Abstract
Background
T cell‐mediated immunity plays a central part in the pathogenesis of tissue damage in inflammatory bowel disease (IBD). The mechanism by which T cells mediate tissue damage during IBD remains unclear, but evidence indicates that T cell‐derived cytokines stimulate fibroblasts to synthesise matrix metalloproteinases (MMPs), which then mediate mucosal degradation. We have previously shown that, in IBD, there is high production of interleukin (IL) 21, a T cell‐derived cytokine, which enhances Th1 activity.
Aim
To investigate whether IL21 controls MMP production by intestinal fibroblasts.
Methods
IL21 receptor (IL21R) was evaluated in intestinal fibroblasts by reverse transcriptase‐polymerase chain reaction (RT‐PCR) and western blotting. Fibroblasts were stimulated with IL21 and MMPs were evaluated by RT‐PCR and western blotting. The effect of a neutralising IL21R fusion protein (IL21R/Fc) on the induction of MMPs in fibroblasts stimulated with IBD lamina propria mononuclear cell (LPMC) supernatants was also evaluated.
Results
Intestinal fibroblasts constitutively express both IL21R and the common γ chain receptor, which are necessary for IL21‐driven signalling. IL21 enhances fibroblast production of MMP‐1, MMP‐2, MMP‐3 and MMP‐9, but not tissue inhibitors of MMP‐1 and MMP‐2. Moreover, IL21 synergises with tumour necrosis factor α to increase synthesis of MMP synthesis. IL21 enhances MMP secretion without affecting gene transcription and protein synthesis. IBD LPMC supernatants stimulate MMP secretion by intestinal fibroblasts, and this effect is partly inhibited by IL21R/Fc.
Conclusions
These results suggest that fibroblasts are a potential target of IL21 in the gut and that IL21 controls MMP secretion by fibroblasts.
Matrix metalloproteinases (MMPs) are a group of enzymes capable of degrading all components of the extracellular matrix.1,2 Excess MMP activity is involved in many human diseases such as rheumatoid arthritis, osteoarthritis, periodontal diseases, and tumour invasion and progression.1 MMPs also play an important part in the tissue degradation in Crohn's disease and ulcerative colitis, the two major forms of inflammatory bowel disease (IBD) in humans.3,4,5,6 Moreover, we have shown that lamina propria T cell activation in human fetal gut explants increases MMP production, followed by matrix degradation.7,8,9,10 Fibroblasts are the major source of MMPs in the human gut.9,10,11
Interleukin 21 (IL21) is a T cell‐derived cytokine whose effects are mediated through a class I cytokine family receptor, IL21R,12 that interacts with the common γ chain receptor.12,13,14,15 Consistent with the distribution of its receptor on immune cells, IL21 has been shown to affect the growth and functional activity of T, B and natural killer lymphocytes.12,13 More recently, however, IL21R has been described in synovial macrophages and fibroblasts of patients with rheumatoid arthritis,16 raising the possibility that IL21 may have additional cell targets in vivo.
We have recently shown that IL21 is produced in excess in the gut of patients with Crohn's disease, and that IL21 helps sustain the Th1 mucosal response in this disease.17 However, high IL21 was also seen in patients with ulcerative colitis,17 a disease that is not associated with a predominant Th1 cell response.18 This raises the possibility that, in the gut, IL21 can sustain additional inflammatory pathways apart from enhancing Th1 cell immunity. Therefore, we have examined whether intestinal fibroblasts express IL21R, and investigated the effect of IL21 on MMP production.
Materials and methods
Patients and samples
Mucosal samples were taken from surgical specimens of 12 patients with Crohn's disease. Eight patients were receiving corticosteroids, and four were taking corticosteroids and azathioprine. Mucosal samples were also taken from four patients with active ulcerative colitis undergoing endoscopy and five patients undergoing colectomy for a chronic disease unresponsive to medical treatment. Five patients were taking corticosteroids and four were taking mesalazine. Normal controls included samples taken from four patients with irritable bowel syndrome, and from macroscopically and microscopically unaffected areas of six patients undergoing colectomy for colon cancer.
Isolation and culture of intestinal fibroblasts
Intestinal fibroblasts were isolated and phenotypically characterised as described elsewhere.19 Briefly, fresh colon was washed in Hank's balanced salt solution (Sigma‐Aldrich, Milan, Italy), and strips of mucosa were cut into small fragments and placed on the bottom of tissue culture dishes with modified Eagle's medium supplemented with 10% heat‐inactivated fetal bovine serum, antibiotics and 1% non‐essential amino acids (all from Sigma‐Aldrich). Fibroblasts grew from the fragments within 3–4 days. Cells used for experiments were all between passages 3 and 8.
To examine whether IL21R expression is regulated by inflammatory stimuli, fibroblasts, isolated from six controls, were starved overnight and then stimulated with IL1β (20 ng/ml; Peprotech EC , London, UK) or tumour necrosis factor (TNF) α (15 ng/ml, R&D Systems, Abingdon, UK) for 24–48 h.
