 (range 10–41) mm and the number of complete portal tracts was 8 (3–25) versus all of the other biopsies, in which the median length was 22 (3–46) mm and the number of complete portal tracts was 8 (0–26). In biopsies of patients with chronic hepatitis C, 88.5% were ⩾15 mm and 38% were ⩾25 mm long.
In 76% of 194 TJLB specimens, we found ⩾6 complete portal tracts; these specimens were significantly longer than those with <6 complete portal tracts (median length 22 v 15 mm, p<0.001). In addition, TJLB specimens with ⩾11 complete portal tracts (26%) were significantly longer than those with <11 complete portal tracts (median length 28 v 20 mm, p<0.001). Although all TJLB specimens with ⩾11 complete portal tracts were >15 mm, only 50% of those ⩾25 mm had ⩾11 complete portal tracts. Finally, we found no significant difference between TJLB specimens from patients without or with a liver transplant regarding the number of complete portal tracts (8.5 v 8, p = 0.19) and PPT specimens (6 v 5, p = 0.17; table 2).
In the PLB specimens, the median total length and the number of complete portal tracts were 17 (range 5–49) mm and 7 (range 0–18) mm, respectively, which were not significantly different compared with TJLB specimens (median length 22 (range 3–46) mm and the median number of complete portal tracts 8 (0–26); p = 0.35).
Correlation between the length and complete portal tracts in TJLB specimens
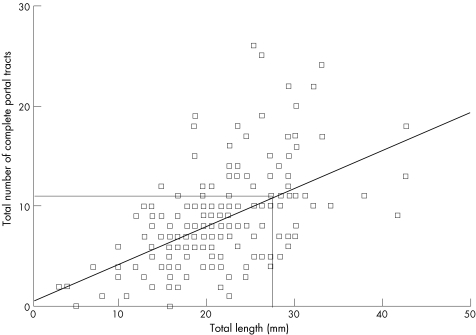
This was assessed in the 194 TJLB specimens (Spearman's r = 0.49, p<0.001; fig 1). A TJLB specimen ⩾28 mm long contained ⩾11 complete portal tracts in 60% cases versus 16% when <28 mm (p<0.001; fig 1).
Figure 1 Non‐parametric correlation between total length and complete portal tracts in the 194 transjugular liver biopsy specimens in which portal tracts could be counted (Spearman's r = 0.49, p⩽0.001).
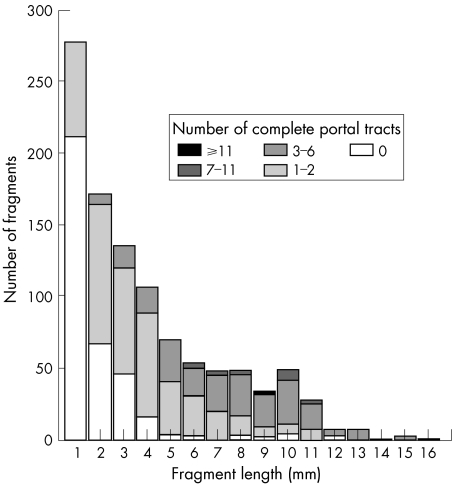
In the 194 TJLB specimens, 1045 fragments were obtained (fig 2). The distribution of fragment length was similar in all TJLB specimens. Generally, longer fragments contained more complete portal tracts (fig 2); 212 of 277 (76.5%) fragments 1 mm long had no complete portal tracts, and the remainder had 2–3 complete portal tracts (fig 2). Considering the 1045 fragments as separate liver specimens, we found good correlation between fragment length and complete portal tracts (r = 0.72, p<0.001). From these data, we can estimate that on average a non‐fragmented specimen ⩾23 mm long would be needed to obtain the optimal number of 11 complete portal tracts—that is, the partial portal tracts at the ends (but not at the sides) of all fragments but three would be considered to be contiguous, assuming each pass would obtain one unfragmented core. Thus, both an increase in length and reducing fragmentation of TJLB are required to improve the number of adequate liver biopsy specimens.
