Abstract
Background
Hypercholesterolaemia often occurs in primary biliary cirrhosis (PBC) as a result of chronic cholestasis, but whether these patients are exposed to greater cardiovascular risk is unknown.
Aim
To establish whether hypercholesterolaemia is associated with subclinical atherosclerosis in PBC.
Patients
103 consecutive patients with PBC (37 with total cholesterol ⩾6.21 mmol/l) and 37 controls with hypercholesterolaemia, and 141 matched controls with normocholesterolaemia.
Methods
Ultrasound imaging of carotid artery to determine intima–media thickness (IMT) and stenosis.
Results
Controls with hypercholesterolaemia had higher IMT and prevalence of carotid stenosis compared with patients with hypercholesterolaemic PBC (mean (SD) 0.850 (0.292) mm v 0.616 (0.137) mm, pc<0.001; 43% v 19%, pc = 0.129) who, in turn, were similar to the 66 patients with normocholesterolaemic PBC (0.600 (0.136) mm; 5%). Compared with subjects with normocholesterolaemia, controls with hypercholesterolaemia, but not patients with hypercholesterolaemic PBC, had an increased risk of raised IMT (odds ratio (OR) 5.4, 95% confidence interval (CI) 2.5 to 11.9, p<0.001; and 0.7, 0.3 to 2.0, p = 0.543) or carotid stenosis (8.2, 3.4 to 20, p<0.001; and 2.5, 0.9 to 6.9, p = 0.075). In PBC, compared with younger patients without hypertension, the risk of increased IMT was OR (CI) 3.1 (0.6 to 17; p = 0.192) in patients with hypertension or old age, but not hypercholesterolaemia, and 4.6 (0.8 to 27; p = 0.096) in patients who also had hypercholesterolaemia. The corresponding figures for risk of stenosis were 3.6 (0.4 to 36; p = 0.277) and 15.8 (1.8 to 141; p = 0.014).
Conclusions
Hypercholesterolaemia is not consistently associated with subclinical atherosclerosis in PBC, but should be treated if other risk factors for cardiovascular disease are also present. The search for factors that may protect patients with hypercholesterolaemic PBC against atherosclerosis should be encouraged.
Primary biliary cirrhosis (PBC) is a chronic cholestatic disease characterised by immune‐mediated, progressive damage of intrahepatic bile ducts.1,2 With our advancing awareness of the disease, most patients are being recognised with increasing frequency at an earlier stage than in the past, and survive several decades after the diagnosis.3
As a result of chronic cholestasis, hypercholesterolaemia is a common finding in patients with PBC, but it is still unclear whether longstanding lipoprotein elevation entails increased cardiovascular risk in this disease and, therefore, should be treated.4,5,6,7 In the past, a few studies have investigated mortality from cardiovascular disease in patients with PBC, suggesting that the frequency of fatal cardiovascular events may not be increased.6,8 We have recently evaluated the incidence of clinically relevant, both fatal and non‐fatal, cardiovascular events in PBC showing that the hypercholesterolaemia in these patients is not associated with an excess risk.7 However, we also pointed out that in a chronic, longlasting but ultimately fatal disease such as PBC, studies based on such outcome measures are subject to bias due to selective removal of patients dying from liver‐related causes. In the presence of competing causes of death, the use of early markers of cardiovascular disease as surrogate end points for atherosclerosis seems necessary to ascertain the clinical effect of hypercholesterolaemia in PBC.
Measurement of the intima–media thickness (IMT) of the carotid artery by B‐mode ultrasound imaging has emerged as a safe, non‐invasive and relatively inexpensive means of assessing subclinical atherosclerosis.9,10,11,12 IMT of the carotid artery was shown to be associated with the presence of cardiovascular risk factors and with the prevalence and incidence of cardiovascular disease.
By using ultrasound imaging of the carotid artery, we sought to determine whether in PBC, hypercholesterolaemia is associated with a substantial risk of cardiovascular disease by assessing the presence of initial atherosclerotic lesions.