To examine whether IL21 regulates production of MMP, confluent fibroblasts were starved overnight and then stimulated with recombinant human IL21 (10–50 ng/ml, R&D Systems) for 2–48 h. In parallel, fibroblasts were cultured with or without IL21 (25 ng/ml) or TNF α (15 ng/ml). In experiments with inhibitors of gene transcription, protein synthesis and secretion, fibroblasts were incubated with or without actinomycin D (5 μg/ml) or cycloheximide (10 μg/ml) or brefeldin A (5 μg/ml; Sigma‐Aldrich) for 2 h before adding IL21 or TNF α for a further 18 h.
To examine whether fibroblast growth was affected by IL21, fibroblasts were cultured in 96‐well dishes (2×103 cells/well) and allowed to adhere overnight. The non‐adherent cells were then removed and fresh media containing IL21 was added for a further 48 h. Bromodeoxyuridine was added to the cells during the last 6 h of incubation, and the level of bromodeoxyuridine‐positive cells was assessed by a colorimetric kit (Roche Diagnostics, Monza, Italy).
Effect of Crohn's disease lamina propria mononuclear cell derived IL21 on MMP production
To examine whether Crohn's disease lamina propria mononuclear cell (LPMC)‐derived IL21 enhances MMP synthesis by fibroblasts, Crohn's disease LPMC, isolated as described previously ,20 were cultured in modified Eagle's medium containing 1% nutridoma (Roche Diagnostics). After 48 h, supernatants were collected and used (1:20 final dilution) to stimulate confluent Crohn's disease intestinal fibroblasts, in the presence or absence of a recombinant human IL21 receptor/Fc fusion protein (IL21R/Fc; 20 μg/ml; R&D Systems) or control immunoglobulin (Ig)G. MMPs were analysed after 48 h by western blotting.
Western blotting
Western blotting for IL21R was carried out using total extracts of fibroblasts isolated from seven patients with Crohn's disease, seven patients with ulcerative colitis, and seven normal controls, of the fetal gut fibroblast cell line, CCD18CO (LGC Promochem, Sesto San Giovanni, Italy) and peripheral blood CD3+ lymphocytes (PBL). IL21R was also examined in total extracts of normal fibroblasts stimulated with TNF α or IL1β. IL21R was detected using a monoclonal mouse anti‐human IL21R (1 μg/ml; R&D Systems) followed by a horseradish peroxidase‐conjugated rabbit anti‐mouse IgG (Dako SpA, Milan, Italy). The reaction was detected with a chemiluminescence kit (Pierce, Rockford, Illinois, USA). After IL21R analysis, blots were stripped and incubated with a monoclonal mouse anti‐human common γ chain antibody (1:500 final dilution; Santa Cruz Biotechnology, Santa Cruz, California, USA) and finally with a mouse anti‐human β‐actin antibody (Sigma‐Aldrich) as internal loading control. Computer‐assisted scanning densitometry was used to analyse the intensity of the immunoreactive bands.
For the detection of MMPs and tissue inhibitor of metalloproteinases (TIMPs), the membranes were incubated with the following monoclonal anti‐human antibodies: MMP‐1, MMP‐2, MMP‐3, MMP‐9, TIMP‐1 and TIMP‐2 (1 μg/ml; R&D Systems).
Gelatin zymography
Fibroblast culture supernatants were electrophoresed under non‐reducing conditions in an 8% acrylamide gel containing 1 mg/ml gelatin (Sigma‐Aldrich). After electrophoresis, the gels were washed for 30 min in 2.5% Triton X‐100, then equilibrated in developing buffer (50 mM Tris‐HCl, pH 7.4, 0.2 M NaCl, 5 mM CaCl2, 0.02% Brij35). After 30 min, the gels were incubated in fresh developing buffer and incubated overnight at 37°C, then stained with 0.25% Coomassie Blue for 1 h and destained in 50% methanol and 10% glacial acetic acid.
RNA extraction, complementary DNA preparation and reverse transcriptase‐polymerase chain reaction
IL21R and common γ chain receptor RNA were analysed in samples extracted from fibroblasts of five patients with Crohn's disease, five patients with ulcerative colitis, five controls, CCD18CO and PBL by reverse transcription‐polymerase chain reaction (RT‐PCR). RNA isolation, reverse transcription of the RNA and RT‐PCR were carried out as described previously. 20 IL21R and common γ chain receptor primers and PCR conditions have been described elsewhere.21,22 A constant amount of RNA (500 ng/sample) was retrotranscribed into complementary DNA (cDNA), and 2 μl of cDNA/sample was amplified using the following conditions: denaturation for 1 min at 94°C, annealing for 1 min at 58°C for both MMP and β actin, and extension for 1 min at 72°C using the following primers. MMP‐1, FWD: 5′‐TTG TCC TCA CTG AGG GAA AC‐3′, REV: 5′‐AGG TTA GCT TAC TGT CAC AC‐3′; MMP‐2, FWD: 5′‐CCT GTT TGT GCT GAA GGA CA‐3′, REV: 5′‐GTA CTT GCC ATC CTT CTC AA‐3′; MMP‐3, FWD: 5′‐GCC CCT GGG CCA GGG ATT AAT GGA GAT GC‐3′; REV: 5′‐ATC TTG AGA CAG GCG GAA CCG AGT CAG G‐3′; MMP‐9, FWD: 5′‐GTC GAA ATC TCT GGG GCC TG‐3′, REV: 5′‐AAA CCG GTC GTC GGT GTC GT‐3′; β‐actin, FWD: 5′:GGC ACC ACA CCT TCT ACA‐3′, REV: 5′: CAGGTCTTTGCGGATGTC‐3′. In preliminary experiments, we established the optimal number of cycles to obtain a PCR product within the linear phase of the amplification. Therefore, cDNA was amplified with β actin primers for 22 cycles and MMP primers for 28 cycles. RT‐PCR products were electrophoresed in 1% agarose gel, bands quantified by densitometry and values expressed as arbitrary units. RT‐PCR product specificity was confirmed by restriction analysis.
