Abstract
Background
Hepatitis C virus (HCV) infection is a major cause of human hepatocellular carcinoma (HCC). The precise mechanism of hepatocarcinogenesis in humans by HCV is currently unclear. It was recently shown, however, that transgenic mice with the HCV core gene often develop HCC, suggesting tumorigenic activity of the HCV core protein. Further, the HCV core protein expressed in HepG2 cells transfected with the core gene was shown to stimulate proliferation of transfectants through activation of nuclear factor‐κB (NF‐κB). The downstream target molecule(s) of NF‐κB activated by the HCV core protein to evoke cell proliferation is not yet identified. Transforming growth factor (TGF) α, which is often overexpressed in various tumour tissues such as HCC, has been shown to stimulate hepatocyte proliferation through activation of the mitogen‐activated protein kinase or extracellular signal‐related protein kinase (MAPK/ERK) cascade.
Aims
To explore the possibility that TGFα might be a target molecule for NF‐κB activated by the HCV core, and that TGFα participates in the growth promotion of the core transfectants in an autocrine manner, activating the MAPK/ERK pathway.
Methods
A HCV core expression vector was transfected into human hepatoma Huh‐7, HepG2 and Hep3B cells. NF‐κB activity was examined by an electrophoretic mobility shift assay. TGFα transcription was assessed by a luciferase reporter assay. TGFα protein was determined by immunoblot and ELISA. MAPK/ERK activity was examined by an in vitro kinase assay. Cell proliferation was assessed by a water‐soluble tetrazolium salt‐1 assay.
Results
In the HCV core transfectants, NF‐κB bound to the κB site in the TGFα proximal promoter region, resulting in an increase in TGFα transcription. Immunoblot as well as ELISA showed increased TGFα expression in the HCV core transfectants. SN50, a specific inhibitory peptide for NF‐κB, cancelled HCV core‐induced TGFα expression. HCV core protein increased cell proliferation as well as ERK activity of the HCV core transfectants as compared with the mock transfectants. The growth‐promoting activity and activation of ERK by the HCV core protein were negated by treatment with anti‐TGFα antibodies.
Conclusions
These results suggest that the HCV core protein promotes proliferation of human hepatoma cells by activation of the MAPK/ERK pathway through up regulation of TGFα transcription via activation of NF‐κB. Our finding provides a new insight into the mechanism of hepatocarcinogenesis by HCV infection.
Hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, liver cirrhosis and hepatocellular carcinoma (HCC) worldwide.1 The precise mechanism by which HCV infection results in the development of HCC remains largely unknown.2 HCV is a member of the Flaviviridae family, containing approximately 9.5 kb of positive‐strand RNA.3 The viral genome encodes a large precursor polyprotein, which is cleaved by both host and viral proteases into functional proteins such as core, envelope (E1, E2) and non‐structural proteins (NS2–NS5).4,5,6
Recent extensive studies on transgenic mice showed that, among these HCV proteins, the core protein may have a role in hepatocarcinogenesis.7 It was also shown that, in these transgenic mice, oxidative stress in hepatocytes was increased, resulting in hepatic steatosis.8 This finding also led us to speculate that longstanding oxidative DNA damage may be the causative factor for hepatocarcinogenesis.
Other lines of study using HCV core gene transfectants disclosed that the core protein had transforming activity as well as anti‐apoptotic activity, and that these activities were linked to activation of nuclear factor‐κB (NF‐κB)9,10,11,12,13 or mitogen‐activated protein kinase or extracellular signal‐regulated kinase (MAPK/ERK) signalling.14,15,16,17 However, it is presently unclear how these two signalling pathways are integrated in the mechanism of increased proliferation of the HCV core gene transfectants.
Transforming growth factor (TGF) α, a member of the epidermal growth factor (EGF) receptor ligand family, has been implicated in carcinogenesis and progression through activation of the MAPK/ERK cascade in various tumour cells, including HCC.18,19 In transgenic mice with the TGFα gene, a high incidence of HCC has been observed.20,21 Furthermore, increased expression of TGFα in the liver of patients with chronic hepatitis C and HCV‐related HCC has been reported.22 These findings suggest that hepatic overexpression of TGFα is responsible for the hepatocarcinogenesis and progression of HCC.
In this study, we attempted to explore the possibility that TGFα might be a target molecule for NF‐κB activated by the HCV core protein and participate in the growth promotion of the core transfectants in an autocrine manner, activating the MAPK/ERK signalling pathway.
Materials and methods
Reagents
The peptide SN50 (Biomol, Plymouth Meeting, Pennsylvania, USA), consisting of the nuclear localisation sequence of p50 (residues 360–369) fused to the hydrophobic region of the signal sequence of Kaposi fibroblast growth factor to provide cell permeability, specifically inhibits nuclear translocation of NF‐κB. SN50M (Biomol), a synthetic analogue with a mutated nuclear localisation sequence, is inactive and served as a negative control. Both reagents were used at a concentration of 50 μg/ml.23 PD98059 (Sigma‐Aldrich, St Louis, Missouri, USA), inhibitor of MAP kinase kinase (MEK) was dissolved in dimethylsulfoxide and used at a concentration of 10 μM.24
Cell culture and transfection
The human hepatoma cell lines Huh‐7, HepG2 and Hep3B (Riken Cell Bank, Tsukuba, Japan) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS). Cells were plated 24 h before transfection. The plasmids were transfected by using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) according to the manufacturer's instructions.
