Abstract
Background
With the recent development of endoscopic submucosal dissection (ESD), large oesophageal cancers can be removed with a single procedure, with few limits on the resectable range. However, after aggressive ESD, a major complication that arises is postoperative inflammation and stenosis that can considerably affect the patient's quality of life.
Aims
To examine a novel treatment combining ESD and the endoscopic transplantation of tissue‐engineered cell sheets created using autologous oral mucosal epithelial cells, in a clinically relevant large animal model.
Methods
Oral mucosal epithelial cells, harvested from beagle dogs, were cultured under normal conditions at 37°C, on temperature‐responsive dishes. After ESD (5 cm in length, 180° in range), cell sheets were harvested by a simple reduction in temperature to 20°C, and transplanted by endoscopy.
Results
The transplanted cell sheets were able to adhere to and survive on the underlying muscle layers in the ulcer sites, providing an intact, stratified epithelium. Four weeks after surgery, complete wound healing, with no observable stenosis, was seen in the animals receiving autologous cell sheet transplantation. By contrast, noticeable fibrin mesh and host inflammation, consistent with the intermediate stages of wound healing, were observed in the control animals that received only ESD.
Conclusions
These findings in a clinically relevant canine model show the effectiveness of a novel combined endoscopic approach for the potential treatment of oesophageal cancers that can effectively enhance wound healing and possibly prevent postoperative oesophageal stenosis.
Considerable progress in the field of endoscopic surgery has now enabled the effective removal of early cancers of the gastrointestinal tract.1 In the case of m1 (epithelial cancers restricted to the intraepithelial layer) and m2 (mucosal cancers with invasion of intermediate depth) oesophageal carcinomas, the use of endoscopic mucosal resection (EMR) is a practical and non‐invasive alternative to open surgery. With EMR, the affected mucosal tissues are removed by resection through the middle or deeper portions of the underlying submucosa, thereby eliminating the need for oesophageal reconstruction.2 However, a major restriction with conventional EMR is the resectable range that is generally limited to about 20 mm in size.3 Large cancerous lesions therefore require piecemeal resections,4,5,6 which present a considerably higher risk of local recurrence.7,8 Furthermore, the need for the retrieval of multiple specimens can also result in damage to the tissues that are harvested for critical diagnostic procedures. Thus, although EMR is a widely accepted technique for the treatment of early gastrointestinal cancers, methods for resection of large lesions with a single operation are needed.
With the recent development of new endoscopic surgical devices, such as the insulated‐tip,5,9 hook10 and flex knives,11 a new method of endoscopic submucosal dissection (ESD) has emerged, permitting large en bloc resections without the limitations on the resectable range encountered with conventional EMR.12 In Japan, ESD is currently used in the treatment of early oesophageal cancers and enables precise diagnosis, with the removal of large cancers using a single procedure.13,14 Yet even with this straightforward method for large en bloc resection, a major complication is the ulcerative constriction that occurs owing to extensive ESD. After aggressive endoscopic resection, severe inflammation causes oesophageal scarring and stenosis,15,16 meaning that the presence of large ulcerations often considerably influences the patient's postoperative quality of life. Although treatment with balloon dilation or temporary stents can partially overcome this problem,17,18,19 extended or prolonged effects generally require repeated treatments, which can lead to further inflammation and postoperative pain. Therefore, even though ESD is an effective treatment for early oesophageal cancers, methods to enhance wound healing are needed to reduce scarring, while possibly preventing painful constriction.
In normal tissues, the epithelia act as a barrier to the external environment and are critically involved in wound healing, indicating that the loss of the oesophageal epithelium during ESD probably has a considerable effect on the observed inflammation and scarring. In this study, we demonstrate the creation and application of transplantable tissue‐engineered cell sheets, using previously described temperature‐responsive culture dishes,20 in a clinically relevant large animal model that has been used in studies on oesophageal reconstruction.21 By combining ESD with the endoscopic transplantation of autologous oral mucosal epithelial cell sheets, we demonstrate a novel endoscopic treatment for large en bloc oesophageal resections, with improved wound healing, in an effort to reduce ulcerative constriction.
Materials and methods
Experimental animals
All experimental protocols were approved by the animal welfare committee of Tokyo Women's Medical University (approval number 05‐73, 2004). Male beagle dogs (1 year old, 10 kg) were injected intramuscularly with 0.04 mg/kg atropine and 15 mg/kg ketamine for premedication, and then injected intravenously with 2.5 mg/kg propofol. An endotracheal tube was inserted and anaesthesia was maintained using sevoflurane and nitrous oxide inhalation.