Statistical analysis
Differences between groups were compared using Student's t test.
Results
IL21R is expressed by gut fibroblasts
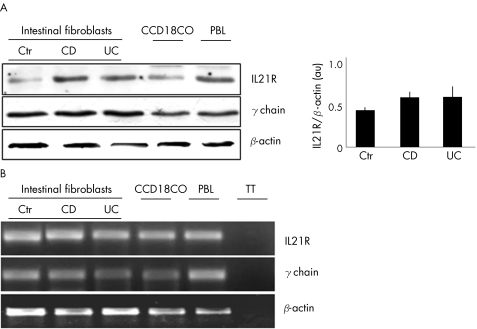
Constitutive expression of IL21R was seen in fibroblasts from patients with IBD and controls, and CCD18CO (fig 1A). Densitometric analysis of IL21R bands showed no significant difference between IBD and normal controls (fig 1A, right inset). Data were confirmed by showing that these cells contain RNA transcripts for IL21R (fig 1B). Intestinal fibroblasts also expressed the common γ‐chain receptor (fig 1 A,B).
Figure 1 (A) Representative western blots showing interleukin 21 receptor (IL21R) (upper blot), common γ chain (middle blot) and β‐actin (lower blot) in fibroblasts isolated from the colon of one control (Ctr), one patient with Crohn's disease (CD), one patient with ulcerative colitis (UC), from fetal gut fibroblast cell lines (CCD18CO) and from peripheral blood lymphocytes (PBL, used as a positive control). The right inset shows the quantitative analysis of IL21R/β‐actin protein ratio in fibroblasts isolated from seven controls, seven patients with Crohn's disease and seven patients with ulcerative colitis as measured by densitometry scanning of western blots. Values are expressed in arbitrary units (au) and indicate mean (standard deviation (SD)) of all experiments. (B) Representative electrophoretic gel showing reverse transcription‐polymerase chain reaction products for IL21R, common γ chain and β‐actin in RNA samples prepared from fibroblasts of one control, one patient with Crohn's disease, and one patient with ulcerative colitis, from CCD18CO and from PBL. TT, tube test, in which amplification was carried using RNA instead of cDNA. One of three separate experiments is shown.
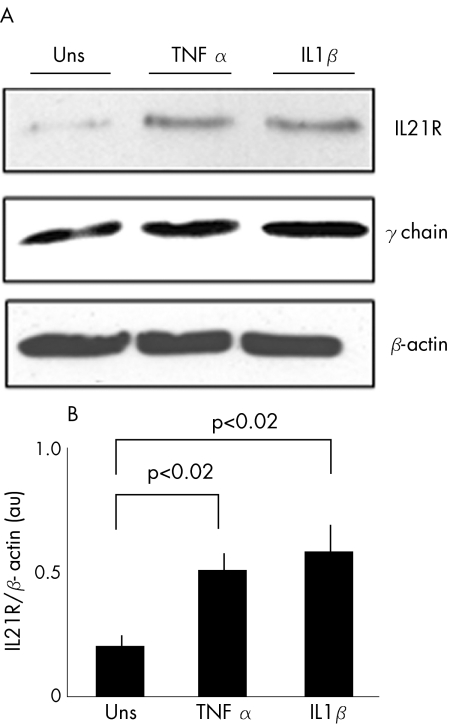
Notably, both IL1β and TNF α, two cytokines which are produced in excess in IBD,23 significantly enhanced IL21R expression (fig 2; p<0.02).
Figure 2 Stimulation of normal intestinal fibroblasts with tumour necrosis factor (TNF) α and interleukin (IL)1β results in enhanced IL21 receptor (IL21R) expression (p<0.02). (A) Representative western blot showing IL21R (upper blot), common γ chain (middle blot) and β‐actin (lower blot) in control fibroblasts either left unstimulated (Uns) or stimulated with TNF α and IL1β for 24 h. (B) Quantitative analysis of IL21R/β‐actin protein ratio in control fibroblasts, cultured as indicated in (A), as measured by densitometry scanning of western blots. Values are expressed in arbitrary units (au) and indicate mean (standard deviation (SD)) of four separate experiments.