Plasmid construction
The plasmid containing an infectious cDNA clone of the genotype 1b strain of HCV, pCV‐J4L6S25 (a gift from Dr Jens Bukh, National Institutes of Health, Bethesda, Maryland, USA), was used as a template in a standard polymerase chain reaction (PCR) to amplify the HCV core (amino acids 1–191) by using the oligodeoxynucleotides: sense primer 5′‐ATG AGC ACG AAT CCT AAA CCT‐3′ and the antisense primer 5′‐AG CGG AAG CTG GGA TGG TCA‐3′. The PCR product was confirmed by direct sequencing and inserted into the TOPO site of the pcDNA3.1TOPO vector (Invitrogen) using standard cloning procedures, and the resulting plasmid was designated as pcDNA3/core. To make a luciferase reporter plasmid for a TGFα promoter assay, a fragment of the human TGFα promoter region (−1085 to +37) was amplified by PCR from genomic DNA of HepG2 cells by using the sense primer (−1118 to −1085) 5′‐GTA GGC CAG AGG CAG GAG AAG AGG GTC AGT‐3′ and the antisense primer (+6 to +37) 5′‐CCC AAG CTT CTC CAG CCT GCC CTA CCT GCG GTG CCC GA‐3′ (the underlined sequence represents the restriction enzyme site for HindIII). Semi‐nested PCR was carried out by using the sense primer (TGF‐SacI) 5′‐CGT GAG CTC CGG GTA CCT GGA GAA CAT‐3′ (the underlined sequence represents the restriction enzyme site for SacI) and the antisense primer (+6 to +37). The amplified product was subcloned into the SacI–HindIII site of the pGL3‐basic vector (Promega, Madison, Wisconsin, USA) to yield a pGL‐NF‐κB/TP reporter plasmid.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay was carried out as described previously.26 Briefly, 2×106 cells were plated onto a 10 cm dish and transfected with 15 μg of the effector plasmid (pcDNA3/core) or the mock plasmid (pcDNA3.1) and incubated for 24 h in the absence of FBS after transfection. Their nuclear extracts were prepared according to mininuclear extraction methods.27 The oligonucleotide used was as follows: 5′‐CTC AGG GGG GCA CCC CCA TCG‐3′ for the NF‐κB‐binding element. This oligonucleotide was end‐labelled with [32P]γ ATP using T4 polynucleotide kinase and incubated with 10 μg of the nuclear extracts for 20 min at room temperature. For competition assay, unlabelled oligonucleotide was added to the reaction mixture in 100‐fold molar excess over the labelled probe to examine the binding specificity. For the supershift assay, each 2 μg of antibody against NF‐κB subunits, NF‐κB p50 (c‐19, Santa Cruz Biotech, Santa Cruz, California, USA) and NF‐κB p65 (A, Santa Cruz Biotech), were added and incubated at 4°C for 1 h. Reaction mixtures were loaded onto a 4% polyacrylamide gel for electrophoresis in a 0.5× TRIS‐borate/EDTA buffer (pH 7.0). Amounts of DNA–NF‐κB complex were semiquantified by scanning densitometry using NIH Image V.1.61 software (National Institutes of Health, Bethesda, Maryland, USA).
Immunoblot
Cells were plated onto the wells of a six‐well tissue‐culture plate (5×105 cells/well) and transfected with indicated amounts of the effector plasmid (pcDNA3/core) or mock plasmid. Cells were washed twice with ice‐cold phosphate‐buffered saline (PBS) and lysed by a lysis buffer containing 50 mM Tris hydrochloric acid (pH 7.4), 150 mM sodium chloride, 1% Nonidet P‐40 and a protease inhibitor cocktail (Roche Diagnostic, Basle, Switzerland). Separation of proteins was carried out by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Transfer of protein to Immobilon‐P membranes (Millipore, Bedford, Massachusetts, USA) from SDS‐PAGE was accomplished with a semi‐dry blotting system (Advantec, Tokyo, Japan). Membranes were then blocked with T‐PBS (PBS with 0.1% Tween‐20) containing 10% skimmed milk for 1 h at 4°C. Blots were probed with the primary antibody of interest at a concentration of 1:500 to 1:5000 in T‐PBS containing 5% skim milk. Primary antibodies were directed against the HCV core (Austral Biologicals, San Ramon, California, USA), TGFα (Calbiochem, San Diego, California, USA), IκBα, phospho‐IκBα and p42 MAP kinase (ERK1; all from Cell Signaling Technology, Beverly, Massachusetts, USA) and β actin (Sigma‐Aldrich). After washing with T‐PBS, blots were incubated with horseradish peroxidase‐conjugated antibodies (Santa Cruz Biotech) for 1 h. Specific signals were detected by ECL western blotting reagents (Amersham Pharmacia Biotech, Uppsala, Sweden). Amounts of protein were semiquantified by scanning densitometry using NIH Image V.1.61 software.