Preparation of oral mucosal epithelial cell sheets
Oral mucosal tissue (10×10 mm2) was excised from the buccal cavity of each animal and the wounds were sutured. Harvested tissue specimens were incubated in Dulbecco's modified Eagle's medium (Sigma, St Louis, Missouri, USA) containing 1000 units/ml dispase I (Godo Shusei, Tokyo, Japan) overnight at 4°C. All epithelial layers were then removed using sterile forceps and treated with 0.25% trypsin–0.1% EDTA (Sigma) for 20 min at 37°C to create single‐cell suspensions. From each tissue biopsy specimen, about 2×106 cells were obtained. Oral mucosal epithelial cells were then seeded on culture dishes (19.6 cm2) having central 24×24 mm2 temperature‐responsive regions surrounded by non‐cell adhesive polyacrylamide (CellSeed, Tokyo, Japan), at a density of 5×105 cells/cm2. Epithelial cells were cultured in the presence of mitomycin C‐treated 3T3 feeder layers22 for 2 weeks at 37°C in a humidified atmosphere of 5% carbon dioxide.
Endoscopic submucosal dissection
An artificial ulceration (180° in range, 5 cm in length) was created in the lower oesophagus by dissection of the oesophageal mucosa, using a thin high‐resolution video endoscope (XQ260 and Q240Z, Olympus,Tokyo, Japan) and the Videoscope System (EVIS LUCERA 260, Olympus). ESD was carried out using the hook‐knife method.10,13 Marks were made around the lesion using a flex knife (KD‐630L, Olympus) and an electrosurgery unit (VIO 300D, ERBE, Tübingen, Germany), set to forced coagulation mode (60 W, effect 3). Glycerol solution containing 0.2 mg/ml indigo carmine dye was then injected into the submucosal layer to separate the mucosa from the underlying muscle layer. Initial mucosal incisions were made on the base (anal side) and top of the lesion site, using the back of the hook knife (KD‐620LR, Olympus) and dry cut mode (60 W, effect 3). The mucosal layer was then grasped with the hook knife from the submucosal layer and repeatedly dissected in the direction of the lumen. The lateral sides of the mucosal incision were then also cut with the hook knife. Finally, submucosal fibres and vessels were cut using the hook knife with either the dry cut or forced coagulation mode (40 W, effect 2) from the oral side. In cases of slight bleeding during submucosal dissection, haemostatic forceps in soft coagulation mode (80 W, effect 5) were applied. After ESD, only faint remnants of submucosal tissues were observed on the muscle layer.
Endoscopic transplantation of autologous oral mucosal epithelial cell sheets
After culture for 2 weeks at 37°C, oral mucosal epithelial cells were transferred to another incubator set at 20°C for 30 min. Cell sheets were then non‐invasively harvested using a polyvinylidene difluoride (PVDF) (Immobilon‐P, DURAPORE; Millipore Corporation, Bedford, Massachusetts, USA) support membrane. Immediately after ESD, two autologous oral mucosal epithelial cell sheets were individually transplanted to the artificial oesophageal ulceration using endoscopy (n = 3). An EMR tube (Create Medic, Tokyo, Japan) was inserted in the oesophagus of each animal, and the PVDF support membrane with an attached autologous oral mucosal epithelial cell sheet was grasped by endoscopic forceps, and carefully manoeuvred to the ulcer site through the EMR tube.23 Cell sheets were then placed directly on to the ulcer sites, and gentle pressure was applied to the overlying PVDF support membranes using the EMR tube. After 10 min, the support membranes were simply removed by using endoscopic forceps, with the cell sheets remaining on the ulcer wound beds. Identical procedures were then carried out for the transplantation of the second oral mucosal epithelial cell sheet. Animals receiving only ESD, without cell sheet transplantation (n = 3), were used as controls. Animals were allowed access to water only for the first 2 days after operation, and allowed free access to food and water thereafter. All animals were observed by endoscopy once a week for 4 weeks and then killed for further analysis.
To follow the presence of transplanted cells, cell sheets were stained with either 10 µg/ml of the DNA‐binding dye Hoechst 33342 (Molecular Probes, Eugene, Oregon, USA) or 10 µg/ml of the fluorescent cell tracer DiI (Molecular Probes), one day before surgery. Animals receiving Hoechst 33342‐labelled cell sheets were observed for 8 days after transplantation and then killed for further analysis. Animals transplanted with DiI‐labelled cells were killed after 4 days and the transplanted areas were then processed into 5 µm‐thick cryosections for examination by fluorescence microscopy.