Methods
Study populations
We studied 103 patients with PBC who consecutively presented to the School of Medicine Ospedale San Paolo, University of Milan, Milan, Italy, for follow‐up evaluation, or first visited between September 2001 and September 2003. PBC was diagnosed on the basis of the usual biochemical, serological, clinical and histological criteria.13
We selected 101 healthy subjects matched for age and sex with the patients with PBC. Subjects were excluded if they had a family history of premature cardiovascular disease (coronary heart disease, peripheral arterial disease, abdominal aortic aneurysm or symptomatic carotid artery disease in male first‐degree relatives before the age of 55 years, or in female first‐degree relatives before the age of 65 years), a smoking habit of >10 cigarettes/day, cardiovascular disease, hypertension (blood pressure of ⩾140/90 mm Hg, or current use of drugs for hypertension), diabetes mellitus (fasting serum glucose concentration ⩾7.0 mmol/l, non‐fasting glucose concentration ⩾11.1 mmol/l or current use of drugs for diabetes), hypercholesterolaemia (serum cholesterol concentration ⩾6.21 mmol/l, or currently receiving cholesterol‐lowering treatment), a history of anticoagulant or antiaggregant use, anaemia (haemoglobin <110 g/l in women or <120 g/l in men), thrombocytosis (platelet count of >500×109/l), vasculitis, a serum creatinine concentration >133 μmol/l, alcohol or drug dependence, or substantial coexisting medical conditions.
We also enrolled 40 patients with chronic hepatitis C who attended the School of Medicine Ospedale San Paolo, University of Milan, Milan, Italy, during the same period. They were matched for age, sex and stage of liver disease with the PBC group. Patients were excluded according to the same criteria used for the group of controls. As serum lipid levels and ultrasound imaging measurements were remarkably similar in controls and patients with chronic hepatitis C, the 141 subjects from the two groups were considered as a single control group.
Finally, we studied 37 patients with hypercholesterolaemia (serum cholesterol ⩾6.21 mmol/l) who presented as outpatients to the Centre for Hyperlipidaemic Disease, School of Medicine Ospedale San Paolo, University of Milan, Milan, Italy, during the same period. These patients were matched for age, sex and serum levels of total cholesterol with the subgroup of patients with PBC showing total serum cholesterol ⩾6.21 mmol/l. The controls with hypercholesterolaemia were excluded from the study if they had liver disease, alcohol or drug dependence, or substantial coexisting medical conditions.
We analysed the lipid profile in more detail in two groups of 30 controls and 45 patients with PBC selected from the overall study population by a random sampling, balanced procedure to include the same proportion of people with hypercholesterolaemia (20 patients in the control group and 30 in the PBC group).
Sample size calculations
In a group of 52 women undergoing statin treatment for hypercholesterolaemia, who had been assessed by ultrasonography of the common carotid artery for a different study carried out at the School of Medicine Ospedale San Paolo, University of Milan, Milan, Italy, we found a mean (standard deviation (SD)) IMT value of 0.9 (0.25) mm. Assuming a risk of type I error of 0.05 (two‐sided), we calculated that to attain a power of 0.9 in detecting in patients with hypercholesterolaemic PBC a difference equal to at least 20% of the IMT value expected in patients with hypercholesterolaemia and no liver disease (ie, if the IMT values were 0.72 and 0.9 mm in the two groups, respectively, with SD 0.25 mm in both), we would need to allocate 41 subjects to each group. On the basis of estimates from our previous study discussing cardiovascular risk in PBC,7 in which the prevalence of hypercholesterolaemia (total serum cholesterol ⩾6.21 mmol/l) was 41% in the patients enrolled after 1990, we deemed that screening 100 consecutive outpatients with PBC was needed to yield about 40 patients with hypercholesterolaemia.
Measurements
Complete clinical history was obtained and physical examination was carried out in all subjects. Body mass index (BMI) was computed from weight and height measurements. The circumference of the waist was taken at the umbilical level. Blood pressure was measured three times using a random‐zero sphygmomanometer, and the mean value of the last two measurements was used for analysis. Blood was taken from fasting patients with PBC and chronic hepatitis C. Plasma total cholesterol, high‐density lipoprotein (HDL)‐cholesterol and triglyceride levels were determined by routine laboratory methods at the time of enrolment and after 2 months. The mean value of the two measurements was used in the study analyses and for matching purposes. Low‐density lipoprotein (LDL) values were calculated as usual by subtracting the sum of the triglyceride values divided by five and HDL‐cholesterol from the total cholesterol. However, this measure was not used in the overall evaluations involving patients with PBC, because calculations are influenced by the presence of high levels of abnormal lipoproteins in their sera. Instead, we used direct measurements of serum LDL‐cholesterol for a detailed analysis of the lipid profile.