IL21 promotes MMP synthesis by intestinal fibroblasts
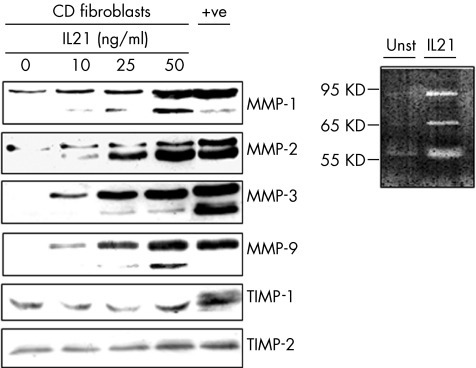
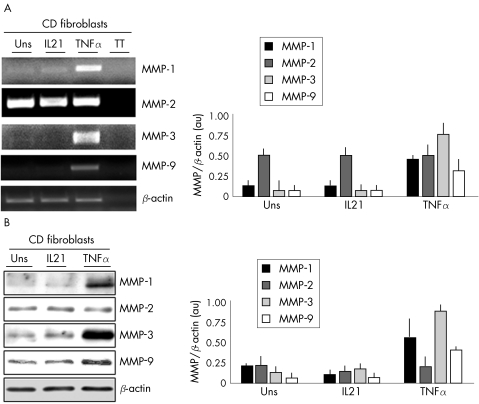
To examine whether fibroblasts respond to IL21, we evaluated the effect of IL21 stimulation on MMP synthesis. Figure 3 shows that IL21 dose dependently enhanced the secretion of distinct MMP isoforms, which correspond to the mature and active enzymes.2,8,9 This effect was seen regardless of whether fibroblasts were isolated from normal patients or from patients with IBD, as well as in CCD18CO cells (not shown). The ability of IL21 to enhance the synthesis of gelatinases (MMP‐2 and MMP‐9) was confirmed by zymography (fig 3, inset). By contrast, no considerable change in the secretion of TIMP‐1 and 2 was seen in IL21‐stimulated fibroblasts. Importantly, IL21 did not increase fibroblast proliferation (0.45 (0.1) arbitrary units in untreated cells v 0.37 (0.23) in IL21‐treated cells), excluding the possibility that differences in the release of MMPs were secondary to changes in cell growth.
Figure 3 Representative western blots showing MMP‐1, MMP‐2, MMP‐3, MMP‐9, TIMP‐1 and TIMP‐2 in fibroblasts isolated from the colon of one patient with Crohn's disease (CD), and either left untreated or treated with graded doses of interleukin (IL) 21 for 48 h. One of 12 separate experiments is shown. In the last lane (+ve), total proteins extracted from the inflamed colon of a patient with Crohn's disease were used as a positive control. The right inset shows a representative zymogram of gelatinolytic activities in supernatants from Crohn's disease fibroblasts either left unstimulated (Unst) or stimulated with 50 ng/ml IL21 for 48 h. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
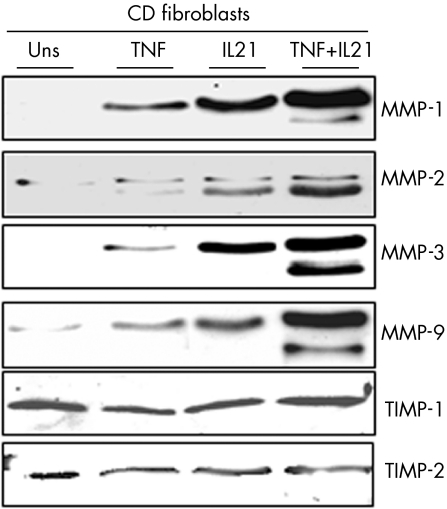
As TNF α is known to upregulate MMP‐1 and MMP‐3 production by intestinal fibroblasts,9,10 we examined whether IL21 cooperates with TNF α in inducing MMPs. Both IL21 and TNF α enhanced the secretion of MMP‐1, MMP‐2, MMP‐3 and MMP‐9. Cells stimulated with IL21 and TNF α together produced more MMPs than either IL21 or TNF α alone. Secretion of TIMP‐1 and 2 remained unchanged (fig 4).
Figure 4 Representative western blots showing MMP‐1, MMP‐2, MMP‐3, MMP‐9, TIMP‐1 and TIMP‐2 in culture supernatants of fibroblasts, isolated from one patient with Crohn's disease, and either left untreated (Uns) or treated with interleukin (IL) 21 (25 ng/ml) or tumour necrosis factor α (TNF α) (15 ng/ml) for 48 h. One of five separate experiments is shown. MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
IL21 does not enhance MMP RNA expression and new protein synthesis
To investigate increased transcription as a potential mechanism for the IL21‐induced secretion of MMPs, MMP RNA expression was assessed by RT‐PCR. MMP RNA transcripts were seen in untreated fibroblasts and were not increased by IL‐21 (fig 5A). By contrast, TNF α enhanced MMP‐1, MMP‐3 and MMP‐9, but not MMP‐2 RNA (fig 5A, B).