Histological and immunohistochemical analyses
Oral mucosal epithelial cell sheets, harvested from temperature‐responsive culture dishes, and resected tissues were fixed in 10% neutral‐buffered formalin (Wako Pure Chemicals, Osaka, Japan) and routinely processed into 3 µm‐thick paraffin‐wax‐embedded sections. Haematoxylin and eosin (H&E) staining was carried out by conventional methods. For immunohistochemical analyses, deparaffinised sections were treated with a 1:500 dilution of anti‐cytokeratin 3 (AE5, Progen Biotechnik, Heidelberg, Germany), a 1:10 dilution of anti‐cytokeratin 4 (6B10, Progen) or a 1:200 dilution of anti‐cytokeratin 7 (LD5‐68, Sigma) antibodies, overnight at 4°C. All sections were then stained with peroxidase using the LSAB 2 kit (DakoCytomation, Glostrup, Denmark), according to the manufacturer's suggested protocol.
Scanning electron microscopy
Harvested oral mucosal epithelial cell sheets were fixed in 2% glutaraldehyde and post‐fixed with 1% osmium tetroxide. The samples were dehydrated with 2‐methyl‐2‐propanol and embedded using the TAAB EPON812 Embedding Kit (TAAB Laboratories Equipment, Berks, UK). Semithin sections (500 nm thick) were then cut, stained with toluidine blue and observed under a conventional microscope.
Ultrathin sections (80 nm thickness) were cut on an ultramicrotome (LKB‐2088, LKB, Bromma, Sweden) and then examined by scanning electron microscopy (Hitachi S‐800, Hitachi, Ibaraki, Japan) at magnifications of ×1500 and ×6000.
Analysis of inflammation
From H&E stained tissue sections, the number of inflammatory cells was counted under the microscope, 4 weeks after the procedures. All inflammatory cells present in the field of view were counted in 12 random locations for each sample and the number of inflammatory cells was compared between the transplant and control groups. Statistical analysis was carried out using an unpaired Student's t test with Dr SPSS II software. Data are presented as mean (standard deviation (SD)) and a p value <0.01 was considered significant.
Results
Creation of transplantable oral mucosal epithelial cell sheets that resemble the native oesophageal epithelium
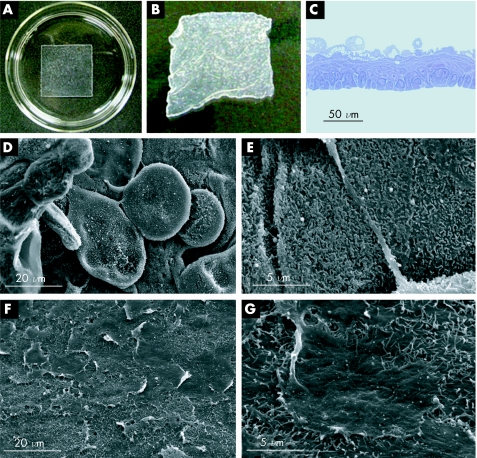
After culture using conventional methods with 3T3 feeder layers, isolated oral mucosal epithelial cells proliferated to form confluent layers on the square‐patterned temperature‐responsive dishes. When the incubation temperature was reduced to 20°C, all of the cultured cells spontaneously detached from the dish surfaces as intact sheets (fig 1B). Each harvested cell sheet was stratified and consisted of 3–5 cell layers (fig 1C), with flattened, polygonal cells on the apical surface (fig 1D). Additionally, the oral mucosal epithelial cell sheets non‐invasively harvested using the low‐temperature treatment also showed numerous microvilli on the apical surface (fig 1E), similar to the native oesophagus. Images of the basal surface of the cell sheets obtained with a scanning electron microscope also showed the presence of a basement membrane‐like extracellular matrix (ECM) (fig 1F,G).
Figure 1 Transplantable oral mucosal epithelial cell sheets. (A) Under normal culture conditions at 37°C, oral mucosal epithelial cells proliferate to confluence on square‐patterned temperature‐responsive dishes. (B) By reducing the temperature to 20°C for 30 min, the cultured cells can be harvested as intact sheets, without the need for enzymatic treatments. (C) Toluidine blue staining shows that the oral mucosal epithelial cell sheets are stratified, with 3–5 cell layers. (D) Scanning electron microscopy (SEM) of the apical surface shows that the cell sheets contain flattened, polygonal superficial cells similar to the native epithelium. (E) Magnified views also show that the apical surface is covered by numerous microvilli. (F, G) SEM of the basal surface of the cell sheets shows that a basement membrane‐like deposition of extracellular matrix is maintained during low‐temperature cell sheet harvest.