A detailed study of the lipid profile was carried out on serum samples collected after a 12‐h fast. Gas chromatography–mass spectrometry analysis was used to measure free and esterified serum cholesterol according to a previously described method.14 Apolipoprotein (Apo) B and Apo A‐1 were assessed by an immunoturbidimetric assay calibrated against World Health Organization International Reference Materials (Sentinel CH, Milan, Italy). LDL‐cholesterol was measured by using a direct colorimetric assay (elimination technique) in homogeneous phase without precipitation (Sentinel CH).
To measure carotid artery IMT, ultrasonography of the common carotid artery, carotid bifurcation and internal carotid artery (left and right arteries) was carried out with a 7.5‐MHz linear‐array transducer. The interfaces of the distal common carotid artery were marked across a length of 10 mm. The beginning of the dilation of the distal common carotid artery served as a reference point for the start of measurement. On a longitudinal, two‐dimensional ultrasound image of the carotid artery, the anterior (near) and posterior (far) walls of the carotid artery are displayed as two bright white lines separated by a hypoechogenic space. The distance between the leading edge of the first bright line of the far wall (lumen–intima interface) and the leading edge of the second bright line (media–adventitia interface) indicates the IMT. Measurements at the far wall are true representatives of the IMT, with a mean agreement between different readers of 0.02 mm.15 Common carotid artery IMT was the average of three IMT measurements. For each subject, the common carotid artery IMT was determined as the average of far‐wall measurements of both the left and right arteries. The common carotid artery, the carotid bifurcation and the internal carotid artery were also evaluated for the presence of atherosclerotic lesions. Plaques were defined as a focal widening relative to adjacent segments, with protrusion into the lumen composed either of only calcified deposits or a combination of calcified and non‐calcified material. Carotid stenosis was quantified by measuring the distance from the near media–adventitia interface to the far media–adventitia interface and the distance from the near intimal surface to the far intimal surface at the most stenotic point of the artery according to Kagawa et al.16 The degree of stenosis was defined in terms of the categories based on the North American Symptomatic Carotid Endarterectomy Trial criteria.17 All of the examinations were carried out by a single trained doctor blinded to all clinical information. Subjects were examined in the supine position on a comfortable bed.
The study was approved by the local ethics committee. Written consent to undergo study investigations was obtained from each subject after full information on the study design and purpose.
Data analysis
Continuous variables are summarised as mean (SD) values. Study data were visually displayed using the box plot method.18 The significance of differences in means was assessed using the Mann–Whitney test to compare two groups, and the Kruskal–Wallis test, a non‐parametric analysis of variance, to compare more than two groups. If the p value of the overall F test in the Kruskal–Wallis analysis was beyond the significance level (p = 0.05), a set of pairwise comparisons was carried out using Mann–Whitney tests to assess which group differed from the others. For each comparison, the significance value was then multiplied for the number of comparisons made, according to the Bonferroni inequality method, to control for multiple testing. If the main interest of the analysis of variance was to assess the presence of a gradient in the continuous variable across ordered groups, a non‐parametric test for trend was used. Differences in proportions were tested using Fisher's exact test or χ2 statistics.
Carotid artery IMT values in the highest quintile of the distribution of IMT measures obtained in the subjects included in the study were defined as increased IMT values (cut‐off value for the quintile with highest thickness = 0.808 mm). To compare the frequency of detection of increased carotid artery IMT or of carotid stenosis in different patient groups, ORs, 95% confidence intervals (CIs) and p values were calculated with logistic regression analysis. To evaluate relationships with the serum lipid profile within the framework of multivariable analysis, carotid artery IMT was also studied as a continuous variable and multiple regression was used in addition to logistic regression.
All the statistical analyses were two‐sided and carried out using Stata Statistical Software V.8.0.
Results
Table 1 reports the main characteristics of the 103 patients with PBC. Compared with the 141 subjects in the control group (101 healthy subjects and 40 patients with chronic hepatitis C), patients with PBC had higher concentrations of total cholesterol (mean (SD), 5.87 (1.29) v 5.25 (1.06) mmol/l; p<0.001), and similar levels of HDL‐cholesterol (1.76 (0.49) v 1.66 (0.39) mmol/l; p = 0.290) and triglycerides (1.16.(0.44) v 1.10 (0.53) mmol/l; p = 0.106). As a result of the matching procedures, age (60 (12) v 59 (11) years) and sex (92% women) of patients were equally distributed between the two groups.