Figure 5 (A) Representative electrophoretic gel showing reverse transcriptase‐polymerase chain raction products for matrix metallopsoteinase (MMP) ‐1, MMP‐2, MMP‐3, MMP‐9 and β‐actin in RNA samples prepared from fibroblasts, isolated from the colon of one patient with Crohn's disease (CD), and either left untreated (Uns) or treated with interleukin (IL) 21 (50 ng/ml) or tumour necrosis factor (TNF) α (15 ng/ml) for 8 h. TT, tube test, in which amplification was carried out using RNA instead of cDNA. One of three separate experiments is shown. The right inset shows the quantitative analysis of MMP/β‐actin RNA transcripts in intestinal fibroblasts with Crohn's disease either left unstimulated or stimulated with IL21 (50 ng/ml) or TNF α (15 ng/ml) for 8 h. Values are expressed in arbitrary units (au) and indicate mean (standard deviation (SD)) of three separate experiments. (B) Representative western blots showing MMP‐1, MMP‐2, MMP‐3, MMP‐9 and β‐actin in total extracts prepared from fibroblasts isolated from the colon of one patient with Crohn's disease and either left unstimulated or stimulated with IL21 (50 ng/ml) or TNF α (15 ng/ml) for 48 h. One of four separate experiments is shown. The right inset shows the quantitative analysis of MMP/β‐actin protein ratio in intestinal fibroblasts with Crohn's disease either unstimulated or activated with IL21 (50 ng/ml) or TNF α (15 ng/ml) for 48 h. Values are expressed in arbitrary units (au) and indicate mean (SD) of four separate experiments.
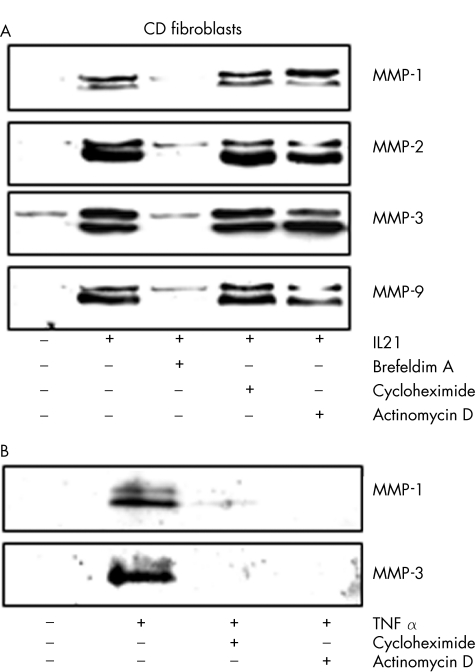
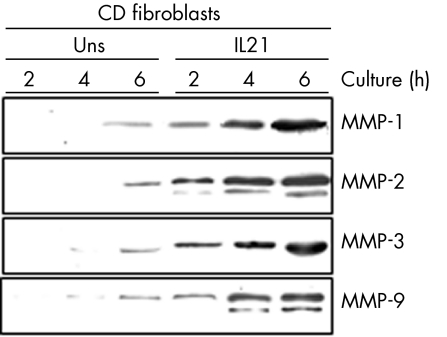
To determine whether IL21 upregulates the level of MMPs before secretion, cell‐associated MMPs were assayed by western blotting using total extracts. IL21 did not alter the cellular content of any MMP, whereas TNF α increased the expression of MMP‐1, MMP‐3 and MMP‐9, but not MMP‐2 (fig 5B). Inhibitors of RNA and protein synthesis or secretion were then used to confirm our findings. The protein secretion inhibitor, brefeldin A, abrogated the effect of IL21 on MMP production (fig 6A). By contrast, neither actinomycin D nor cycloheximide inhibited the IL21‐mediated MMP secretion (fig 6A). Both actinomycin D and cycloheximide inhibited the TNF α‐induced MMP‐1 and MMP‐3 secretion, indicating that these compounds were active in our system (fig 6B). Data suggest that IL21 affects neither gene transcription nor de novo protein synthesis, but it may enhance the secretion of preconstituted or newly synthesised MMPs. This hypothesis was also supported by the demonstration that IL21 induced a rapid secretion of MMPs, which was evident as early as 2 h after stimulation (fig 7).
Figure 6 (A) Representative western blots showing matrix metalloproteinase (MMP)‐1, MMP‐2, MMP‐3 and MMP‐9 in culture supernatants of fibroblasts isolated from one patient with Crohn's disease (CD) and either untreated (Uns) or preincubated with medium alone, brefeldin A (5 μg/ml), actinomycin D (5 μg/ml) or cycloheximide (10 μg/ml) for 2 h, and then stimulated with interleukin (IL)21 (50 ng/ml) for a further 18 h. (B) Representative western blots showing MMP‐1 and MMP‐3 in culture supernatants of fibroblasts isolated from one patient with Crohn's disease and either untreated or preincubated with medium alone, actinomycin D (5 μg/ml) or cycloheximide (10 μg/ml) for 2 h, and then stimulated with TNF α (15 ng/ml) for a further 18 h. Two of four representative experiments are shown.