Luciferase reporter assay
Luciferase reporter assay was carried out as previously described.28 Briefly, 5×105 cells were plated onto the wells of a six‐well tissue culture plate and transfected with a reporter plasmid, pCMVβ‐gal, and indicated amounts of the effector plasmid (pcDNA3/core) or mock plasmid. After 24 h of transfection, cells were lysed directly in the well. Luciferase activities were measured using a luminometer according to the manufacturer's protocols. An aliquot of the same cell lysates was used for measurement of β galactosidase activities to normalised luciferase results.
ELISA for TGFα
Cells were plated onto a 6 cm dish (1×106 cells/dish) and transfected with indicated amounts of the effector plasmid (pcDNA3/core) or the mock plasmid. After 24 h of transfection, the culture media of transfectants were collected and concentrated up to 10‐fold using a Centriprep YM‐3 Centrifugal Filter Device (Millipore). Sandwich ELISA for TGFα was carried out using a TGFα ELISA kit (Oncogene Science, Uniondale, California, USA) according to the manufacturer's protocol. Spectrophotometric absorbance at 490 nm was measured against a standard curve using known amounts of lyophilised control TGFα peptide reconstituted in double distilled water and then serially diluted in assay buffer to concentrations of 250, 125 and 63 pg/ml. All assays were carried out in triplicate.
In vitro kinase assay
The activity of ERK was determined using the p44/p42 MAP kinase assay kit (Cell Signalling Technology) according to the manufacturer's protocol. Briefly, 2×106 cells were plated onto a 10 cm dish and transfected with 15 μg of the effector plasmid (pcDNA3/core) or the mock plasmid and cell lysates were immunoprecipitated with anti‐p44/p42 MAPK (ERK1/2) monoclonal antibody. The in vitro kinase assay was carried out using Elk‐1 protein as a substrate. The samples were separated by 10% SDS‐PAGE and transferred to Immobilon‐P membranes. Phosphorylated Elk‐1 was detected by western blotting.
Cell proliferation assay
The cell proliferation assay was carried out using a Premix water‐soluble tetrazolium salt (WST)‐1 Cell Proliferation Assay System (Takara Bio, Ohtsu, Japan). Cells were seeded on 96‐well plates at a density of 5×103 cells/well in 100 μl culture media with 10% FBS. After 24 h of incubation, cells were washed and transfected with 0.3 μg of the effector plasmid (pcDNA3/core) or the mock plasmid in the culture media with 0.1% FBS in the presence or absence of anti‐TGFα neutralising antibody (Ab‐3, Oncogene Science), SN50 and PD98059. The media were replaced every other day. To evaluate cell proliferation, cells were incubated for 1–3 days after transfection and exposed to 10 μl of WST‐1 reagent for 1.5 h. The absorbance of the treated samples against a blank control was measured at 420 nm as the detection wavelength and 650 nm as the reference wavelength for the assay.
Statistical analysis
Data are presented as mean (standard deviation (SD)) unless otherwise indicated. The results were analysed for statistical significance using the two‐tailed Student's t test or analysis of variation. The criterion for significance was p<0.05.
Results
Activation of NF‐κB in human hepatoma cells transfected with HCV core gene
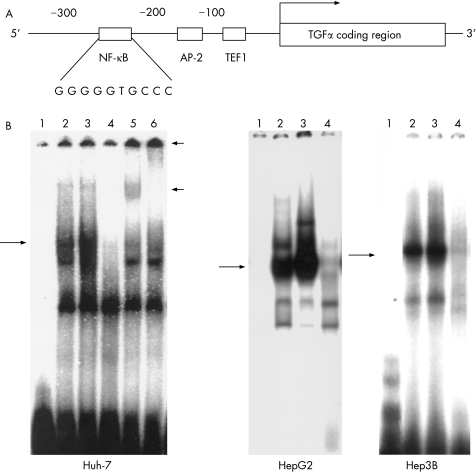
We first searched for transcription factor‐binding sites in the genomic promoter of the TGFα gene by using the computer program Transcription Factor Search (http://www.cbrc.jp/research/db/TFSEARCH.html). Figure 1A shows an element identified between−215 and−206, which has about 90% similarity as the consensus κB sequence (GGG GGT GCC C), in the proximal promoter region of the TGFα gene, in addition to the trefoil factor 1 and the activator protein‐2‐binding sites, which have been reported to activate TGFα transcription in HeLa cells and in rat kidney cells.29,30 We then examined if NF‐κB binds to this κB homologous sequence by using an electrophoretic mobility shift assay. When the nuclear extract from Huh‐7 cells transfected with a mock vector (pcDNA3.1) was incubated with a 32P‐labelled κB DNA probe, the band corresponding to the DNA–NF‐κB complex was faintly detected, and the density of the NF‐κB band was clearly enhanced in the pcDNA3/core transfectants (fig 1B). Further, the NF‐κB band was supershifted by the addition of either antibodies to NF‐κB subunits p65 and p50, suggesting that NF‐κB consisting of a p65 and p50 heterocomplex was activated in the HCV core transfectants. Similar results were obtained in two other hepatoma cell lines (HepG2 and Hep3B) in addition to Huh‐7 (fig 1B). On the other hand, neither activator protein 2 nor trefoil factor 1 were activated in Huh‐7 cells by transfection of the HCV core gene (data not shown).
