Abstract
Background and aims
Intralumenal bile acid (BA) concentrations have a profound effect on cholesterol absorption. We performed studies to assess the effects of markedly reduced lumenal BA on cholesterol absorption in children with inborn errors in BA synthesis and the role of micellar solubilisation of cholesterol on its absorption in an animal model using human intestinal contents.
Methods
We studied five subjects: two with 3β hydroxy‐C27 steroid dehydrogenase isomerase deficiency (3‐HSD), two with Δ4‐3‐oxosteroid 5β reductase deficiency (5β reductase), and one with 2‐methylacyl CoA racemase deficiency (racemase). Subjects were studied on supplemental BA therapy and three weeks after withdrawal of supplements. During each treatment period a liquid meal was consumed. Duodenal samples were collected and analysed, and cholesterol absorption and cholesterol fractional synthetic rates were measured. Human intralumenal contents were infused in a bile diverted rat lymph fistula model to assess micellar versus vesicular absorption of cholesterol.
Results
Without BA supplementation, intralumenal BA concentrations were below the critical micellar concentration (CMC) whereas intralumenal BAs increased to above the CMC in all subjects on BA supplementation. Lumenal cholesterol was carried primarily as vesicles in untreated subjects whereas it was carried as both micelles and vesicles in treated subjects. Cholesterol absorption increased ≈55% in treated compared with untreated subjects (p = 0.041), with a simultaneous 70% decrease in synthesis rates (p = 0.029). In the rat lymph fistula model, minimal vesicular cholesterol was absorbed whereas vesicular and micellar fatty acid and phospholipid were comparably absorbed.
Conclusions
Increasing micellar cholesterol solubilisation by supplemental BA in subjects with inborn errors of BA synthesis leads to an improvement in cholesterol absorption and reduction in cholesterol synthesis due to improved micellar solubilisation of cholesterol.
Keywords: cholesterol synthesis, low density lipoprotein receptor, lumenal contents, bile acid
Bile acids (BA) are synthesised from cholesterol.1 Within the gut lumen, BA form macromolecular aggregates with digested food products and other bile components, including cholesterol, phospholipids, monoglycerides, and fatty acids. The now aqueous soluble lipid aggregates diffuse through the lumenal contents and unstirred water layer to the intestinal epithelia where digested lipid products cross the enterocyte membrane and enter the cells.2,3 Although early studies indicated that the intestinal lumen contains primarily micelles, more recent studies have demonstrated the presence of a variety of different sized particles in the lumen after a meal. Two major macromolecular aggregates appear to be involved with lipid absorption within the intestinal lumen. Unilamellar vesicles are larger BA poor particles which are several hundred angströms in diameter and contain phospholipids, fatty acids, monoglycerides, cholesterol, and BA. Mixed micelles are much smaller BA rich particles with diameters less than 100 angströms and are comprised primarily of BA and cholesterol with lesser amounts of phospholipids. BA also exist in very low concentration as monomeric forms depending on the concentration of BA in solution.4,5 Different types of aggregates appear to be present in a fluid equilibrium that shifts between different sized particles as the concentration of BA and other lipid components within the lumen changes.4 When BA concentrations exceed the critical micellar concentration (CMC), micelles form with some monomeric BA. BA concentrations below the CMC result in equilibrium that is preferential towards vesicle formation.
BA appear to be required for efficient cholesterol absorption. In the absence of BA, murine cholesterol absorption is markedly reduced.6 Little is known about absorption of cholesterol from vesicles in humans. Based on these in vivo and in vitro results, we have proposed that poor lipid absorption efficiency is due to poor micellar incorporation of cholesterol. This hypothesis has not been directly tested because of technical difficulties involved in obtaining lumenal samples from both humans and mice, and because healthy adult humans synthesise adequate levels of primary BA.