Table 1 Main characteristics of patients with primary biliary cirrhosis according to serum level of total cholesterol.
| All patients | Total cholesterol level | p Value | ||
|---|---|---|---|---|
| <6.21 mmol/l | ⩾6.21 mmol/l | |||
| Subjects (n) | 103 | 66 | 37 | |
| Females (n (%)) | 95 (92) | 58 (88) | 37 (100) | 0.048 |
| Mean age (years) | 60 (12) | 58 (12) | 62 (10) | 0.150 |
| Age >60 years (n (%)) | 53 (51) | 33 (50) | 20 (54) | ― |
| Mean duration of disease (years) | 11 (7) | 12 (7) | 10 (7) | ― |
| Total bilirubin (μmol/l) | 15 (17) | 17 (20) | 12 (7) | ― |
| Advanced histological stage (n (%))* | 45 (44) | 35 (53) | 10 (27) | 0.013 |
| Complications of portal hypertension (n (%)) | 9 (9) | 8 (12) | 1 (3) | 0.152 |
| HDL‐cholesterol (mmol/l) | 1.76 (0.49) | 1.71 (0.54) | 1.81 (0.41) | ― |
| Triglycerides (mmol/l) | 1.16 (0.44) | 1.02 (0.33) | 1.41 (0.50) | <0.001 |
| Mean BMI (kg/m2) | 25.4 (4.3) | 24.7 (3.7) | 26.8 (5.1) | 0.034 |
| BMI ⩾25 kg/m2 (n (%)) | 47 (46) | 25 (38) | 22 (59) | 0.041 |
| Arterial hypertension (n (%))† | 31 (30) | 16 (24) | 15 (41) | 0.117 |
| Diabetes (n (%)) | 3 (3) | 2 (3) | 1 (3) | ― |
| Family history of premature cardiovascular disease (n (%))‡ | 23 (22) | 23 (22) | 23 (22) | ― |
| Personal history of cardiovascular events (n (%)) | 8 (8) | 4 (6) | 4 (11) | ― |
BMI, body mass index; HDL, high‐density lipoprotein.
Values are mean (SD) unless otherwise stated. p Values ⩽0.20 are shown.
*Patients with stage III or IV according to the Ludwig classification on histological examination.
†Blood pressure values ⩾140/90 mm Hg, or treatment for hypertension.
‡Coronary heart disease, peripheral arterial disease, abdominal aortic aneurysm or symptomatic carotid artery disease in male first‐degree relatives before the age of 55 years, or in female first‐degree relatives before the age of 65 years.
In the PBC population, 66 patients had serum concentrations of total cholesterol <6.21 mmol/l (mean (SD) 5.15 (0.83) mmol/l; range 2.43–6.18 mmol/l) and 37 had concentrations ⩾6.21 mmol/l (7.19 (0.83) mmol/l; range 6.28–9.67 mmol/l) (table 1). The 37 patients had significantly higher serum triglyceride levels and BMI. None of the patients with PBC from either subset had a history of excessive alcohol consumption (daily alcohol intake exceeding 80 g in male or 50 g in female patients), and all of them had stopped alcohol consumption since the time of PBC diagnosis. The proportion of patients who had never smoked was similar in patients with PBC, with or without hypercholesterolaemia (61% v 59%). At the time of enrolment, 28 patients (76%) with hypercholesterolaemia and 52 (79%; p = 0.806) without it were receiving ursodeoxycholic acid for treatment of liver disease. The mean (SD) duration of treatment lasting for at least 6 months was 7.2 (4.4) and 6.5 (4.4) years, respectively (p = 0.348).
There was a significant relationship between disease stage and serum cholesterol levels. Prevalence of liver cirrhosis or fibrosis was significantly lower in the hypercholesterolaemic group (table 1), and patients with increasing severity of liver disease (without liver cirrhosis, n = 58; with uncomplicated cirrhosis, n = 36; with cirrhosis and major complications of portal hypertension, n = 9) showed decreasing levels of both total cholesterol (6.23 (1.03), 5.66 (1.32) and 4.53 (1.50) mmol/l, respectively; p<0.001 for trend) and HDL‐cholesterol (1.84 (0.44) mmol/l, 1.78 (0.52) mmol/l and 1.16 (0.26) mmol/l, respectively; p<0.001 for trend). Severity of liver disease was not associated with age (58 (12), 62 (12) and 60 (9) years, respectively; p = 0.430).