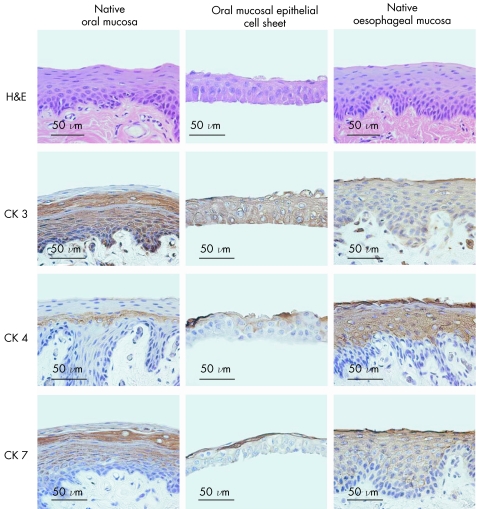
Although the engineered oral mucosal epithelial cell sheets were stratified, they were slightly thinner than both the native oral mucosal and oesophageal mucosal epithelia, which consist of 7–10 cell layers (fig 2). However, even though the cell sheets contained fewer cell layers, they possessed cuboidal basal cells, flattened middle cells and polygonal, flattened superficial cells, similar to both native oral and oesophageal mucosal epithelia. Additionally, cytokeratin 3, a marker of epithelial tissues from the oral cavity, was expressed in the basal and suprabasal layers of the native oral mucosa, and all layers of both the tissue engineered cell sheets and the native oesophagus. Cytokeratin 4, a marker of non‐keratinised, stratified epithelia and cytokeratin 7, which is expressed by many simple epithelia, was present only in the middle layers of the oral mucosa, but was observed in all suprabasal layers of the normal oesophageal tissues. Harvested cell sheets, however, showed positive staining of both cytokeratins 4 and 7 in the middle and superficial layers, indicating that engineered oral mucosal epithelial cell sheets closely resembled both the native oral mucosal and oesophageal epithelia.
Figure 2 Phenotypic examination of cultured oral mucosal epithelial cell sheets. Native oral mucosal tissues, cultured oral mucosal epithelial cell sheets and native oesophageal mucosa were compared by haematoxylin and eosin staining, as well as immunostaining for cytokeratins (CK) 3, 4 and 7.
Effective transplantation of autologous oral mucosal epithelial cell sheets using endoscopy
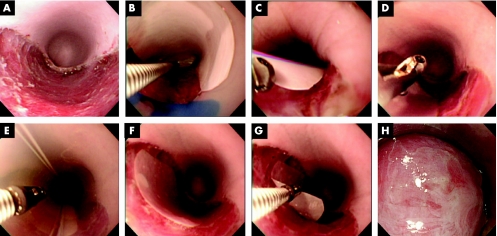
After ESD was carried out using the hook‐knife method, a flat ulcer, characteristic of ESD, was observed in the oesophagus (fig 3A). Cultured autologous oral mucosal epithelial cell sheets were then harvested using circular PVDF membranes, grasped by endoscopic forceps and manoeuvred to the ulcer sites (fig 3B, C). After carefully placing the cell sheets along with the attached PVDF membranes on the wound sites (fig 3D), the EMR tube was used to gently press the cell sheets against the ulcer wound bed (fig 3E), and the cell sheets were then left undisturbed on the ESD ulcer beds (fig 3F). After 10 min, the cell sheets produced stable attachment to the underlying muscle layers without any sutures or clips and could be clearly observed after removal of the PVDF membranes (fig 3G, H; for video 1, see http://gut.bmjjournals.com/supplemental).
Figure 3 Endoscopic transplantation of autologous oral mucosal epithelial cell sheets. After endoscopic submucosal dissection, a flat oesophageal ulcer is created (A). The cultured oral mucosal epithelial cell sheet, attached to a white polyvinylidene difluoride (PVDF) support membrane, is then grasped by endoscopic forceps and transferred to the dissection site (B) and gently placed on the ulcer wound bed (C). After carefully withdrawing the endoscopic forceps (D), the endoscopic mucosal resection tube is used to apply gentle pressure to the PVDF support membrane and the underlying cell sheet (E). The cell sheet along with the support membrane is then left undisturbed for 10 min to allow for direct attachment to the host tissues (F). The support membrane is then easily removed (G), leaving the autologous cell sheet on the ulcer wound bed (H) (for supplementary video 1, see http://gut.bmjjournals.com/supplemental).