In recent years, several inborn errors of BA metabolism have been identified and characterised.7,8,9,10,11 In each of these conditions, markedly reduced intralumenal primary and secondary BA concentrations are observed and intermediary metabolites accumulate with chemical structures characteristic of the substrate for the deficient enzyme. The provision of exogenous supplemental BA enhances lipid absorption and downregulates the synthesis of the hepatotoxic atypical BA leading to increased bile flow and resolution of cholestasis and no residual liver disease.9,12 Using a recently tested method to measure lumenal micellar and vesicular cholesterol in human lumenal samples,13 the effect of markedly reduced BA on cholesterol absorption and cholesterol distribution between micellar and vesicular particles was determined in individuals with various inborn errors in BA synthesis. Additionally, we infused human lumenal contents carrying cholesterol as either micelles or vesicles into the small intestine of rats using the lymph fistula model with endogenous bile diverted out of the body. In this model, the relative importance of micellar and vesicular cholesterol absorption could be determined without the interference of other BA containing particles in the lumen.
Methods
Human subjects
Subjects were aged 6–19 years with inborn errors of BA synthesis previously diagnosed by FAB‐MS analysis of urine and bile (table 1). All subjects had been treated with cholic acid (CA; 3α,7α 12α‐trihydroxy‐5β‐cholanoic acid) daily or ursodeoxycholic acid (UDCA) every other day since their diagnosis. All subjects receiving CA were compliant although it was clear that those receiving UDCA were less compliant and may have been taking UDCA as infrequently as every 3–4 days. All subjects were in good health with no other known cardiac, pulmonary, renal, gastrointestinal, or pancreatic disease. None was on any medications except BA supplements. All subjects grew normally and were otherwise healthy. Subjects were studied during two different treatment phases. In the first phase, subjects were studied while receiving their standard BA replacement therapy. During the second phase, subjects were taken off of their BA treatment for three weeks. All subjects were on unrestricted diets because all came from locations distant from the study centre and dietary control was not practical.
Table 1 Statistics of subjects with inborn errors in bile acid metabolism.
| Measurement | Subject | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Age at diagnosis | 13 y | 2 mo | 2 y | 3 mo | 3 mo |
| Age at study | 19 y | 6 y | 6 y | 13 y | 13 y |
| Sex | M | F | M | M | M |
| Diagnosis | 3‐HSD | Racemase | 3‐HSD | 5β reductase | 5β reductase |
| BA therapy | CA | CA | CA | UDCA | UDCA |
| ALT on therapy (U/l)* | <3 | 7 | 21 | 24 | 25 |
| ALT off therapy(U/l)* | 140 | 19 | 61 | 23 | 25 |
| GGT on therapy(U/l)* | 45 | 23 | 29 | 28 | 21 |
| GGT off therapy(U/l)* | 37 | 15 | 24 | ND | 21 |
BA, bile acid; 3‐HSD, 3β hydroxy‐C27‐steroid dehydrogenase/isomerase; 5β reductase, Δ4‐3‐oxosteroid 5β reductase; racemase, 2‐methylacyl CoA racemase; CA, cholic acid; UDCA, ursodeoxycholic acid; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; ND, not determined.
*Age matched normal values for Children's Hospital Clinical Laboratory: ALT 10–45 U/l, GGT 11–34 U/l.
On day 1 of the study, subjects were seen after a 16 hour fast at the General Clinical Research Center. Their last BA dose had been given on the previous evening. Serum was obtained for total, low density lipoprotein (LDL)‐ and high density lipoprotein (HDL)‐cholesterol and triglyceride concentrations and monocytes were obtained for HMG CoA reductase and LDL receptor mRNA levels. After intravenous sedation with midazolam and topical anaesthesia of the nare, a nasoduodenal tube was placed with fluoroscopic guidance with the tube tip placed at the ligament of Treitz. Subjects ingested a previously described standardised meal.4 After a 15 minute baseline collection, duodenal drainage was collected for 75 minutes. Samples were processed as described previously.13,14 On day 2, oral and intravenous doses of stable isotope labelled cholesterol were given to assess cholesterol absorption, and blood was collected at baseline and at +24, +48, and +72 hours. On day 5, baseline blood was drawn for red blood cell (RBC) cholesterol isotope enrichment and subjects were given oral deuterated water (D2O) to assess cholesterol fractional synthetic rates (FSRs). On day 6, blood was obtained at the same time as isotope administration to determine FSR. The process was repeated as described above three weeks after subjects discontinued BA supplements. Serum tests of liver function were obtained on day 1 to assess any evidence of liver injury while off BA therapy. The Institutional Review Boards of the University of Cincinnati and the Cincinnati Children's Hospital Medical Center approved the study. Informed consent was obtained from the adult subject and from the parent for subjects less than age 18 years who also gave assent to participate.