Figure 2 Fragment length and number in 1045 fragments in 194 biopsy specimens that could be evaluated, and relationship between number of complete portal tracts and fragment length and number.
Discussion
Liver biopsy is an essential diagnostic tool in acute or chronic liver disease and in particular to estimate grading or staging.1,10 Adequate specimen size is crucial for accurate histological interpretation and elimination of sampling error and intraobserver or interobserver variability,24,25 but liver biopsy may still be suboptimal.14,15,26 Apart from length, width and fragmentation also determine the quality of a liver biopsy specimen. For this reason, counting complete portal tracts is a better and more appropriate parameter for evaluation of adequacy of a liver biopsy and, thus, is the most suitable parameter to compare different kinds of liver biopsy specimens (eg, percutaneous versus transjugular, or Menghini versus Tru‐cut, or using different needle sizes) rather than solely the length or width of the specimen.10,15 Liver biopsy specimens with at least six complete portal tracts are considered to be adequate for diagnosis of diffuse liver disease.10
Assessing antiviral treatment has renewed interest in liver biopsy quality. Changes in inflammation (grading) and fibrosis (staging) after antiviral treatment represent end points in most clinical trials. Recent studies14,15 estimated that grade and stage were adequately assessed only in samples 20–25 mm long or containing ⩾11 complete portal tracts. According to these criteria, both PLB and TJLB usually provide inadequate specimens: using a 17‐G Menghini needle, only 42% of PLB specimens had ⩾10 complete portal tracts,23 and in the largest PLB series 45% were <20 mm long.27 In our study, 37.1% of TJLB specimens contained ⩾10 complete portal tracts and only 26% had ⩾11 complete portal tracts. Using three passes, the median number of complete portal tracts was 8. From the correlation (fig 1), a median number of 11 complete portal tracts could be achieved after >3 passes, as 60% of TJLB specimens ⩾28 mm long have ⩾11 complete portal tracts.
TJLB has often been considered to be suboptimal as a result of a higher frequency of inadequate specimens, related to the initial aspiration technique yielding small specimens, which were often excessively fragmented.28,29 The development of a Tru‐cut TJLB has improved the quality without any increase in complications.19,30 Despite this, the perception of being a second‐class biopsy persists, and is reinforced further, considering the current “optimal” PLB criteria.14,15 However, TJLB has some intrinsic characteristics that paradoxically could make it a more appropriate technique for liver biopsy, obtaining optimal specimens even with the current standards. Multiple cores can be obtained, in contrast with PLB in which >1 pass causes increased complications.6,12,31 In TJLB, provided the liver capsule is not punctured (does not occur with experienced operators), complications do not increase with multiple passes.17,26,31,32,33 Minor complications such as neck pain are also infrequent as previously audited by our group.33 However, the diameter of the TJLB needle is usually smaller than that used in PLB, and fragmentation is also thought to be worse.10
Only 11 studies have evaluated Tru‐cut TJLB in terms of the type of needle, number of passes, length, fragmentation and number of portal tracts (table 3).16,17,18,19,34,35,36,37,38,39,40 In these studies, an average of 2.7 passes per patient yielded specimens of mean length 14 (standard deviation (SD 2.6) mm) and a mean number of 6.4 (SD 2.3) complete portal tracts (table 3). Only three studies included ⩾100 TJLB specimens; one reported results using a 1.2‐mm diameter needle (number of passes not recorded), the mean length was 16.5 (SD 6.1) mm37; the second study reported a mean number of 5.6 portal tracts in 123 TJLB specimens38 and the last study evaluated 193 TJLB specimens with a mean length of 18 mm.19 In the last two studies, needle diameter and number of passes are not mentioned. In addition, only one study documented the number of fragments (mean 2.5), but the number of passes was not given.17
Table 3 Systematic review of eleven series of transjugular liver biopsies using Tru‐cut needle, and in which at least one of the following characteristics was documented: length, number of portals tracts, passes or fragments of liver biopsy tissue.