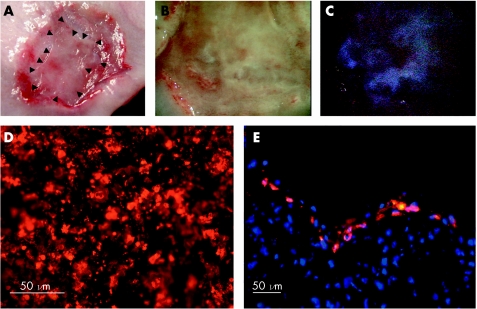
Cell sheets were stained with the DNA‐binding dye Hoechst 33342 to determine whether the cell sheets can remain attached to the ulcer wound beds in the relatively unstable oesophageal environment. Eight days after transplantation, resected tissues showed the presence of epithelial cells covering most of the wound site (fig 4A, B). Under stereoscopy with ultraviolet light, the Hoechst 33342‐derived blue fluorescence was observed at the transplant sites (fig 4C), indicating the survival of the oral mucosal epithelial cells on the underlying muscle layer of the ESD ulcer sites. Additionally, when cell sheets stained with the cell tracer DiI (fig 4D) were transplanted, red fluorescence showed that the stratified epithelial cells present in the ulcer sites were completely derived from the autologous cell sheets (fig 4E).
Figure 4 Survival of transplanted epithelial cell sheets on ulcer wound beds. Cell sheets were stained with the DNA‐binding dye Hoechst 33342 (A–C) or the phospholipid‐binding dye DiI (D, E), 1 day before transplantation. Eight days after transplantation, the excised oesophageal tissues show an ulcerative area in the process of wound healing. Arrowheads indicate the border of the transplanted cell sheet (A). Under normal light and higher magnification, a portion of the ulcer site seems to be covered by a mature and relatively normal epithelium (B). When ultraviolet light is used, Hoechst 33342‐derived blue fluorescence can be detected, indicating the presence of the transplanted cells in the wound site. (D) Before transplantation, DiI is present at a high density in the cultured cell sheets. (E) Four days after transplantation, cross‐sectional views show that the oral mucosal epithelial cell sheet survives at the transplant site, providing an intact, stratified epithelium. Cell nuclei in (E) are stained blue.
Transplantation of autologous oral mucosal epithelial cell sheets enhances wound healing after ESD
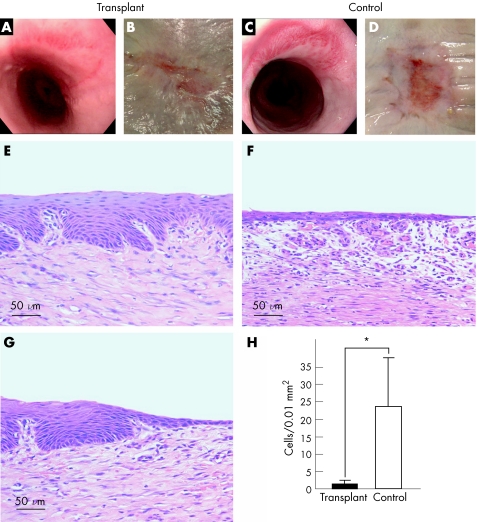
After transplantation, iodine staining of the PVDF support membranes showed that nearly all of the cells from the cell sheets had been transplanted to the ulcer sites (data not shown). Four weeks after transplantation, endoscopic examination showed complete wound healing, with no observable stenosis or fibrin mesh (fig 5A). By contrast, control animals receiving only ESD were still in the intermediate stages of wound healing, with a noticeable fibrin mesh (fig 5C). After the tissues were resected, the original ESD site was completely covered by epithelial cells in the transplant group (fig 5B). Although epithelial cells had migrated to cover some of the peripheral portions of the ESD site, an ulcerative area remained in the centre of the dissected region (fig 5D). Histological analysis of the tissues 4 weeks after ESD showed the presence of an intact, stratified and mature epithelium, which closely resembled the native oesophageal surface, at the centre of the transplant site (fig 5E). Additionally, at the border of the cell‐sheet transplanted areas, migrating epithelial cells were seen, covering the remainder of the ESD site (fig 5G). In comparison, control ulcer sites showed the presence of only an immature squamous epithelium in the central ESD site (fig 5F), as well as considerably higher accumulation of inflammatory cells, compared with the transplant groups (fig 5H).