Analytical methods: humans
Serum and mononuclear cell measurements
Serum total, LDL‐cholesterol, and HDL‐cholesterol, and triglyceride concentrations were measured using methods validated by the NIH‐Lipid Research Clinics. Copies of LDL receptor mRNA and HMG CoA reductase mRNA in mononuclear cells were measured as described previously.14
Duodenal aspirates
Intermicellar BA concentrations of each sample were determined14 and used to make a buffer to separate micelles and non‐micellar particles, including vesicles, by size exclusion chromatography.15 Cholesterol was measured in each fraction by gas liquid chromatography using stigmastanol as an internal standard.16 Phospholipid and free fatty acid concentrations were measured as described previously.13,14
Serum chemistries
Serum “liver function tests”, including total and direct bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase, gamma glutamyl transpeptidase, total protein, and albumin were measured in the Clinical Laboratory of the Children's Hospital Medical Center.
Cholesterol absorption
Analysis of cholesterol absorption was performed as previously described.14 Briefly, 15 mg of intravenous cholesterol‐D7 (Cambridge Isotopes, Andover, Massachusetts, USA) were dissolved in 20% Intralipid and administered over 30 minutes, and 75 mg of cholesterol‐13C2 (Mass Trace, Woburn, Massachusetts, USA) orally were dissolved in corn oil and added to an English muffin as part of a mixed meal. Blood samples were collected for 72 hours, and free cholesterol was extracted from RBCs. 13C enrichments of free cholesterol were measured by differential isotope ratio mass spectrometry using an automated dual inlet system (SIRA 12, Isomass, Cheshire, UK) and absorption calculated, as described previously.17
Cholesterol biosynthesis (FSR)
Endogenous cholesterol synthesis determination was performed as described previously.14,18 On day 5, the subject was dosed with 0.71 g D2O per kg by mouth, and continued to consume water containing 0.7 g D2O/kg. Deuterium enrichment was measured in RBC free cholesterol and plasma water before and 24 hours after consumption of D2O, and cholesterol FSR was calculated.
BA composition and concentration
Bile from lumenal samples collected at baseline (−15 to 0 minutes), +15 to +30 minutes, and +45 to +60 minutes after meal administration was analysed by gas chromatography (GC) for total and individual BA after addition of the internal standard, nordeoxycholic, and extraction on solid phase Bond Elut‐C18 cartridges, as described elsewhere.9,19,20 Samples were solvolysed, hydrolysed, and underwent enzymic hydrolysis with cholyglycine hydrolase. The unconjugated BA were extracted on a cartridge of octadecylsilane bonded silica and passed through a small column of the lipophilic anion exchange gel, diethylaminohydroxypropyl Sephadex LH‐20 (Lipidex DEAP), to remove neutral steroids and sterols. After addition of a second internal standard (coprostanol 0.1–10 μg), BA were converted to the methyl ester trimethylsilyl ether derivatives for analysis by GC.
Gas chromatography (GC)
Me‐TMS ether derivatives were separated by GC on 30 m×0.4 mm DB‐1 (film thickness 0.25 μm) fused silica capillary column (J and W Scientific, Folsom, California, USA) operated in a temperature programming mode from 225–295°C. BA were quantified from a comparison of their peak area response with that obtained for the known amount of the added internal standard, nordeoxycholic acid.