Figure 1 Enhancement of nuclear factor (NF)‐κB DNA‐binding activity by hepatitis C virus (HCV) core protein. (A) Schematic representation of the NF‐κB site in the transforming growth factor (TGF) α promoter region. We searched for the κB site in the promoter region of TGFα using a computer program and found an element with about 90% homogeneity to the consensus κB sequence between−215 and−206. (B) Electrophoretic mobility shift assay (EMSA) using NF‐κB binding sequence in the promoter region of TGFα. Huh‐7, HepG2 and Hep3B cells were transfected with the effector plasmid (pcDNA3/core) or the mock plasmid. After 24 h of transfection, nuclear extracts were prepared and assayed for the NF‐κB DNA‐binding activity by EMSA. Lane 1, 32P‐labelled free oligonucleotide; lane 2, nuclear extract prepared from cells transfected with mock plasmid; lane 3, nuclear extract prepared from cells transfected with pcDNA3/core; lane 4, competition assay (100‐fold of unlabelled oligonucleotide); lanes 5 and 6, supershift assay (lane 5, anti‐p50 antibody; lane 6, anti‐p65 antibody). Long arrows indicate DNA–NF‐κB complex. Short arrows indicate DNA–NF‐κB–antibody complex.
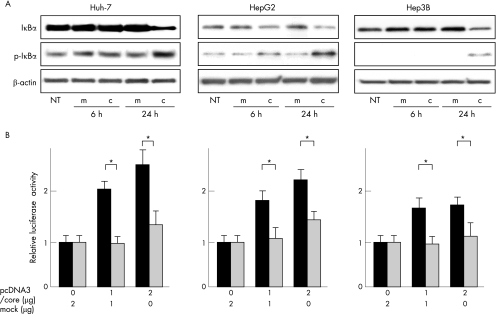
IκB has been shown to bind to NF‐κB to inactivate it, and activation of NF‐κB is preceded by dissociation from IκB via its phosphorylation and degradation.31 We thus conducted western blot analyses for IκB and phospho‐IκB in the pcDNA3/core transfectants of three hepatoma cell lines (Huh‐7, HepG2 and Hep3B). As expected, the expression of IκB decreased in the pcDNA3/core transfectants compared with that of the mock transfectants 24 h after transfection. In contrast, the expression of phospho‐IκB increased 24 h after transfection, suggesting that the HCV core protein activated NF‐κB through phosphorylation and degradation of IκB (fig 2A).
Figure 2 (A) Phosphorylation and degradation of IκB in hepatoma cells transfected with hepatitis C virus (HCV) core plasmid. Huh‐7, HepG2 and Hep3B cells were transfected with the effector plasmid (pcDNA3/core) or mock plasmid. Whole cell lysates were collected 6 and 24 h after transfection and analysed by western blotting using anti‐IκB, anti‐phospho‐IκB and anti‐β‐actin antibodies. (B) Activation of transforming growth factor (TGF) α gene transcription through nuclear factor (NF)‐κB by HCV core protein. Huh‐7, HepG2 and Hep3B cells were transfected with 0.5 μg of pGL‐NFκB/TP (black column) or pGL‐NFκBm/TP (grey column) and an indicated amount of the effector plasmid (pcDNA3/core) or mock plasmid. After 24 h of transfection, luciferase activity was measured. It was normalised by assigning the activity of cells transfected with mock plasmid alone a value of 1 (relative luciferase activity). Error bars represent the mean (standard deviation) from three independent experiments. *p<0.05. c, core transfectant; m, mock transfectant; NT, no treatment.
Activation of TGFα gene transcription through NF‐κB in human hepatoma cells transfected with HCV core gene
To examine whether the HCV core protein enhances the transcriptional activity of the TGFα gene through this NF‐κB site, we carried out a luciferase reporter assay using transfectants with a pcDNA3/core and pGL‐NF‐κB/TP encoding the TGFα promoter including NF‐κB site (fig 3). As shown in fig 2B, after cotransfection of pcDNA3/core and pGL‐NF‐κB/TP into Huh‐7, HepG2 and Hep3B cells, the luciferase activity considerably increased in a manner dependent on the dose of pcDNA3/core plasmid transfected. To test the specificity of NF‐κB‐dependent TGFα transcription, we constructed a mutated form of pGL‐NF‐κB/TP, pGL‐NF‐κBm/TP, in which there was a replacement of two bases (GG→AA) in the κB site (GAA GGT GCC C). When pcDNA3/core and pGL‐NF‐κBm/TP were cotransfected into cells, the enhanced effect of the transcriptional activity of the TGFα gene was negated (fig 2B), indicating that the HCV core affected TGFα gene up regulation through this κB site.