Figure 5 Transplantation of oral mucosal epithelial cell sheets promotes wound healing and reduces postoperative inflammation. Left and right panels represent transplant and control groups, respectively. (A, C) Endoscopic photographs taken 4 weeks after operation. (B, D) Macroscopic images of the oesophageal sites receiving endoscopic submucosal dissection, after 4 weeks. (E, F) Haematoxylin and eosin (H&E) staining of the central portions of the ulcer sites. (G) H&E staining of the border region between the transplanted cell sheet and the outer portions of the ulcer site. (H) Comparison of the number of inflammatory cells present in the surgical sites between transplant and control groups. *p<0.01 (for supplementary video 1, see http://gut.bmjjournals.com/supplemental).
Discussion
In Japan, the occurrence of squamous cell carcinoma of the oesophagus is considerably higher than that in either Europe or the Americas,24,25,26,27,28 accounting for 89.2% of all patients with oesophageal cancer.29 Owing to the relative invasiveness of open oesophageal surgery compared with the stomach and colon, endoscopic surgery has become a preferred method of treatment in these patients. Although the recent development of ESD has enabled the removal of cancerous lesions with few limitations on the resectable size, oesophageal constriction due to the natural course of wound healing remains an important complication. Although the use of balloon dilation or stenting can partially solve this problem, these procedures must also be repeated for a prolonged period after endoscopic surgery. Together, these consequences can affect the patient's postoperative quality of life.
In cases of gastric ESD, it has been previously shown that regardless of the original size, artificial ulcers after ESD generally heal within 8 weeks, in humans.30 With larger ulcers, the specific aspect that accelerates the wound healing progression is actually a reduction in the size of the wound, with the surrounding mucosa contracting to be in close proximity with other areas of the raw surfaces. Therefore, ulcer constriction is an important first step in the normal healing process after aggressive gastric ESD. By contrast, in this study, we show that the transplantation of cultured autologous oral mucosal epithelial cell sheets can enhance wound healing, with complete re‐epithelialisation achieved by 4 weeks after ESD, in a dog model. In comparison to humans, although the size of the canine oesophagus is nearly identical, the rate of postoperative healing in control animals after ESD is slightly slower, with less constriction, owing to the thicker muscle layers of the dog oesophagus. Taken together, these results imply that cell sheet transplantation has the potential to inhibit constriction after ESD, as well as to prevent postoperative bleeding and perforations, owing to considerably accelerated epithelial recovery. Although the cell sheets present an intact epithelial barrier over only some portions of the post‐ESD ulcers, the secretion of various growth factors and cytokines by the transplanted cell sheets also likely has a role in recruiting host epithelial cells to proliferate and migrate into the wound site, analogous to the use of cultured keratinocytes in the treatment of burns.31
With the use of temperature‐responsive dishes, cultured cell sheets can be harvested in a transplantable form via simple low‐temperature treatment.20 With the presence of deposited ECM on the basal surface, the cell sheets can therefore be transplanted without the need for sutures or clips. The cell sheets remain stably attached to the oesophageal ulcer bed, which is in constant motion and subjected to shear stress from swallowing. Additionally, by avoiding the use of any implantable biomaterial carriers, the associated host inflammatory responses32 can be eliminated. Therefore, owing to the transplantation of only autologous cells, normal inflammatory reactions that may lead to pain and oesophageal scarring can be reduced.
In this study, we applied the use of the oral mucosa as an alternative cell source owing to its similarities to the normal oesophageal mucosa. Additionally, cultured oral mucosal epithelial cell sheets also resemble the native oesophageal epithelium, with a stratified, squamous, non‐keratinised structure and similar expression of cytokeratins. Previously, we have described the use of autologous oral mucosal epithelial cell sheets to treat diseases of the corneal surface33 and we now show the use of the oral mucosa as a readily available cell source, suitable for oesophageal tissue‐engineering applications. In cases of oesophageal cancer, the application of oral mucosa provides several key advantages. Firstly, the oral cavity is markedly isolated from the oesophagus, thereby eliminating the risk of transplanting carcinoma cells. Secondly, compared with the resection of oesophageal tissues, oral biopsy specimens can be obtained with relative ease and without pain or scarring. Therefore, in conjunction with expansion of the harvested epithelial cells on temperature‐responsive culture dishes, cell sheets can be formed from extremely small, non‐invasive biopsy specimens.