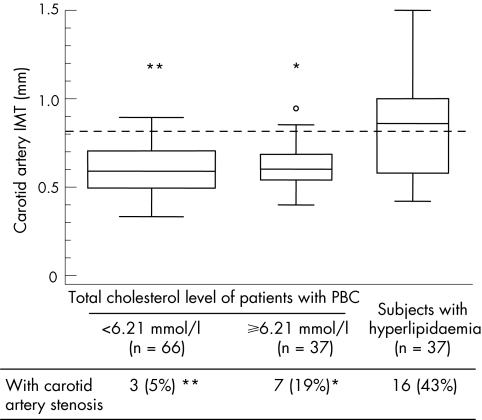
On average, the whole population of patients with PBC did not show increased carotid artery IMT values, which were actually lower compared with the control group (0.606 (0.136) v 0.653 (0.161) mm; p = 0.027). The proportion of subjects with increased carotid artery IMT—that is, with an IMT value in the highest quintile of the distribution of the measures obtained in the study populations—was 11% in the patients with PBC and 18% in the controls (p = 0.146). Carotid artery stenosis was detected in 45% and 55% of the subjects from the two groups (p = 0.822). Figure 1 shows the distribution of carotid artery IMT values in patients with PBC, with or without hypercholesterolaemia, and in subjects with hyperlipidaemia, without liver disease. Frequency of increased carotid artery IMT was significantly (pc<0.001) lower in both PBC groups (14% and 9%, respectively) than in controls with hyperlipidaemia (54%). Table 2 further analyses carotid artery measurements in the different study populations.
Figure 1 Carotid artery measurements in patients with primary biliary cirrhosis (PBC) and in controls with hypercholesterolaemia.*pc = 0.002; **pc<0.001 for the comparison with subjects with hyperlipidaemia. pc are Bonferroni‐adjusted p values to control for multiple testing. The horizontal dotted line denotes the cut‐off value for the quintile with highest thickness (0.808 mm). Each box extends from the 25th to the 75th centile, an interval referred to as the interquartile range (IQR), and the line in the middle of the box represents the median of the data. The lines emerging from the box extend to the upper and lower adjacent values (the largest data point ⩽75th centile plus 1.5 times the IQR, and the smallest data point ⩾25th centile minus 1.5 times the IQR, respectively). If the examined data come from a normal distribution, we would expect the interval between adjacent values to include 99.3% of the data. Observed points more extreme than the upper or lower adjacent values are plotted individually. Box width has been made proportional to the number of observations. IMT, intima–media thickness.
Table 2 Risk of increased carotid artery intima–media thickness or stenosis in the different study populations as assessed by logistic regression analysis.
| No (%) with increased IMT* | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Controls (n = 141) | 25 (18) | 1 | ||
| PBC, total cholesterol <6.21 mmol/l (n = 66) | 6 (9) | 0.5 | 0.2 to 1.2 | 0.111 |
| PBC, total cholesterol ⩾6.21 mmol/l (n = 37) | 5 (14) | 0.7 | 0.3 to 2.0 | 0.543 |
| Subjects with hyperlipidaemia (n = 37) | 20 (54) | 5.4 | 2.5 to 11.9 | <0.001 |
| No (%) with carotid stenosis | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Controls (n = 141) | 12 (9) | 1 | ||
| PBC, total cholesterol <6.21 mmol/l (n = 66) | 3 (5) | 0.5 | 0.1 to 1.9 | 0.313 |
| PBC, total cholesterol ⩾6.21 mmol/l (n = 37) | 7 (19) | 2.5 | 0.9 to 6.9 | 0.075 |
| Subjects with hyperlipidaemia (n = 37) | 16 (43) | 8.2 | 3.4 to 20 | <0.001 |
IMT, intima–media thickness; PBC, primary biliary cirrhosis.
*IMT values in the highest quintile of the distribution of carotid artery measures obtained in the subjects under study (cut‐off value for the quintile with highest thickness = 0.808 mm).
Study of the relationship between ultrasonographic findings and the most prevalent cardiovascular risk factors besides hypercholesterolaemia in the PBC population—that is, age ⩾61 years and arterial hypertension—showed higher carotid artery IMT values in elderly patients with PBC than in their younger counterparts (mean (SD) 0.651 (0.127) v 0.558 (0.129) mm, p<0.001), and no significant difference between patients with PBC, with or without arterial hypertension (0.626 (0.131) v 0.597 (0.138) mm; p = 0.292). Table 3 shows the association between such risk factors and ultrasonographic findings at the carotid level.