Rat lymph fistula model
Male Sprague‐Dawley rats (300–350 g) were purchased from Harlan (Indianapolis, Indiana, USA) and were fed Purina rodent chow. Animals were fasted overnight. A lymph cannula and duodenal feeding tube were implanted into the animals under halothane anaesthesia, and the common bile duct was cannulated for diversion of bile out of the body, as previously described.21,22,23 The major intestinal lymph duct lying just superior to the superior mesenteric artery was cannulated. Additionally, a tube was placed through the fundus of the stomach and extended 2.0 cm into the duodenum. Finally, the common bile duct was cannulated and bile diverted. Animals recovered overnight at ∼30°C while being infused with 5% dextrose saline (145 mM NaCl, 4 mM KCl, and 0.28 M dextrose) at 3 ml/h. The next day, the dextrose solution was replaced with either 10 mM taurocholic acid or saline at a rate of 3 ml/h. Lymph was collected for one hour. Concurrent with the animal surgeries, intralumenal contents were collected after a meal stimulus from normal adult subjects, as described above. Samples were mixed with either saline or 10 mM glycocholic acid (GCA) and injected into the duodenum within 24 hours over five minutes. Immediately following, rats were infused with either saline or 10 mM taurocholic acid. Lymph was collected from rats on an hourly basis for eight hours.
Analytical methods: animal studies
Lymph composition
Lymph was collected from rats on an hourly basis and quantitated by assay kits for phospholipid (Wako Chemicals USA, Richmond, Virginia, USA), cholesterol (Sigma‐Aldrich, St Louis, Missouri, USA), and triglyceride (Randox, San Francisco, California, USA).
Statistics
Data are presented as means (SEM). Comparisons of human data were performed using two tailed paired Student's t tests (cholesterol absorption, FSR, plasma lipid concentrations) or Student's t tests (lumenal lipids, rat lymph fistula lipids). For intralumenal lipid measurements, areas under the curve (AUC) were calculated using the trapezoidal rule, with baseline drawn between values at time 0 and +75 minutes. Only four pairs of data were compared because intralumenal samples could not be obtained from one patient (C) when off therapy. Lymph fistula triglyceride, phospholipid, and cholesterol comparisons were made between AUC using the trapezoidal rule with baseline drawn between the 0 and +8 hour data points. Comparisons between mean AUC for phospholipids and triglycerides were made using unpaired Student's t tests. As AUC values were not normally distributed for cholesterol, the Shapiro‐Wilk statistic was used. Log transformed data were normally distributed and unpaired Student's t tests were used for comparisons.
Results
Subject characteristics
Subjects were studied while on BA treatment (table 1) and three weeks after BA withdrawal. Subjects did not lose weight while off BA treatment. ALT levels increased in two subjects after BA withdrawal (table 1). Serum total cholesterol was unchanged (p = 0.31) during BA supplementation (129.2 (13.5) mg/dl) versus when BA were not administered (117.2 (8.2) mg/dl). There was a trend (p = 0.15) for serum LDL‐cholesterol concentrations to be increased in treated (61.6 (6.1) mg/dl) versus untreated (44.6 (8.6) mg/dl) subjects.
Lumenal lipid composition
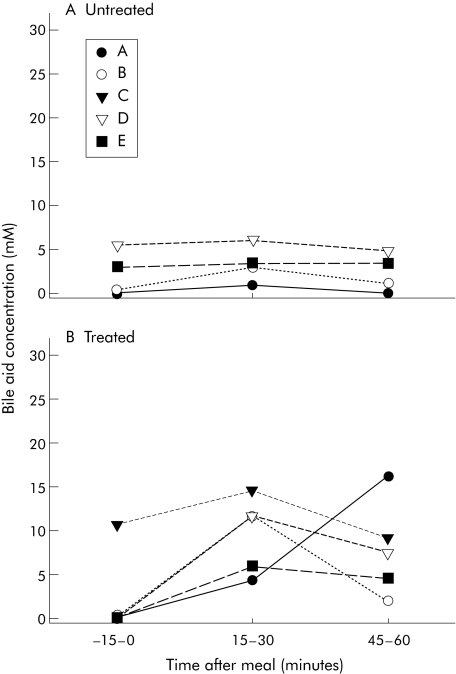
Lumenal BA compositions and concentrations varied markedly in subjects while supplemented with BA and when off BA therapy. The type of inborn errors in BA metabolism also had an impact on BA concentrations and compositions. When untreated, all subjects had small quantities of primary BA and some secondary BA in bile (table 2, fig 1). Additional BA identified by GC included 3β,7α‐dihydroxy‐ and 3β,7α,12α‐trihydroxy‐5‐cholenoic acids in one of the patients with 3β hydroxy‐C27‐steroid dehydrogenase/isomerase (3‐HSD) deficiency and cholestanoic acids in the subject with 2‐methylacyl CoA racemase (racemase) deficiency. As seen in table 2, other intermediary metabolites of the BA synthetic pathway were present in minor concentrations but their definitive identification by GC‐mass spectrometry was not performed. During BA therapy the amount of abnormal BA in the lumen decreased and supplemented BA increased.