Our results therefore show a novel combined endoscopic treatment for the removal of extensive cancerous lesions. By enhancing ulcer wound healing after ESD, this treatment enables en bloc resection and coverage of the raw dissected surfaces with an intact epithelial layer. Although tissue‐engineering approaches have been previously proposed for oesophageal reconstruction,21 the current method shows the first successful approach of delivering engineered tissues in a novel and non‐invasive fashion, with endoscopy. We are currently planning to apply this technique to patients with early oesophageal cancer with either m1 or m2 depths of invasion. Although the occurrence of Barrett's oesophagus is less common in Japan than squamous cell carcinoma, this technique may also be applicable, as endoscopic surgery is becoming an increasingly popular treatment for Barrett's oesophagus,4,34,35 and an alternative to photodynamic treatment.36 Similarly, in cases of EMR for Barrett's epithelium at the oesophageal–gastric junction where balloon dilation is required to prevent strong constriction, this method may be effective in improving the quality of life for patients.16,37
In conclusion, we have demonstrated a novel endoscopic treatment for the en bloc resection of large oesophageal lesions with reduced inflammation and the associated complications. By combining the endoscopic transplantation of cultured autologous oral mucosal epithelial cell sheets with ESD, this new approach broadens the applicability of endoscopic resection in the treatment of various gastrointestinal diseases.
Supplementary Material
Acknowledgements
We thank Chinatsu Kohno (Institute of Advanced Biomedical Engineering and Science, Tokyo Women's Medical University) and Seiichi Kosaka (Medical Research Laboratory, Tokyo Women's Medical University) for technical assistance.
Abbreviations
ECM - extracellular matrix
EMR - endoscopic mucosal resection
ESD - endoscopic submucosal dissection
H&E - haematoxylin and eosin
PVDF - polyvinylidene difluoride
Footnotes
Funding: This work was supported in part by the Center of Excellence (COE) Program for the 21st Century, the High‐Tech Research Center Program, and the Support Program for Contemporary Educational Needs (Gendai GP) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; the Core Research for Evolution Science and Technology Program (CREST) by the Japan Science and Technology Agency (JST); and the Core To Core Program from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Soetikno R, Kaltenbach T, Yeh R.et al Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol 2005234490–4498. [DOI] [PubMed] [Google Scholar]
- 2.Soetikno R M, Gotoda T, Nakanishi Y.et al Endoscopic mucosal resection. Gastrointest Endosc 200357567–579. [DOI] [PubMed] [Google Scholar]
- 3.Inoue H, Takeshita K, Hori H.et al Endoscopic mucosal resection with a cap‐fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc 19933958–62. [DOI] [PubMed] [Google Scholar]
- 4.Ell C, May A, Gossner L.et al Endoscopic mucosal resection of early cancer and high‐grade dysplasia in Barrett's esophagus. Gastroenterology 2000118670–677. [DOI] [PubMed] [Google Scholar]
- 5.Gotoda T, Kondo H, Ono H.et al A new endoscopic mucosal resection procedure using an insulation‐tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 199950560–563. [DOI] [PubMed] [Google Scholar]
- 6.Rosch T, Sarbia M, Schumacher B.et al Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated‐tip knives: a pilot series. Endoscopy 200436788–801. [DOI] [PubMed] [Google Scholar]
- 7.Katada C, Muto M, Manabe T.et al Local recurrence of squamous‐cell carcinoma of the esophagus after EMR. Gastrointest Endosc 200561219–225. [DOI] [PubMed] [Google Scholar]
- 8.Nomura T, Boku N, Ohtsu A.et al Recurrence after endoscopic mucosal resection for superficial esophageal cancer. Endoscopy 200032277–280. [DOI] [PubMed] [Google Scholar]
- 9.Ono H, Kondo H, Gotoda T.et al Endoscopic mucosal resection for treatment of early gastric cancer. Gut 200148225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyama T, Kikuchi Y. Aggressive endoscopic mucosal resection in the upper GI tract—hook knife EMR method. Minim Invasive Ther Allied Technol 200211291–295. [DOI] [PubMed] [Google Scholar]
- 11.Yahagi N, Fujishiro M, Kakushima N.et al Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Dig Endosc 20041634–38. [Google Scholar]