Figure 7 Representative western blots showing matrix metallo‐proteinase (MMP)‐1, MMP‐2, MMP‐3 and MMP‐9 in culture supernatants of fibroblasts isolated from one patient with Crohn's disease (CD) and either left untreated (Uns) or treated with 50 ng/ml interleukin (IL)21 for the indicated time points. One of four representative experiments is shown.
Blocking IL21 reduces fibroblast MMP secretion induced by Crohn's disease LPMC supernatants
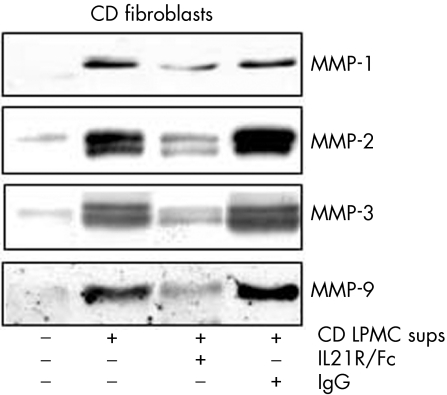
To confirm further that IL21 regulates matrix metalloproteinase (MMP) production, Crohn's disease fibroblasts were stimulated with Crohn's disease LPMC supernatants in the presence or absence of IL21R/Fc. Unstimulated fibroblasts released low levels of MMPs, but responded to Crohn's disease LPMC supernatant stimulation with enhanced synthesis of all MMPs (fig 8). The addition of IL21R/Fc to the fibroblast cultures decreased the production of MMPs induced by Crohn's disease LPMC supernatants (fig 8).
Figure 8. Neutralisation of interleukin (IL)21 activity reduces fibroblast matrix metalloproteinase (MMP) secretion induced by Crohn's disease (CD) lamina propria mononuclear cell (LPMC) supernatants. Fibroblasts were incubated with or without Crohn's disease LPMC supernatants (1:20 final dilution) in the presence or absence of a blocking IL21R fusion protein (IL21R/Fc) or control immunoglobulin G (IgG) for 48 h. At the end, fibroblast‐free supernatants were analysed by western blotting for MMP‐1, MMP‐2, MMP‐3 and MMP‐9. One representative of three separate experiments is shown.
Discussion
In this study, we examined whether IL21 regulates the synthesis of MMPs by intestinal fibroblasts, given that fibroblasts are a major source of MMPs in the gut and that MMP production can be enhanced by T cell‐produced cytokines.3,4,5,6,7,8,9,10 Initially we showed that IL21R is constitutively expressed by intestinal fibroblasts, and that such expression can be enhanced by TNF α and IL1β. In this context, however, it is worth noting that IL21R protein expression was semiquantitatively assessed by western blotting, as our attempts to characterise IL21R by flow cytometry using commercially available antibodies were unsuccessful. We cannot therefore exclude the possibility that the arbitrary units we measured may not exactly reflect the biological quantities of IL21R protein in those cells. Intestinal fibroblasts also express the common γ chain receptor, suggesting that these cells may be potential targets of IL21 in vivo. Indeed, intestinal fibroblasts responded to IL21 by increasing the production of MMPs. The effects of IL21 on MMP synthesis were observed with concentrations of IL21 that are similar to those used by other authors to test the biological effects of this cytokine in vitro.15 We do not know whether such concentrations reflect the amount of IL21 produced in the gut of patients with IBD, as no commercial kit is yet available to quantify human IL21. However, our previous western blotting analysis of IL21 showed that the intensity of immunoreactive bands in patients with IBD was not different from that obtained with 50 ng/ml rhIL‐2117—that is, the maximal dose we used to stimulate fibroblasts. We restricted our analysis of the effect of IL21 on the synthesis of MMP‐1, MMP‐2, MMP‐3 and MMP‐9, as these proteases are produced in excess in the gut of patients with IBD.3,4,6,11
MMP production or activity can be regulated at multiple levels including gene activation and transcription, mRNA stability, secretion, proenzyme activation and inactivation by endogenous inhibitors.1,2 We thus examined how IL21 regulates MMP synthesis in gut fibroblasts. Our data suggest that regulation of MMPs by IL21 does not occur at the transcriptional level. In fact, stimulation of fibroblasts with IL21 did not alter the expression of MMP RNA transcripts. Additionally, the intracellular level of MMP proteins was not increased by IL21, and the IL21‐induced MMP synthesis was not affected by inhibitors of gene transcription and de novo protein synthesis. It is thus plausible that IL21 preferentially increases the secretion of either preconstituted or newly synthesised MMPs, as treatment of fibroblasts with brefeldin A inhibited IL21‐induced MMP secretion, and increased secretion of MMP was seen after exposure to IL21 for a short time.
Although it was once considered that MMPs were transcriptionally regulated and rapidly secreted, recent evidence shows that MMPs are stored in secreted granules, ready for rapid release. For example, coculture of a monocytic cell line with metastatic colon cancer cells results in an increased production of MMP‐2 without any increase in mRNA levels.24 Subcellular compartmentalisation of MMPs within caveolar structures has been described in endothelial cells, and activation of these cells by various stimuli results in enhanced MMP secretion, with no change in MMP RNA or de novo protein synthesis.25,26,27,28 By contrast, TNF α augmented the RNA expression of MMP‐1, MMP‐3 and MMP‐9, but not MMP‐2, supporting data of previous reports showing that MMP‐2 can be regulated via a different pathway from that of other MMPs, including MMP‐9.1,2,29,30 These data suggest that inflammatory cytokines such as TNF α may transcriptionally increase MMP production, but this effect is amplified by IL21 at the post‐transcriptional level.