Table 2 Lumenal bile acid concentrations in subjects with different inborn errors in bile acid metabolism.
| Bile acid | Untreated (mmol/l) | Treated (mmol/l) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | D | E | |
| CA | 0.8 | 1.8 | ND | 2.2 | 1.7 | 4.6 | 8.0 | 14.8 | 2.5 | 10.2 |
| CDCA | – | 0.9 | 3.0 | 1.3 | – | 2.8 | – | 2.1 | 11.5 | |
| DCA | 0.2 | 0.4 | 1.0 | 0.4 | – | 1.0 | – | 0.9 | 0.7 | |
| UDCA | – | – | 0.1 | 0.1 | – | – | – | 0.6 | 3.3 | |
| 3β‐delta | 3.1 | – | – | – | 0.4 | – | 3.5 | – | – | |
| Cholestanoic acid | – | 4.0 | – | – | – | 4.8 | – | – | – | |
Lumenal samples were collected 15–30 minutes after a liquid meal. Subjects were the same as those described in fig 1.
CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; UDCA, ursodeoxycholic acid; ND, not determined; 3β‐delta, 3β‐delta 5 CDCA or CA.
Figure 1 Lumenal bile acid concentrations with inborn errors in bile acid metabolism. After bile acid withdrawal for three weeks (untreated (A)) or while on bile acid treatment (treated (B)), lumenal samples were collected from a nasoduodenal tube prior to consumption of a liquid meal and every 15 minutes after the meal. Concentrations and composition were measured for three different time points. Concentrations for primary bile acids, secondary bile acids, and ursodeoxycholic acid are shown. Each line depicts a single subject and subject letter refers to table 1, which give subject descriptions.
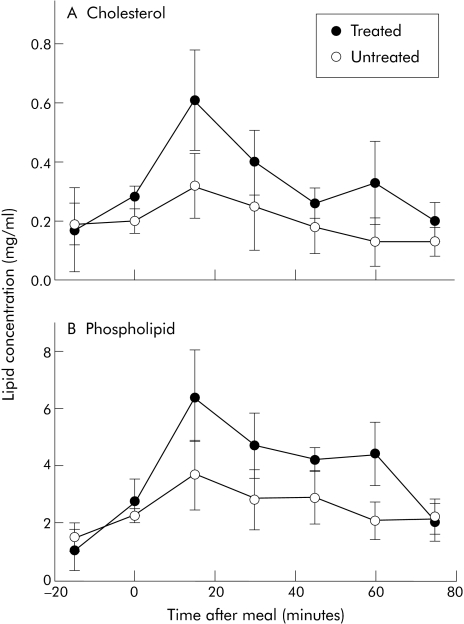
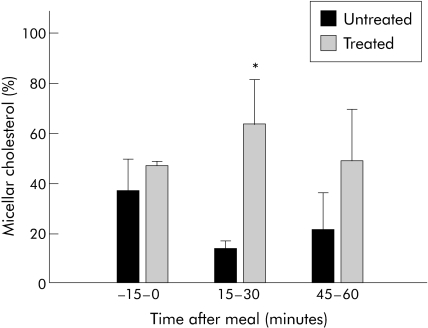
Intralumenal primary, secondary, and ursodeoxycholic acid concentrations were reduced in subjects with inborn errors of BA metabolism in response to a meal stimulus when not receiving BA therapy compared with when treated (fig 1). Additionally, there was no increase in concentrations in response to a meal, as during the treated phase. Lumenal lipid concentrations were measured after a meal stimulus. When untreated, there was a minimal increase in cholesterol concentrations in the aqueous phase in the intestinal lumen. When treated, subphase cholesterol concentrations increased. Results from four subjects were compared with and without BA supplementation. There was a marked difference (p = 0.007) in the subphase cholesterol AUC between untreated (18.5 (7.3) mg/ml per 90 minutes) and treated (30.3 (8.4) mg/ml per 90 minutes) phases (fig 2A). As with cholesterol, the typical increase in subphase phospholipid concentration in response to a meal stimulus was blunted when subjects were off BA treatment compared with when subjects were treated (fig 2B). AUC values of subphase phospholipid concentration were higher (p = 0.028) in subjects treated versus when not treated (358 (55) v 235 (63) mg/ml per 90 minutes, respectively). Free fatty acid concentrations were similar regardless of treatment (data not shown). As might be expected in lumenal contents with enough BA to form micelles, a greater proportion of cholesterol was carried as micelles at peak lumenal BA concentration (15–30 minutes post‐meal) in subjects treated with BA compared with without BA therapy (14.5 (8.1) v 64.0 (18.3)% in untreated v treated subjects, respectively) (p = 0.049) (fig 3).