Table 3 Association between different cardiovascular risk factors and the presence of increased carotid artery intima–media thickness or stenosis in primary biliary cirrhosis.
| No (%) with increased IMT* | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Total cholesterol (mmol/l) | ||||
| <6.21 (n = 66) | 6 (9) | 1 | ||
| ⩾6.21 (n = 37) | 5 (14) | 1.6 | 0.4 to 5.5 | 0.488 |
| Age (years) | ||||
| ⩽60 (n = 50) | 3 (6) | 1 | ||
| >60 (n = 53) | 8 (15) | 2.8 | 0.7 to 11.2 | 0.148 |
| Arterial hypertension† | ||||
| No (n = 72) | 7 (10) | 1 | ||
| Yes (n = 31) | 4 (13) | 1.4 | 0.7 to 11.2 | 0.633 |
| No (%) with carotid stenosis | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Total cholesterol (mmol/l) | ||||
| <6.21 (n = 66) | 3 (5) | 1 | ||
| ⩾6.21 (n = 37) | 7 (19) | 4.9 | 1.2 to 20 | 0.028 |
| Age (years) | ||||
| ⩽60 years (n = 50) | 2 (4) | 1 | ||
| >60 years (n = 53) | 8 (15) | 4.3 | 0.9 to 21 | 0.076 |
| Arterial hypertension† | ||||
| No (n = 72) | 2 (3) | 1 | ||
| Yes (n = 31) | 8 (26) | 12.1 | 2.4 to 61 | 0.002 |
IMT, intima–media thickness; PBC, primary biliary cirrhosis.
*IMT values in the highest quintile of the distribution of carotid artery measures obtained in the subjects under study (cut‐off value for the quintile with highest thickness = 0.808 mm).
†Blood pressure values of ⩾140/90 mm Hg, or treatment for hypertension.
To further characterise the association between cardiovascular risk factors and ultrasound findings at the carotid level in PBC, we identified the subset of patients with PBC presenting with arterial hypertension or old age, but did not have hypercholesterolaemia (n = 38), and the subset presenting with the same risks in addition to hypercholesterolaemia (n = 22). The 43 patients with PBC who had neither hypertension nor old age, regardless of their cholesterol status, were considered the reference group. Mean (SD) carotid artery IMT values were 0.555 (0.130) mm in the reference group, and 0.644 (129) and 0.639 (0.130) mm, respectively, in the two groups presenting hypertension or old age without hypercholesterolaemia or in addition to it (pc = 0.005 and pc = 0.029, respectively, v reference group). Logistic regression analysis showed that, compared with the reference group, the risk of having a higher value of carotid artery IMT was 3.1 times greater (95% CI 0.6 to 17; p = 0.192) in the patients presenting hypertension or old age but no hypercholesterolaemia, and 4.6 times greater (0.8 to 27; p = 0.096) in those who also had hypercholesterolaemia. The corresponding figures relative to the risk of carotid stenosis were 3.6 (0.4 to 36; p = 0.277) and 15.8 (1.8 to 141; p = 0.014), respectively.
Detailed lipid profile was studied in two subgroups of 45 patients with PBC and 30 controls that included the same proportion of subjects with hypercholesterolaemia (30 and 20 patients, respectively). Free:esterified serum cholesterol ratio was 0.13 (0.06) in the PBC and 0.10 (0.05) in the control subgroup (p = 0.293). Both HDL‐cholesterol and LDL‐cholesterol concentrations were significantly higher in PBC (1.86 (0.44) v 1.45 (0.36) mmol/l, p<0.001; and 4.11 (0.96) v 3.10 (1.37) mmol/l; p = 0.001). Apo A‐I was 1.05 (0.70) g/l in the PBC group and 2.20 (1.09) g/l in the control group (p<0.001), whereas Apo B did not differ between them (1.73 (0.76) v 1.67 (0.56) g/l, respectively; p = 0.473). Multivariable analysis failed to reveal any significant relationship between lipid profile and higher value of carotid artery IMT or carotid stenosis. Use of IMT as a continuous variable gave similar results.
Discussion
This study was designed to clarify the clinical significance of hypercholesterolaemia in PBC. We used ultrasound imaging of the carotid artery as a non‐invasive indicator of subclinical atherosclerosis to compare patients with PBC with normocholesterolaemic subjects without any known cardiovascular risk factors and subjects with hypercholesterolaemia. We found increased IMT values and high prevalence of carotid stenosis in controls with hypercholesterolaemia but not in patients with PBC with corresponding levels of serum cholesterol. Rather, results of ultrasonographic examination in the patients who had hypercholesterolaemia with PBC were remarkably similar to normocholesterolaemic patients with PBC and controls.