| First author | TJLB, n | Needle (G) | Pass, n | Fragment, n | Mean length, mm | Mean PT, n |
|---|---|---|---|---|---|---|
| Kardache16 | 29 | 18 | 1 | – | 12 | ⩾8* |
| De Hoyos17 | 52 | 18 | – | 2.5 | 17 | 6.2 |
| Bruzzi18 | 50 | 18 | 2.2 | – | 1–20† | 10.4 |
| Bull19 | 193 | – | – | – | 18 | – |
| Choo34 | 7 | 18 | 2.9 | – | 12 | – |
| Dimichele35 | 13 | 19 | >3–5 | – | 13.6 | 6 |
| Chau36 | 18 | 18 | 1–3 | – | 10 | 4 |
| Elshakawy‡37 | 100 | – | – | – | 16 | – |
| Regan38 | 123 | – | – | – | – | 5.6 |
| Little39 | 43 | 18, 19, 20 | 2.7 | – | 11 (18‐G), 15 (19‐G) | – |
| Gorriz40 | 77 | 18 | 5.2 | – | 15.2 | – |
TJLB, transjugular liver biopsy; G, gauge; PT, portal tracts.
G, gauge (translation of gauge (G) to external diameter of needle in millimetre (mm): 14 G = 2.1 mm; 15 G = 1.83 mm; 16 G = 1.65 mm; 17 G = 1.47 mm; 18 G = 1.24 mm; 19 G = 1.06 mm).
*In 14 patients with cirrhosis.
†Length per core.
‡1.2‐mm needle diameter.
Our study on TJLBs using three passes per procedure in 326 biopsy specimens (274 patients) is the largest study evaluating quality. We focused our evaluation on the portal tract assessment using published criteria,21 as Bravo et al10 showed that portal tract number was the most reliable parameter, and Colloredo et al14 stated that “The critical factor influencing the negative effect of smaller sizes is probably the significant drop in number of complete portal tracts in the smaller specimens”.
In our study, liver specimens were sufficient for diagnosis in 98.5% of cases similar to other studies on TJLB using 18‐G needles,18,30,37 or a 19‐G needle20 as in ours. The median length was 22 mm, and most importantly, the median number of complete portal tracts was 8. These results confirm the diagnostic efficacy of TJLB according to published guidelines10 and show that TJLB with three passes provides samples comparable with those obtained by PLB from our centre and from other series on PLB.23,27,41 In addition, as we showed in a recent review, the median length of a PLB specimen was 19.8 mm for Menghini and 14.5 mm for a Tru‐cut biopsy specimen, and the median number of complete portal tracts was 6.6 for Menghini and 5.8 for a Tru‐cut biopsy,42 so that about 50% of PLB specimens reported in series in the literature are suboptimal for diagnostic purposes. In our study, we found no major difference in the length or in the number of fragments between patients with or without cirrhosis, whereas differences have been reported for percutaneous biopsy specimens.42 This may be due to our standardised procedure for handling TJLB. Fragmentation may occur after biopsy during transport to the histopathology laboratory, and usually it has been reported on the fixed specimen and not at the time of biopsy.42 In addition, the previously asserted inadequacy of TJLB for diagnosis of cirrhosis29 was not confirmed. Our pathologists, using H&E, reticulin and Victoria blue stains, were always able to confirm the clinical diagnosis of cirrhosis, even from one sufficiently large fragment.
We found no differences in the number of complete portal tracts or in the length or proportions of specimens ⩾15 or ⩾25 mm long, in the biopsies carried out mainly for diagnosis, mainly for staging or grading, or from these groups when carried out for both. Thus, TJLB with three passes almost always gives optimal biopsy specimens for diagnosis (in contradistinction to data on PLB in the literature), but only results in adequate biopsy specimens for staging or grading in 38% (⩾25 mm) or 25% (⩾11 complete portal tracts).