The proteolytic activity of MMPs is tightly controlled by TIMPs.1,2 One of the most striking features of this study is that TIMP‐1 and TIMP‐2 protein remained unchanged after IL21 stimulation. These findings are consistent with previous reports showing high MMP production without concomitant TIMP elevation in other systems. For example, in IBD tissue, increased expression of MMP‐3 occurs with no change in TIMP‐1 production.5,6 Stimulation of scleral fibroblasts from patients with necrotising scleritis by TNF α resulted in a twofold increase in TIMP‐1 mRNA compared with a sevenfold increase in stromelysin‐1 mRNA.31 There was no similar increase in TIMP‐1 mRNA in the aqueous humour of patients with uveitis, although MMPs were increased.32
In conclusion, we have shown that IL21 increases MMP production by fibroblasts, thus confirming and expanding on our previous data showing that cytokines are important mediators of the cross talk between immune and non‐immune cells in the gut.5,9,18 The in vivo relevance of our findings relates to the fact that supernatants of Crohn's disease LPMC rapidly increase MMP production by fibroblasts and this is partially inhibited by IL21R/Fc. The fact that the IL21R/FC reduces but does not abrogate the effect of Crohn's disease LPMC supernatants on fibroblast MMP production indicates that Crohn's disease LPMC make additional MMP‐inducing molecules other than IL21. Indeed, molecules such as IL1β, IL6, IL17, IL22 and TNF α, all of which could regulate MMPs, are produced in excess in Crohn's disease.2,5,10,18,33,34
Although IL21 was originally described as an important regulator of T, B and NK cells, recent studies have shown that IL21 modulates the activity of multiple cell types and triggers several inflammatory pathways.14,35,36 These observations, with the demonstration of upregulation of IL21 in IBD tissue and enhancement of MMPs in fibroblasts by IL21, suggest that blocking IL21 can help in limiting the tissue‐damaging inflammatory response in IBD.
Acknowledgements
This work received support from the “Fondazione Umberto di Mario”, Rome, the Broad Medical Research Program Foundation (Grant No IBD‐0154R), and Giuliani SpA, Milan, Italy.
Abbreviations
cDNA - complementary DNA
IBD - inflammatory bowel disease
Ig - immunoglobulin
IL21R - interleukin 21 receptor
IL21R/Fc - IL21R fusion protein
LPMC - lamina propria mononuclear cell
MMPs - matrix metalloproteinases
PBL - peripheral blood CD3+ lymphocytes
RT‐PCR - reverse transcriptase‐polymerase chain reaction
TIMP - tissue inhibitor of metalloproteinase
TNF - tumour necrosis factor
Footnotes
Competing interests: None.
References
- 1.Nagase H, Woessner J F J. Matrix metalloproteinases. J Biol Chem 199927421491–21494. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G, Reynolds J J. Extracellular matrix degradation. In: Royce PM, Steinmann B, eds. Connective tissue and its heritable disorders: molecular, genetic, and medical aspects. New York: Wiley‐Liss 1993287–316.
- 3.Louis E, Ribbens C, Godon A.et al Increased production of matrix metalloproteinase‐3 and tissue inhibitor of metalloproteinase‐1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol 2000120241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Lampe B, Barthel B, Coupland S E.et al Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 20004763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pender S L, MacDonald T T. Matrix metalloproteinases and the gut—new roles for old enzymes. Curr Opin Pharmacol 20044546–550. [DOI] [PubMed] [Google Scholar]
- 6.Heuschkel R B, MacDonald T T, Monteleone G.et al Imbalance of stromelysin‐1 and TIMP‐1 in the mucosal lesions of children with inflammatory bowel disease. Gut 20004757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmela M T, MacDonald T T, Black D.et al Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut 200251540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteleone G, MacDonald T T, Wathen N C.et al Enhancing Lamina propria Th1 cell responses with interleukin 12 produces severe tissue injury. Gastroenterology 19991171069–1077. [DOI] [PubMed] [Google Scholar]
- 9.Pender S L, Tickle S P, Docherty A J.et al A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 19971581582–1590. [PubMed] [Google Scholar]
- 10.Pender S L, Fel J M, Chamow S M.et al A p55 TNF receptor immunoadhesin prevents T cell‐mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol 19981604098–4103. [PubMed] [Google Scholar]
- 11.Kirkegaard T, Hansen A, Bruun E.et al Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut 200453701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habib T, Nelson A, Kaushansky K. IL‐21: a novel IL‐2‐family lymphokine that modulates B, T, and natural killer cell responses. J Allergy Clin Immunol 20031121033–1045. [DOI] [PubMed] [Google Scholar]