Early atherosclerotic lesions of the carotid artery are expected to be detectable in PBC well before a distorted collection of patients remains as a result of patient removal by liver‐related mortality. Therefore, our findings offer strong support for the contention that hypercholesterolaemia associated with chronic cholestatic conditions is not atherogenic, and agree with results of earlier retrospective studies on cardiovascular morbidity and mortality in PBC.6,8 The available evidence invites consideration of some unknown factors protecting patients with PBC from the atherogenic effects of longlasting hypercholesterolaemia. Among these factors, the particular lipoprotein pattern characteristic of patients with PBC, with raised HDL‐cholesterol levels and the presence of lipoprotein‐X, has been the object of interest and warrants further investigation.19,20 Complex interactions between atherogenic lipoproteins and macrophages beyond cholesteryl ester accumulation and foam cell formation have been described.21 Although detailed analysis of serum lipid profiles in a subset of our patients did not show any relationship with carotid artery IMT or stenosis specific to PBC, in these patients we found higher HDL‐cholesterol and LDL‐cholesterol and lower Apo A‐1 levels than in controls. This picture resembles, at least partly, the altered lipid profile described by Crippin et al6 in an uncontrolled study on PBC. Lower Apo A‐1 content, despite higher cholesterol content, of HDL in our patients with PBC compared with controls may be secondary to initial impairment of protein synthesis. A thorough lipid analysis, however, was beyond the scope of this study, and we could not examine the hypothesis that different types of HDL, less rich in Apo A‐1, or altered LDL contribute to a less atherogenic profile in PBC.
Ursodeoxycholic acid, the only approved medical treatment for PBC, has been reported to improve hyperlipidaemia in this condition, probably as the result of general improvement of cholestatic features.22 However, the magnitude of changes induced by this bile acid in the lipid pattern of patients with PBC is negligible compared with cholesterol‐lowering agents.23,24,25 Moreover, atherosclerosis requires several decades to develop, a period of time that is much longer than the average duration of bile acid treatment in the present PBC series. Finally, it should be noted that in this study each patient with hypercholesterolaemic PBC was matched with a control with hypercholesterolaemia according to a set of criteria that included serum total cholesterol value measured at the time of enrolment.
Showing that serum cholesterol levels may offer inaccurate prediction of cardiovascular risk has substantial implications for the management of patients with PBC. In view of the high prevalence of hyperlipidaemia in the general population, many patients with PBC should be expected to present raised cholesterol levels as the expression of a genetically determined trait conditioning increased risk of cardiovascular disease. In recent years, it has become increasingly apparent that PBC is coming to medical attention more often, showing a less severe clinical spectrum than in the past.1,2 Although progression to the latest disease stages is still to be expected even in presymptomatic patients, survival has improved and non‐liver‐related deaths are being recognised as an important issue.3 Our data show that in the subset of patients with PBC with hypertension or old age, hypercholesterolaemia may be associated with increased IMT and frequency of stenosis at the carotid level. Therefore, this patient subset deserves closer follow‐up, with an active approach for prevention of cardiovascular disease and therapeutic intervention, including the use of inhibitors of 3‐hydroxy‐3‐methylglutaryl coenzyme A to lower serum cholesterol.4
In conclusion, this study shows that hypercholesterolaemia associated with PBC does not condition, in itself, the development of subclinical atherosclerosis as evidenced by ultrasound imaging of carotid arteries. Cholesterol‐lowering treatment should be undertaken in those patients with hypercholesterolaemia who also present other risk factors for cardiovascular disease. Further research on the possible antiatherogenic properties of lipoprotein patterns specific for chronic cholestatic conditions, or of other factors that may result in protection at the endothelial surface, should be encouraged.
Abbreviations
Apo - apolipoprotein
BMI - body mass index
HDL - high‐density lipoprotein
IMT - intima–media thickness
IQR - interquartile range
LDL - low‐density lipoprotein
PBC - primary biliary cirrhosis
Footnotes
Competing interests: None.