Figure 2 Concentrations of cholesterol and phospholipid in the subphase of lumenal samples taken before and after a liquid meal. Samples were collected as described in fig 1. Cholesterol (A) and phospholipid (B) concentrations were measured as described in materials and methods.
Figure 3 Distribution of cholesterol in micelles of lumenal samples of subjects after a liquid meal. Lumenal samples were separated into micellar and non‐micellar particles at three different time points during the lumenal collection. Data are presented as μg cholesterol per sample for subjects untreated or treated with bile acid therapy. Significant difference between untreated and treated percentages (*p<0.05). Data are means (SEM).
Cholesterol absorption/metabolism
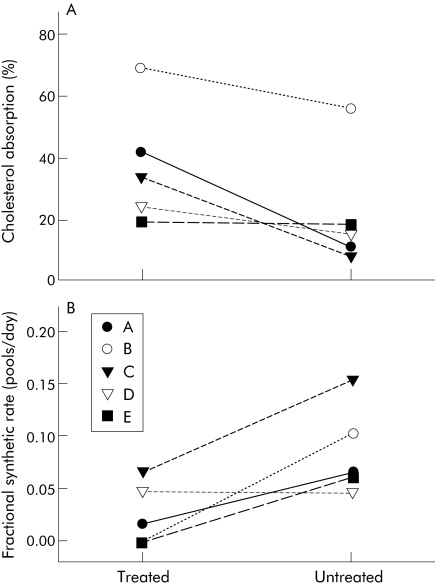
Cholesterol absorption and cholesterol FSRs were determined in all five subjects. In treated subjects, a significantly greater (p = 0.041; 37.4 (8.7)%) percentage of cholesterol was absorbed, regardless of genetic defect, compared with no BA treatment (21.5 (8.7)%) (fig 4A). Conversely, FSRs were significantly lower (p = 0.029) in subjects treated with BA (0.0262 (0.0133) pools/day) compared with on BA withdrawal (0.0856 (0.0192) pools/day) (fig 4B). In parallel with FSR changes, the amount of mononuclear cell HMG‐CoA reductase mRNA was greater (p<0.001) in subjects without BA treatment (15131 (761) copies/ml) compared with when on BA treatment (7037 (454) copies/ml). Mononuclear cell LDL receptor mRNA was also greater (p = 0.001) in untreated (5198 (335) copies/ml) versus treated (2811 (296) copies/ml) subjects.
Figure 4 Cholesterol absorption percentages and fractional synthetic rates are shown for each subject while receiving bile acid treatment or no bile acid supplement. Cholesterol absorption percentages (A) and fractional synthetic rates (B) were determined with stable isotope. Data are presented as per cent cholesterol absorbed or as pools of cholesterol synthesised each day. Each line depicts a single subject and subject letters refer to table 1, which provides subject descriptions.