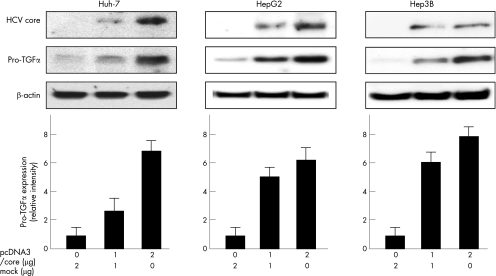
Figure 3 Expression of transforming growth factor (TGF) α protein in hepatitis C virus (HCV) core‐expressing cells. Huh‐7, HepG2 and Hep3B cells were transfected with indicated amounts of the effector plasmid (pcDNA3/core) or mock plasmid. After 24 h of transfection, whole cell lysates were collected and analysed by western blotting. The β‐actin was used as an internal control. The lower panels show densitometric analyses of the bands. The values were normalised by assigning the intensity of cells transfected with mock plasmid alone a value of 1. Error bars represent the mean (standard deviation) from three independent experiments.
Expression of TGFα protein induced by HCV core protein in human hepatoma cells
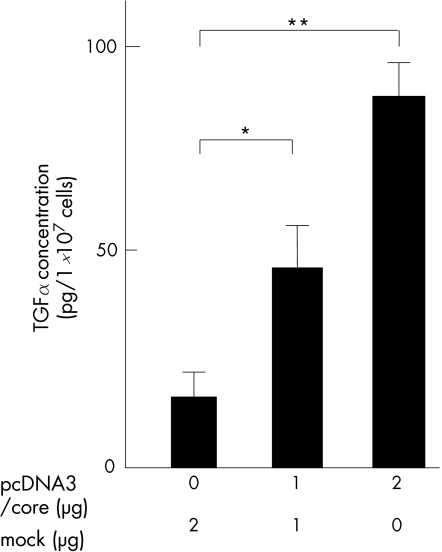
Next, we investigated the effect of the HCV core protein on the expression of TGFα protein in Huh‐7, HepG2 and Hep3B. After 24 h of transfection, expression of the TGFα protein was assessed by western blot analyses in the 1 or 2 μg pcDNA3/core transfectants. Western blot analyses showed that cytoplasmic expression of pro‐TGFα protein (about 30 kDa) remarkably increased in parallel to the expression of the HCV core protein in the pcDNA3/core transfectants. Further, we measured TGFα concentration in the culture media in Huh‐7 cells by ELISA. As shown in fig 4, TGFα concentration in the culture media significantly increased in a manner dependent on the dose of pcDNA3/core plasmid transfected.
Figure 4 Secretion of transforming growth factor (TGF) α from hepatitis C virus (HCV) core‐expressing cells analysed by ELISA. Huh‐7 cells were transfected with indicated amounts of the effector plasmid (pcDNA3/core) or the mock plasmid. After 24 h of transfection, transforming growth factor (TGF) α concentrations in the culture media were measured by using a TGFα ELISA kit according to the manufacturer's protocol. Error bars represent the mean (standard deviation) from three independent experiments. *p<0.05; **p<0.01.
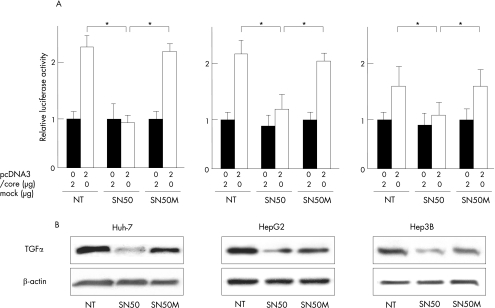
Effect of an NF‐κB inhibitor (SN50) on TGFα transcription and protein expression induced by HCV core
To confirm whether NF‐κB is required for TGFα transactivation, we treated cells with a specific inhibitor for nuclear translocation of NF‐κB, SN50 and its inactive mutated form, SN50M. As shown in fig 5A, the luciferase activity decreased considerably in the cells treated with SN50 compared with cells with no treatment and SN50M. Furthermore, western blot analyses showed that SN50 was able to inhibit expression of the pro‐TGFα, but SN50M was unable to do so in HCV core transfectants, indicating that the nuclear translocation of NF‐κB is required for activation of the TGFα expression (fig 5B).
Figure 5 Inhibition of transforming growth factor (TGF) α transcription and protein expression by SN50 in hepatitis C virus (HCV) core‐expressing hepatoma cells. (A) Huh‐7, HepG2 and Hep3B cells were transfected with 0.5 μg of pGL‐nuclear factor (NF)‐κB/TP and indicated amounts of the effector plasmid (pcDNA3/core) or the mock plasmid and incubated for 24 h in the presence or absence of SN50, an inhibitor of nuclear translocation of NF‐κB or SN50M (mutated and inactive form) at a concentration of 50 μg/ml. *p<0.05. (B) Cells were transfected with 2 μg of the effector plasmid (pcDNA3/core) and incubated for 24 h in the presence or absence of SN50 or SN50M (50 μg/ml). After 24 h of transfection, whole cell lysates were collected and analysed by western blotting. The result shown is representative of three independent experiments. NT, no treatment.