References
- 1.Talkwalkar J A, Lindor K D. Primary biliary cirrhosis. Lancet 200336253–61. [DOI] [PubMed] [Google Scholar]
- 2.Selmi C, Invernizzi P, Keefe E B.et al Epidemiology and pathogenesis of primary biliary cirrhosis. J Clin Gastroenterol 200438264–271. [DOI] [PubMed] [Google Scholar]
- 3.Prince M I, Chetwynd A, Craig W L.et al Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 200453865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 20012852486–2497. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen T A. Lipid absorption, bile acid and cholesterol metabolism in patients with chronic liver disease. Gut 197213682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crippin J S, Lindor K D, Jorgensen R.et al Hypercholesterolemia and atherosclerosis in primary biliary cirrhosis: what is the risk? Hepatology 199215858–862. [DOI] [PubMed] [Google Scholar]
- 7.Longo M, Crosignani A, Battezzati P M.et al Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 200251265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dam G M, Gips C H. Primary biliary cirrhosis in the Netherlands. Scand J Gastroenterol 19973277–83. [DOI] [PubMed] [Google Scholar]
- 9.Mannami T, Konishi M, Baba S.et al Prevalence of asymptomatic carotid atherosclerotic lesions detected by high‐resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study city. Stroke 199728518–525. [DOI] [PubMed] [Google Scholar]
- 10.Burke G L, Evans G W, Riley W A.et al Arterial wall thickness is associated with prevalent cardiovascular disease in middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke 199526386–391. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary D H, Polak J F, Kronmal R A.et al Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 199934014–22. [DOI] [PubMed] [Google Scholar]
- 12.Hodis H N, Mack W J, LaBree L.et al The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med 1998128262–269. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan M. Medical progress: primary biliary cirrhosis. N Engl J Med 19963351570–1580. [DOI] [PubMed] [Google Scholar]
- 14.De Fabiani E, Caruso D, Cavaleri M.et al Cholesta‐5,7,9(11)‐trien 3beta‐ol found in plasma of patients with Smith‐Lemli‐Opitz syndrome indicates formation of sterol hydroperoxide. J Lipid Res 1996372280–2287. [PubMed] [Google Scholar]
- 15.Montauban van Swijndregt A D, De Lange E E, De Groot E.et al An in vivo evaluation of the reproducibility of intima‐media thickness measurements of the carotid artery segments using B‐mode ultrasound. Ultrasound Med Biol 199925323–330. [DOI] [PubMed] [Google Scholar]
- 16.Kagawa R, Moritake K, Shima T.et al Validity of B‐mode ultrasonographic findings in patients undergoing carotid endarterectomy in comparison with angiographic and clinicopathologic features. Stroke 199627700–705. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter J P, Lexa F J, Davis J T. Determination of duplex Doppler ultrasound criteria appropriate to the North American Symptomatic Carotid Endarterectomy Trial. Stroke 199627695–699. [DOI] [PubMed] [Google Scholar]
- 18.Williamson D F, Parker R A, Kendrick J S. The box plot: a simple visual method to interpret data. Ann Intern Med 1989110916–921. [DOI] [PubMed] [Google Scholar]
- 19.Agorastos J, Fox C, Harry D S.et al Lecitin‐cholesterol acyltransferase and the lipoprotein abnormalities of obstructive jaundice. Clin Sci Mal Med 197854369–379. [DOI] [PubMed] [Google Scholar]
- 20.Sörös P, Böttcher J, Maschek H.et al Lipoprotein‐X in patients with cirrhosis: its relationship to cholestasis and hypercholesterolemia. Hepatology 1998281199–1205. [DOI] [PubMed] [Google Scholar]
- 21.Rader D J, Puré E. Lipoprotein, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab 20051223–230. [DOI] [PubMed] [Google Scholar]
- 22.Balan V, Dickson E R, Jorgensen R A.et al Effects of ursodeoxycholic acid on serum lipids of patients with primary biliary cirrhosis. Mayo Clin Proc 199469923–929. [DOI] [PubMed] [Google Scholar]
- 23.Poupon R E, Oguerram K, Chrétien Y.et al Cholesterol‐lowering effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology 199317577–582. [DOI] [PubMed] [Google Scholar]
- 24.Miettinen T A, Färkkilä M, Vuoristo M.et al Serum cholestanol, cholesterol precursors, and plant sterols during placebo‐controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology 1995211261–1268. [PubMed] [Google Scholar]
- 25.Del Puppo M, Galli‐Kienle M, Crosignani A.et al Cholesterol metabolism in primary biliary cirrhosis during simvastatin and UDCA administration. J Lipid Res 200142437–441. [PubMed] [Google Scholar]