Our study is also the first in which the number of complete portal tracts and PPTs has been evaluated in a TJLB series. The median ratio of complete portal tracts to PPTs was 1.6:1, but we found only a modest correlation between the total length and total number of complete portal tracts (r = 0.49). This finding may be due to two reasons: (1) the small diameter of liver samples or (2) fragmentation. The 19‐G needle has an external diameter of 1 mm and an internal diameter (which in fact determines the maximum width of the liver core) of approximately 0.9 mm. However, the width in several fragments was <0.5 mm or was variable, even in the same fragment. Crawford et al21 and Menghini,43 also found variable and smaller widths of biopsy specimens (0.9 (SD 0.3) mm and average 0.75 mm diameter, respectively) than the internal needle diameter when using 14‐G or a 1.5‐mm‐diameter Menghini needle. In our study, using Tru‐cut needle, this phenomenon may be exacerbated because the slot in the Tru‐cut needle is not cylindrical—the height of the space is less than its width. The range of widths is because the microtome cutting planes through the biopsy core do not always go through its maximum diameter, so that the internal needle diameter is not equivalent to biopsy width. In addition, there is tissue shrinkage with fixation embedding and some twisting of the tissue core. As a result, in our study, several lengthy fragments contained very few or even no complete portal tracts, but nevertheless ⩾11 complete portal tracts were obtained with core widths ⩽1 mm, in contradistinction to that by Colloredo et al.14 Using an 18‐G needle could improve this, but requires further evaluation because it is less flexible and increases the fragmentation rate.20,40
From our assessment of the number of complete portal tracts and PPTs in each fragment, using three passes, it can be estimated that 50% of three non‐fragmented cores of 23 mm total length would contain 11 complete portal tracts. However, in clinical practice, fragmentation can not be eliminated completely, but improvements might be made by more careful handling of TJLB samples. In our cohort, considering fragmentation, the median total length (to contain 11 complete portal tracts) was 28 mm.
In summary, obtaining at least three cores at each TJLB was safe, and resulted in a median length of 22 mm independent of the presence of cirrhosis. The median number of complete portal tracts in TJLB was 8 and that in PPT was 5. We found an average of five fragments in each TJLB. Reduction in fragmentation or modification of the length of the notch in the Tru‐cut device would increase the number of complete portal tracts. The characteristics of our TJLB with three passes are better than those obtained by the average PLB reported in the literature.23,27,41,42
Abbreviations
H&E - haematoxylin and eosin
PLB - percutaneous liver biopsy
PPT - partial portal tracts
TJLB - transjugular liver biopsy
Footnotes
Competing interests: None.
References
- 1.Campbell M S, Reddy K R. Review article: the evolving role of liver biopsy. Aliment Pharmacol Ther 200420249–259. [DOI] [PubMed] [Google Scholar]
- 2.Lebrec D. Various approaches to obtaining liver tissue—choosing the biopsy technique. J Hepatol 199625(Suppl 1)20–24. [PubMed] [Google Scholar]
- 3.Silecchia G, Raparelli L, Perrotta N.et al Accuracy of laparoscopy in the diagnosis and staging of lymphoproliferative diseases. World J Surg 200327653–658. [DOI] [PubMed] [Google Scholar]
- 4.DeWitt J, LeBlanc J, McHenry L.et al Endoscopic ultrasound‐guided fine needle aspiration cytology of solid liver lesions: a large single‐center experience. Am J Gastroenterol 2003981976–1981. [DOI] [PubMed] [Google Scholar]
- 5.Babb R R, Jackman R J. Needle biopsy of the liver. A critique of four currently available methods. West J Med 198915039–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut 199945(Suppl 4)IV1–NaN11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burroughs A K, Dagher L. Liver biopsy. In: Classen M, Tytgat G, Lightdale C, eds. Gastrointestinal Endoscopy. New York: Thieme, 2002252–259.