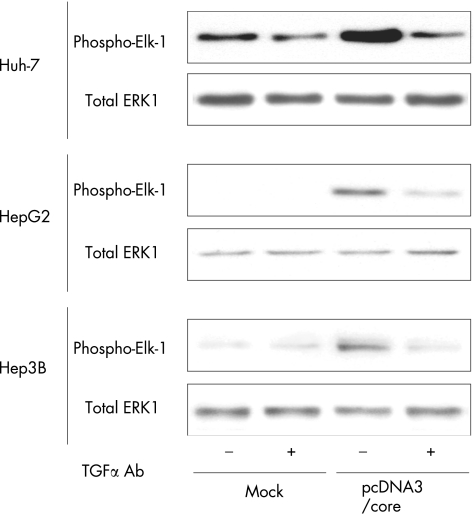
Activation of MAPK/ERK pathway in HCV core‐expressing hepatoma cells
We assessed the kinase activity of MAPK/ERK in the cell extracts of pcDNA3/core transfectants. The anti‐ERK1/2 immunoprecipitate prepared from pcDNA3/core transfectants effectively phosphorylated recombinant Elk‐1 (a specific substrate for MAPK/ERK) compared with those measured in the mock transfectants (fig 6). Further, phosphorylation of Elk‐1 in the pcDNA3/core transfectants was abrogated by treatment with anti‐TGFα neutralising antibodies, suggesting that TGFα stimulation enhanced MAPK/ERK activity in the pcDNA3/core‐transfected Huh‐7, HepG2 and Hep3B cells.
Figure 6 Activation of mitogen‐activated protein kinase/extracellular signal‐regulated kinase (MAPK/ERK) pathway in hepatitis C virus (HCV) core expressing hepatoma cells. The activities of ERK were determined by an in vitro kinase assay. Cells were transfected with the effector plasmid (pcDNA3/core) or the mock plasmid. Whole cell lysates collected 48 h after transfection were immunoprecipitated with anti‐p44/p42 MAPK monoclonal antibody. In vitro kinase assay was carried out using Elk‐1 protein as a substrate. Phosphorylated Elk‐1 was detected by immunoblotting. Total ERK1 of the cell lysate was used as control immunoblot. The results shown are representative of three independent experiments.
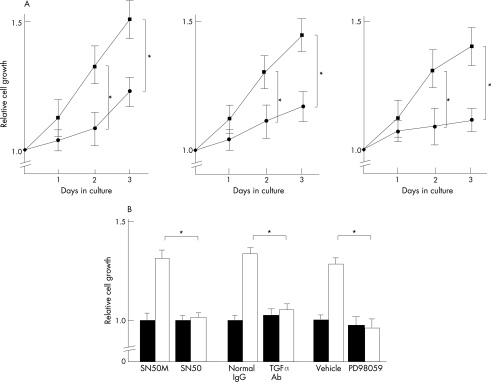
Cell proliferation of HCV core transfectants
To investigate if TGFα secreted from hepatoma cells could act as an autocrine or a juxtacrine growth factor, we carried out a cell proliferation assay using Huh‐7, HepG2 and Hep3B cells that had been transfected with pcDNA3/core or mock plasmid. As shown in fig 7A, the cell proliferation of the pcDNA3/core transfectants considerably increased on days 2 and 3 compared with the mock transfectants. When the pcDNA3/core transfectants were treated with anti‐TGFα neutralising antibody, SN50 and PD98059, the promoting effects of the cell proliferation were cancelled, suggesting that the HCV core promotes cell proliferation of TGFα expression via the NF‐κB and MAPK/ERK pathways.
Figure 7 Cell proliferation of hepatitis C virus (HCV) core‐transfected cells and inhibition of cell growth by SN50, anti‐transforming growth factor (TGF) α antibody and PD98059. (A) Huh‐7, HepG2 and Hep3B cells were transfected with pcDNA3/core (square symbols) or mock plasmid (circle symbols). A WST‐1 assay was carried out daily for 3 days and the relative absorbance was plotted. Mean (standard deviation) were determined from four independent experiments. At each time point, the statistical significance between the HCV core and mock transfectants were analysed by the two‐tailed Student's t test. *p<0.05 (B) Huh‐7 cells were transfected with the pcDNA3/core (open column) or the mock plasmid (filled column) and cultured for 24 h. Then, cells were further incubated for 24 h in the presence or absence of SN50 (50 μg/ml), SN50M (50 μg/ml), anti‐TGFα antibody (10 μg/ml) and PD98059 (10 μM). Cell proliferation was then analysed by the WST‐1 assay. Mean (SD) were determined from three independent experiments. *p<0.05.
Discussion
In this study, using human hepatoma cell lines, we showed that the HCV core protein activated NF‐κB, which in turn induced TGFα expression, thereby promoting cell proliferation through activation of the MAPK/ERK pathway.