- 12.Eguchi T, Gotoda T, Oda I.et al Is endoscopic one‐piece mucosal resection essential for early gastric cancer? Dig Endosc 200315113–116. [Google Scholar]
- 13.Oyama T, Tomori A, Hotta K.et al Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 20053S67–S70. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto H. Endoscopic submucosal dissection of early cancers and large flat adenomas. Clin Gastroenterol Hepatol 20053S74–S76. [DOI] [PubMed] [Google Scholar]
- 15.Katada C, Muto M, Manabe T.et al Esophageal stenosis after endoscopic mucosal resection of superficial esophageal lesions. Gastrointest Endosc 200357165–169. [DOI] [PubMed] [Google Scholar]
- 16.Seewald S, Akaraviputh T, Seitz U.et al Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high‐grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc 200357854–859. [DOI] [PubMed] [Google Scholar]
- 17.Repici A, Conio M, De Angelis C.et al Temporary placement of an expandable polyester silicone‐covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc 200460513–519. [DOI] [PubMed] [Google Scholar]
- 18.Satodate H, Inoue H, Yoshida T.et al Circumferential EMR of carcinoma arising in Barrett's esophagus: case report. Gastrointest Endosc 200358288–292. [DOI] [PubMed] [Google Scholar]
- 19.Siersema P D. Endoscopic therapeutic esophageal interventions: what is new? What needs further study? What can we forget? Curr Opin Gastroenterol 200521490–497. [PubMed] [Google Scholar]
- 20.Yang J, Yamato M, Okano T. Cell‐sheet engineering using intelligent surfaces. MRS Bull 200530189–193. [Google Scholar]
- 21.Badylak S F, Vorp D A, Spievack A R.et al Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res 200512887–97. [DOI] [PubMed] [Google Scholar]
- 22.Rheinwald J G, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 19756331–343. [DOI] [PubMed] [Google Scholar]
- 23.Makuuchi H, Yoshida T, Ell C. Four‐step endoscopic esophageal mucosal resection (EEMR) tube method of resection for early esophageal cancer. Endoscopy 2004361013–1018. [DOI] [PubMed] [Google Scholar]
- 24.Iwakiri K, Sugiura T, Hayashi Y.et al Esophageal motility in Japanese patients with Barrett's esophagus. J Gastroenterol 2003381036–1041. [DOI] [PubMed] [Google Scholar]
- 25.Okabayashi T, Gotoda T, Kondo H.et al Early carcinoma of the gastric cardia in Japan: is it different from that in the West? Cancer 2000892555–2559. [PubMed] [Google Scholar]
- 26.Tajima Y, Nakanishi Y, Yoshino T.et al Clinicopathological study of early adenocarcinoma of the gastric cardia: comparison with early adenocarcinoma of the distal stomach and esophagus. Oncology 2001611–9. [DOI] [PubMed] [Google Scholar]
- 27.van Soest E M, Dieleman J P, Siersema P D.et al Increasing incidence of Barrett's oesophagus in the general population. Gut 2005541062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devesa S S, Blot W J, Fraumeni J F. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998832049–2053. [PubMed] [Google Scholar]
- 29.Japanese Society for Esophageal Disease Comprehensive registry of esophageal cancer in Japan (1998, 1999) & long‐term results of esophagectomy in Japan (1988–1997). Tokyo: Kanehara, 2002
- 30.Kakushima N, Yahagi N, Fujishiro M.et al Healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc 200416327–331. [Google Scholar]
- 31.De Luca M, Albanese E, Bondanza S.et al Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns 198915303–309. [DOI] [PubMed] [Google Scholar]
- 32.Mikos A G, McIntire L V, Anderson J M.et al Host response to tissue engineered devices. Adv Drug Deliv Rev 199833111–139. [DOI] [PubMed] [Google Scholar]
- 33.Nishida K, Yamato M, Hayashida Y.et al Corneal reconstruction with tissue‐engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 20043511187–1196. [DOI] [PubMed] [Google Scholar]
- 34.Spechler S J. Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol 2005100927–935. [DOI] [PubMed] [Google Scholar]
- 35.Mino‐Kenudson M, Brugge W R, Puricelli W P.et al Management of superficial Barrett's epithelium‐related neoplasms by endoscopic mucosal resection: clinicopathologic analysis of 27 cases. Am J Surg Pathol 200529680–686. [DOI] [PubMed] [Google Scholar]
- 36.Gossner L, Ell C. Photodynamic therapy of gastric cancer. Gastrointest Endosc Clin N Am 200010461–480. [PubMed] [Google Scholar]
- 37.Giovannini M, Bories E, Pesenti C.et al Circumferential endoscopic mucosal resection in Barrett's esophagus with high‐grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy 200436782–787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.