- 8.Mayoral W, Lewis J H. Percutaneous liver biopsy: what is the current approach? Results of a questionnaire survey. Dig Dis Sci 200146118–127. [DOI] [PubMed] [Google Scholar]
- 9.Tobkes A I, Nord H J. Liver biopsy: review of methodology and complications. Dig Dis 199513267–274. [DOI] [PubMed] [Google Scholar]
- 10.Bravo A A, Sheth S G, Chopra S. Liver biopsy. N Engl J Med 2001344495–500. [DOI] [PubMed] [Google Scholar]
- 11.McAfee J H, Keeffe E B, Lee R G.et al Transjugular liver biopsy. Hepatology 199215726–732. [DOI] [PubMed] [Google Scholar]
- 12.Piccinino F, Sagnelli E, Pasquale G.et al Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 19862165–173. [DOI] [PubMed] [Google Scholar]
- 13.Schlichting P, Holund B, Poulsen H. Liver biopsy in chronic aggressive hepatitis. Diagnostic reproducibility in relation to size of specimen. Scand J Gastroenterol 19831827–32. [DOI] [PubMed] [Google Scholar]
- 14.Colloredo G, Guido M, Sonzogni A.et al Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol 200339239–244. [DOI] [PubMed] [Google Scholar]
- 15.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003381449–1457. [DOI] [PubMed] [Google Scholar]
- 16.Kardache M, Soyer P, Boudiaf M.et al Transjugular liver biopsy with an automated device. Radiology 1997204369–372. [DOI] [PubMed] [Google Scholar]
- 17.De Hoyos A, Loredo M L, Martinez‐Rios M A.et al Transjugular liver biopsy in 52 patients with an automated Trucut‐type needle. Dig Dis Sci 199944177–180. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzi J F, O'Connell M J, Thakore H.et al Transjugular liver biopsy: assessment of safety and efficacy of the Quick‐Core biopsy needle. Abdom Imaging 200227711–715. [DOI] [PubMed] [Google Scholar]
- 19.Bull H J, Gilmore I T, Bradley R D.et al Experience with transjugular liver biopsy. Gut 1983241057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choh J, Dolmatch B, Safadi R.et al Transjugular core liver biopsy with a 19‐gauge spring‐loaded cutting needle. Cardiovasc Intervent Radiol 19982188–90. [DOI] [PubMed] [Google Scholar]
- 21.Crawford A R, Lin X Z, Crawford J M. The normal adult human liver biopsy: a quantitative reference standard. Hepatology 199828323–331. [DOI] [PubMed] [Google Scholar]
- 22.MacSween R, Desmet V, Roskams T. Developmental anatomy and normal structure. In: MacSween RN, Burt A, Portmann B, Ishak K, Scheuer PJ, Anthony P, eds. Pathology of the liver. London: Churchill Livingstone, 20021–66.