Lymph fistula studies
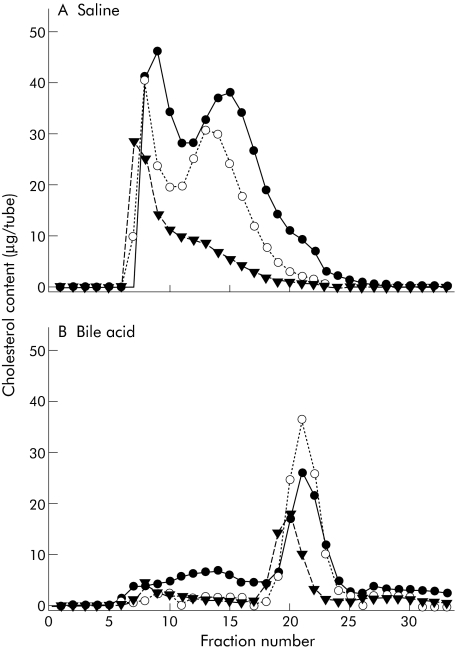
Based on the findings of our experiments in humans, we inferred that micellar cholesterol was the preferred source of cholesterol for absorption in our patients with inborn errors of BA metabolism and that this paradigm is likely to occur in healthy children and adults. To test this hypothesis, lumenal contents were collected from normal adults and mixed with either saline, to ensure that essentially all cholesterol was carried as vesicles, or with 10 mM GCA, to ensure that cholesterol was carried as micelles.13,15 Cholesterol carrying particle composition was confirmed by column chromatography (fig 5).
Figure 5 Cholesterol carried as micelles or non‐micellar particles, including vesicles, in lumenal samples that were mixed with either 0.9% saline or 10 mM glycocholic acid (GCA), and the resultant particles separated by size using the Superose 4B gel filtration columns. Cholesterol concentrations in the fractions from the gel exclusion chromatography were measured by gas chromatography in fractions eluted with 0.9% saline (A) or 10 mM GCA (B).
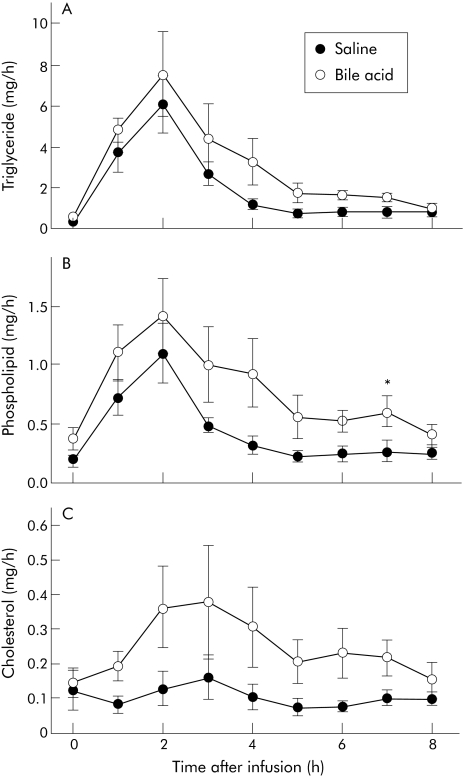
Micellar or vesicular human intralumenal contents were infused into the rat lumen (with bile diverted) and the amount of triglyceride, phospholipid, and cholesterol secreted into the lymph was measured. Enterohepatic circulation of BA was interrupted by bile diversion out of the body and thus the BA present in the lumen would predominantly be from those infused. The amount of triglycerides and phospholipids secreted into the lymph increased dramatically after the infusion for several hours and then decreased (fig 6A, B). The amount of phospholipid and triglyceride secreted was similar, regardless of the type of infusion: AUC was 15.8 (5.2) v 18.9 (6.5) mg/h per eight hours for vesicular versus micellar fatty acid, respectively, and AUC was 3.2 (1.0) v 5.0 (2.0) mg/h per eight hours for vesicular versus micellar phospholipid, respectively (fig 5C). Unlike lymphatic triglyceride and phospholipid outputs, there was no increase in the amount of cholesterol secreted into the lymph in rats infused with vesicles, with an AUC for cholesterol of 0.85 (0.22) mg/h for eight hours, whereas a significant (p = 0.042) increase did occur in rats infused with micelles, with an AUC curve for cholesterol of 2.05 (0.64) mg/h for eight hours.