The NF‐κB signalling pathway has been recognised as “cell survival and anti‐apoptosis signalling” through up regulation of several genes involved in cell proliferation and cell transformation.32 However, until now, there has been no report suggesting that TGFα is a target molecule for NF‐κB. Our study clearly discloses that NF‐κB activated by the HCV core protein up regulated TGFα gene transcription. However, there have been some diverse reports regarding the role of the HCV core on activation of NF‐κB. You et al10 showed that expression of the HCV core protein enhanced the tumour necrosis factor (TNF) and that the lymphotoxin‐β ligand induced NF‐κB activity in Huh‐7 and HeLa cells. Yoshida et al12 showed that NF‐κB activity was enhanced through activation of TNF receptor‐associated factor 2/6 and IκB kinase‐β in HeLa cells. These are compatible with our results. The findings by Shrivastava et al33 and by Zhu et al34 that the HCV core protein suppressed TNF‐dependent NF‐κB activation does not necessarily contradict ours, as the experimental attempt they aimed at was different from ours. They examined the effect of the HCV core protein on TNF‐induced NF‐κB activation, whereas we investigated the effect of the HCV core protein itself on NF‐κB. In other words, their observation was made merely on the competitive effect of the core protein with TNF on NF‐κB activation. Puzzling results were those by Tsutsumi et al,16 which showed that, in the liver of HCV core transgenic mice, NF‐κB was not activated. The only plausible explanation for the discrepancy would be the difference in experimental conditions. The effect of a transgene on cellular events in transgenic mice may be different from those in somatic cells transiently transduced with an exogenous gene. Detailed elucidation on this issue is certainly needed before we implicate our findings in in vivo hepatocarcinogenesis.
Erhardt et al15 showed cell proliferation induced by the HCV core protein as in our study. They suggested that the HCV core protein activated the MAPK/ERK cascade, resulting in the promotion of cell proliferation. The MAPK/ERK pathway is one of the major downstream‐signalling pathways from the EGF receptor, which is stimulated by the EGF ligand family, including TGFα. In our study, the anti‐TGFα neutralising antibody abrogated the almost promoting effect of cell proliferation as well as activation of MAPK/ERK phosphorylation, suggesting that TGFα is a major growth factor produced from hepatoma cell lines transfected with the HCV core gene. Further, our results have suggested that the NF‐κB pathway activated by the HCV core is closely linked with the MAPK/ERK cascade through TGFα stimulation.
Incidentally, however, another functional protein of HCV, NS5A alone or the HCV subgenomic replicon lacking the core gene was also recently found to activate NF‐κB through IκB phosphorylation.35 Thus, it is plausible that in the clinical setting where HCV infection is persistent, several non‐structural proteins encoded by the HCV genome that include NS5A may play a part in hepatocyte growth in concert with core protein. Future studies using full‐length replicons that delete or include the sequence coding each viral protein may elucidate the whole scheme of growth promotion of HCV proteins.
In conclusion, chronic stimulation by TGFα that is autocrined or paracrined from HCV‐infected hepatocytes seems to contribute to the progression of cancer cells. Further studies are necessary to know whether activation of the NF‐κB–TGFα pathway actually occurs in the liver of patients with chronic HCV infection, but data in this study may provide a new insight into a mechanism of hepatocarcinogenesis by HCV infection.
Acknowledgements
We thank Kevin S Litton, BA, for editorial assistance with this manuscript.
Abbreviations
EGF - epidermal growth factor
ERK - extracellular signal‐regulated kinase
FBS - fetal bovine serum
HCC - hepatocellular carcinoma
HCV - hepatitis C virus
MAPK - mitogen‐activated protein kinase
MEK - MAPK kinase
NF‐κB - nuclear factor‐κB
PBS - phosphate‐buffered saline
PCR - polymerase chain reaction
SDS‐PAGE - sodium dodecyl sulphate‐polyacrylamide gel electrophoresis
TGFα - transforming growth factor α
TNF - tumour necrosis factor
Footnotes
Funding: Supported in part by a grant‐in‐aid for scientific research (14570487 to JK) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Competing interests: None declared.
References
- 1.Di Bisceglie A M. Hepatitis C and hepatocellular carcinoma. Hepatology 19972634S–8S. [DOI] [PubMed] [Google Scholar]
- 2.Choo Q L, Kuo G, Weiner A J.et al Isolation of a cDNA clone derived from a blood‐borne non‐A, non‐B viral hepatitis genome. Science 1989244359–362. [DOI] [PubMed] [Google Scholar]
- 3.Kato N, Hijikata M, Ootsuyama Y.et al Molecular cloning of the human hepatitis C virus genome from Japanese patients with non‐A, non‐B hepatitis. Proc Natl Acad Sci USA 1990879524–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hijikata M, Kato N, Ootsuyama Y.et al Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA 1991885547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choo Q L, Richman K H, Han J H.et al Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA 1991882451–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hijikata M, Mizushima H, Tanji Y.et al Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci USA 19939010773–10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriya K, Fujie H, Shintani Y.et al The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med 199841065–1067. [DOI] [PubMed] [Google Scholar]
- 8.Moriya K, Nakagawa K, Santa T.et al Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus‐associated hepatocarcinogenesis. Cancer Res 2001614365–4370. [PubMed] [Google Scholar]