- 23.Rocken C, Meier H, Klauck S.et al Large‐needle biopsy versus thin‐needle biopsy in diagnostic pathology of liver diseases. Liver 200121391–397. [DOI] [PubMed] [Google Scholar]
- 24.Petz D, Klauck S, Rohl F W.et al Feasibility of histological grading and staging of chronic viral hepatitis using specimens obtained by thin‐needle biopsy. Virchows Arch 2003442238–244. [DOI] [PubMed] [Google Scholar]
- 25.Siddique I, El Naga H A, Madda J P.et al Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol 200338427–432. [DOI] [PubMed] [Google Scholar]
- 26.Demetris A J, Ruppert K. Pathologist's perspective on liver needle biopsy size? J Hepatol 200339275–277. [DOI] [PubMed] [Google Scholar]
- 27.Colombo M, Del Ninno E, de Franchis R.et al Ultrasound‐assisted percutaneous liver biopsy: superiority of the Tru‐Cut over the Menghini needle for diagnosis of cirrhosis. Gastroenterology 198895487–489. [DOI] [PubMed] [Google Scholar]
- 28.Lebrec D, Goldfarb G, Degott C.et al Transvenous liver biopsy: an experience based on 1000 hepatic tissue samplings with this procedure. Gastroenterology 198283338–340. [PubMed] [Google Scholar]
- 29.Gamble P, Colapinto R F, Stronell R D.et al Transjugular liver biopsy: a review of 461 biopsies. Radiology 1985157589–593. [DOI] [PubMed] [Google Scholar]
- 30.Smith T P, Presson T L, Heneghan M A.et al Transjugular biopsy of the liver in pediatric and adult patients using an 18‐gauge automated core biopsy needle: a retrospective review of 410 consecutive procedures. Am J Roentgenol 2003180167–172. [DOI] [PubMed] [Google Scholar]
- 31.McGill D B, Rakela J, Zinsmeister A R.et al A 21‐year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990991396–1400. [DOI] [PubMed] [Google Scholar]
- 32.Gilmore I T, Bradley R D, Thompson R P. Improved method of transvenous liver biopsy. Br Med J 19782249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papatheodoridis G V, Patch D, Watkinson A.et al Transjugular liver biopsy in the 1990s: a 2‐year audit. Aliment Pharmacol Ther 199913603–608. [DOI] [PubMed] [Google Scholar]
- 34.Choo S W, Do Y S, Park K B.et al Transjugular liver biopsy: modified Ross transseptal needle versus quick‐core biopsy needle. Abdom Imaging 200025483–485. [DOI] [PubMed] [Google Scholar]
- 35.DiMichele D M, Mirani G, Wilfredo C P.et al Transjugular liver biopsy is safe and diagnostic for patients with congenital bleeding disorders and hepatitis C infection. Haemophilia 20039613–618. [DOI] [PubMed] [Google Scholar]
- 36.Chau T N, Tong S W, Li T M.et al Transjugular liver biopsy with an automated trucut‐type needle: comparative study with percutaneous liver biopsy. Eur J Gastroenterol Hepatol 20021419–24. [DOI] [PubMed] [Google Scholar]
- 37.Elsharkawy A, Austin A, Ryder S. Clinical impact of trasnjugular liver biopsies in a non‐transplant center [abstract]. Hepatology 2002P728
- 38.Regan J, Mihalov M, Limjoko A.et al Transjugular liver biopsy: evaluation and comparison with percutaneous biopsy [abstract]. Hepatology 199726(Suppl 1)P281 [Google Scholar]
- 39.Little A F, Zajko A B, Orons P D. Transjugular liver biopsy: a prospective study in 43 patients with the quick‐core biopsy needle. J Vasc Interv Radiol 19967127–131. [DOI] [PubMed] [Google Scholar]
- 40.Gorriz E, Reyes R, Lobrano M B.et al Transjugular liver biopsy: a review of 77 biopsies using a spring‐propelled cutting needle (biopsy gun). Cardiovasc Intervent Radiol 199619442–445. [DOI] [PubMed] [Google Scholar]
- 41.Farrell R J, Smiddy P F, Pilkington R M.et al Guided versus blind liver biopsy for chronic hepatitis C: clinical benefits and costs. J Hepatol 199930580–587. [DOI] [PubMed] [Google Scholar]
- 42.Cholongitas E, Senzolo M, Standish R.et al A systematic review of the quality of liver biopsy specimens. Am J Clinical Pathology 2006125710–721. [DOI] [PubMed] [Google Scholar]
- 43.Menghini R. One‐second needle biopsy of the liver. Gastroenterology 195835190–199. [PubMed] [Google Scholar]