- 13.Collins M, Whitters M J, Young D A. IL‐21 and IL‐21 receptor: a new cytokine pathway modulates innate and adaptive immunity. Immunol Res 200328131–140. [DOI] [PubMed] [Google Scholar]
- 14.Parrish‐Novak J, Foster D C, Dolly H R.et al Interleukin‐21 and the IL‐21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol 200272856–863. [PubMed] [Google Scholar]
- 15.Habib T, Senadheera S, Weinberg K.et al The common gamma chain (gamma c) is a required signaling component of the IL‐21 receptor and supports IL‐21‐induced cell proliferation via JAK3. Biochemistry 2002418725–8731. [DOI] [PubMed] [Google Scholar]
- 16.Jungel A, Distler J H, Kurowska‐Stolarska M.et al Expression of interleukin‐21 receptor, but not interleukin‐21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum 2004501468–1476. [DOI] [PubMed] [Google Scholar]
- 17.Monteleone G, Monteleone I, Fina D.et al Interleukin‐21 enhances T‐helper cell type I signaling and interferon‐gamma production in Crohn's disease. Gastroenterology 2005128687–694. [DOI] [PubMed] [Google Scholar]
- 18.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 20033521–533. [DOI] [PubMed] [Google Scholar]
- 19.Strong S A, Pizarro T T, Klein J S.et al Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology 19981141244–1256. [DOI] [PubMed] [Google Scholar]
- 20.Monteleone G, Biancone L, Marasco R.et al Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology 19971121169–1178. [DOI] [PubMed] [Google Scholar]
- 21.Brenne A T, Ro T B, Waage A.et al Interleukin‐21 is a growth and survival factor for human myeloma cells. Blood 2002993756–3762. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa A, Tada H, Tamai R.et al Expression of IL‐2 receptor beta and gamma chains by human gingival fibroblasts and up‐regulation of adhesion to neutrophils in response to IL‐2. J Leukoc Biol 200374352–359. [DOI] [PubMed] [Google Scholar]
- 23.Monteleone G, MacDonald T T. Manipulation of cytokines in the management of patients with inflammatory bowel disease. Ann Med 200032552–560. [DOI] [PubMed] [Google Scholar]
- 24.Swallow C J, Murray M P, Guillem J G. Metastatic colorectal cancer cells induce matrix metalloproteinase release by human monocytes. Clin Exp Metastasis 1996143–11. [DOI] [PubMed] [Google Scholar]
- 25.Phillips P G, Birnby L M. Nitric oxide modulates caveolin‐1 and matrix metalloproteinase‐9 expression and distribution at the endothelial cell/tumor cell interface. Am J Physiol Lung Cell Mol Physiol 2004286L1055–L1065. [DOI] [PubMed] [Google Scholar]
- 26.Lopez‐Rivera E, Lizarbe T R, Martinez‐Moreno M.et al Matrix metalloproteinase 13 mediates nitric oxide activation of endothelial cell migration. Proc Natl Acad Sci USA 20051023685–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen M, Arkell J, Jackson C J. Active and tissue inhibitor of matrix metalloproteinase‐free gelatinase B accumulates within human microvascular endothelial vesicles. J Biol Chem 19982735400–5404. [DOI] [PubMed] [Google Scholar]
- 28.Taraboletti G, Sonzogni L, Vergani V.et al Posttranscriptional stimulation of endothelial cell matrix metalloproteinases 2 and 1 by endothelioma cells. Exp Cell Res 2000258384–394. [DOI] [PubMed] [Google Scholar]
- 29.Leveque T, Le Pavec G, Boutet A.et al Differential regulation of gelatinase A and B and TIMP‐1 and ‐2 by TNFalpha and HIV virions in astrocytes. Microbes Infect 20046157–163. [DOI] [PubMed] [Google Scholar]
- 30.Limb G A, Daniels J T, Pleass R.et al Differential expression of matrix metalloproteinases 2 and 9 by glial Muller cells: response to soluble and extracellular matrix‐bound tumor necrosis factor‐alpha. Am J Pathol 20021601847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Girolamo N, Lloyd A, McCluskey P.et al Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral fibroblasts. Am J Pathol 1997150653–666. [PMC free article] [PubMed] [Google Scholar]
- 32.Di Girolamo N, Verma M J, McCluskey P J.et al Increased matrix metalloproteinases in the aqueous humor of patients and experimental animals with uveitis. Curr Eye Res 1996151060–1068. [DOI] [PubMed] [Google Scholar]
- 33.Fujino S, Andoh A, Bamba S.et al Increased expression of interleukin 17 in inflammatory bowel disease. Gut 20035265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andoh A, Zhang Z, Inatomi O.et al Interleukin‐22, a member of the IL‐10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005129969–984. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Tschoi M, Spolski R.et al In vivo antitumor activity of interleukin‐21 mediated by natural killer cells. Cancer Res 2003639016–9022. [PubMed] [Google Scholar]
- 36.Pelletier M, Bouchard A, Girard D. In vivo and in vitro roles of IL‐21 in inflammation. J Immunol 20041737521–7530. [DOI] [PubMed] [Google Scholar]