- 9.Marusawa H, Hijikata M, Chiba T.et al Hepatitis C virus core protein inhibits Fas‐ and tumor necrosis factor alpha‐mediated apoptosis via NF‐kappaB activation. J Virol 1999734713–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You L R, Chen C M, Lee Y H. Hepatitis C virus core protein enhances NF‐kappa B signal pathway triggering by lymphotoxin‐beta receptor ligand and tumor necrosis factor alpha. J Virol 1999731672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato N, Yoshida H, Kioko Ono‐Nita S.et al Activation of intracellular signaling by hepatitis B and C viruses: C‐viral core is the most potent signal inducer. Hepatology 200032405–412. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida H, Kato N, Shiratori Y.et al Hepatitis C virus core protein activates nuclear factor kappa B‐dependent signaling through tumor necrosis factor receptor‐associated factor. J Biol Chem 200127616399–16405. [DOI] [PubMed] [Google Scholar]
- 13.Chung Y M, Park K J, Choi S Y.et al Hepatitis C virus core protein potentiates TNF‐alpha‐induced NF‐kappaB activation through TRAF2‐IKKbeta‐dependent pathway. Biochem Biophys Res Commun 200128415–19. [DOI] [PubMed] [Google Scholar]
- 14.Giambartolomei S, Covone F, Levrero M.et al Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the hepatitis C virus (HCV) core protein. Oncogene 2001202606–2610. [DOI] [PubMed] [Google Scholar]
- 15.Erhardt A, Hassan M, Heintges T.et al Hepatitis C virus core protein induces cell proliferation and activates ERK, JNK, and p38 MAP kinases together with the MAP kinase phosphatase MKP‐1 in a HepG2 Tet‐Off cell line. Virology 2002292272–284. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi T, Suzuki T, Moriya K.et al Alteration of intrahepatic cytokine expression and AP‐1 activation in transgenic mice expressing hepatitis C virus core protein. Virology 2002304415–424. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi T, Suzuki T, Moriya K.et al Hepatitis C virus core protein activates ERK and p38 MAPK in cooperation with ethanol in transgenic mice. Hepatology 200338820–828. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R, Goeddel D V, Ullrich A.et al Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res 198747707–712. [PubMed] [Google Scholar]
- 19.Salomon D S, Kim N, Saeki T.et al Transforming growth factor‐alpha: an oncodevelopmental growth factor. Cancer Cells 19902389–397. [PubMed] [Google Scholar]
- 20.Jhappan C, Stahle C, Harkins R N.et al TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 1990611137–1146. [DOI] [PubMed] [Google Scholar]
- 21.Lee G H, Merlino G, Fausto N. Development of liver tumors in transforming growth factor alpha transgenic mice. Cancer Res 1992525162–5170. [PubMed] [Google Scholar]
- 22.Tanaka S, Takenaka K, Matsumata T.et al Hepatitis C virus replication is associated with expression of transforming growth factor‐α and insulin‐like growth factor‐II in cirrhotic liver. Dig Dis Sci 199642208–215. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y Z, Yao S Y, Veach R A.et al Inhibition of nuclear translocation of transcription factor NF‐kappa B by a synthetic peptide containing a cell membrane‐permeable motif and nuclear localization sequence. J Biol Chem 199527014255–14258. [DOI] [PubMed] [Google Scholar]
- 24.Pang L, Sawada T, Decker S J.et al Inhibition of MAP kinase kinase blocks the differentiation of PC‐12 cells induced by nerve growth factor. J Biol Chem 199527013585–13588. [DOI] [PubMed] [Google Scholar]
- 25.Yanagi M, St Claire M, Shapiro M.et al Transcripts of a chimeric cDNA clone of hepatitis C virus genotype 1b are infectious in vivo. Virology 1998244161–172. [DOI] [PubMed] [Google Scholar]
- 26.Takimoto R, El‐Deiry W S. Wild‐type p53 transactivates the KILLER/DR5 gene through an intronic sequence‐specific DNA‐binding site. Oncogene 2000191735–1743. [DOI] [PubMed] [Google Scholar]
- 27.Schreiber E, Matthias P, Muller M M.et al Rapid detection of octamer binding proteins with ‘mini‐extracts', prepared from a small number of cells. Nucleic Acids Res 1989176419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagiwara S, Nakamura K, Hamada H.et al Inhibition of type I procollagen production by tRNAVal CTE‐HSP47 ribozyme. J Gene Med 20035784–794. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Shin T H, Kudlow J E. Transcription factor AP‐2 controls transcription of the human transforming growth factor‐alpha gene. J Biol Chem 199727214244–14250. [DOI] [PubMed] [Google Scholar]
- 30.Wang D, Kudlow J E. Purification and characterization of TEF1, a transcription factor that controls the human transforming growth factor‐alpha promoter. Biochim Biophys Acta 1999144950–62. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, Baltimore D. Activation in vitro of NF‐kappa B by phosphorylation of its inhibitor I kappa B. Nature 1990344678–682. [DOI] [PubMed] [Google Scholar]
- 32.Pahl H L. Activators and target genes of Rel/NF‐kappaB transcription factors. Oncogene 1999186853–6866. [DOI] [PubMed] [Google Scholar]
- 33.Shrivastava A, Manna S K, Ray R.et al Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors. J Virol 1998729722–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu N, Khoshnan A, Schneider R.et al Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF‐induced apoptosis. J Virol 1998723691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waris G, Livolsi A, Peyron J F.et al Hepatitis C virus NS5A and subgenomic replicon activate NF‐kB via tyrosine phosphorylation of IkB and its degradation by calpain protease. J Biol Chem 200327840778–40787. [DOI] [PubMed] [Google Scholar]