Figure 6 Triglyceride (A), phospholipid (B), and cholesterol (C) secretion in rats infused with either micellar or non‐micellar particles. Rats that had their bile diverted were infused with human lumenal contents mixed with either saline (non‐micellar) or 10 mM glycocholic acid (GCA, micellar) and lymph was collected prior to and for eight hours after infusion. Data are presented as means (SEM) (n = 6).
Discussion
The results of the current study have demonstrated that multiple years after diagnosis, patients with inborn errors of BA metabolism have low intralumenal BA concentrations with predominantly abnormal metabolites when removed from supplemental BA therapy. With therapy, intralumenal BA concentrations of the supplemented BA increased to levels above their CMC and comparable with control children.24 Concurrent with improved intralumenal concentrations, lumenal cholesterol that was solubilised increased significantly as did micellar cholesterol. As a presumed consequence of the improved solubilisation of cholesterol, cholesterol absorption increased and the expected reduction in cholesterol FSR and HMG‐CoA reductase mRNA was observed.
Children with inborn errors of BA metabolism commonly present in infancy with cholestasis and fat and fat soluble vitamin deficiencies.7,9,25,26 As the BA biosynthetic pathways are compromised because of specific enzyme deficiencies, minimal primary BA are produced and large quantities of intermediary metabolites are produced which have been shown to be cholestatic and hepatotoxic.27,28 As a consequence, minimal amounts of BA are secreted into the intestinal lumen in response to gall bladder contraction. The amount of secreted BA can be corrected with exogenous BA and the associated pathophysiology of bile salt deficiency corrected. This group of subjects is particularly well suited to study the role of micellar solubilisation of cholesterol on its absorption.
The most significant observation of the current study relates to the key role of micellar solubilisation in cholesterol absorption. Cholesterol absorption has been presumed to be dependent on the presence of bile and bile salts in animals for as long as 50 years.6,29 Much of our current understanding of the uptake of dietary lipids is derived from the pivotal work of Hofmann and Borgstrom who identified and emphasised the importance of micellar solubilisation in the uptake of lipid digestive products by enterocytes.30,31,32 The validity of this important concept was later challenged by Stafford and colleagues who discovered the co existence of unilamellar liposome vesicles with bile salt lipid mixed micelles in the intestine.33 However, how the composition and structure of the bile salt mixed micelle affect cholesterol uptake by enterocytes was far from being well understood. A number of investigators have demonstrated that trihydroxy BA are more effective in promoting cholesterol absorption than dihydroxy BA; however, the degree of solubilisation was not measured in these experiments.34,35,36 Watt and Simmonds36 demonstrated that micellar solubilisation is important in enterocyte uptake by showing a linear relationship between the amount of cholesterol taken up by the small intestine and micellar cholesterol concentration. Although we know that micellar solubilisation is important for cholesterol uptake by enterocytes, we do not know if cholesterol in vesicles can be taken up by rat or human enterocytes. To our knowledge, no direct study of the role of BA solubilisation of cholesterol on its absorption has been done in humans.
The current investigation provides additional proof that cholesterol absorption is dependent on micellar solubilisation in the human/rodent translational model. In the samples collected in human subjects with inborn errors of BA metabolism, low intralumenal levels of micelle forming BA and minimal cholesterol were solubilised into micelles with cholesterol mostly in vesicular form in the intestinal contents. Although we presume the principal cause of reduced absorption relates to poor micelle formation, it is clearly possible that differences in expression of enterocyte cholesterol transporters, including ABC G5/G8 and NPC1L1 recently described in mice, may have contributed to our observed differences in cholesterol absorption.37,38
To confirm that the observed results were due to low micellar cholesterol, we took advantage of a model in which absorption of cholesterol and other lipids could be directly assessed in lymph duct cannulated rodents with bile diverted outside the body. In this model, human intestinal contents from normal adults whose lipid components were incorporated into either vesicles or micelles were perfused intraduodenally into bile and lymph fistula rats. We found that there was reduced cholesterol absorbed and secreted into the lymph in animals infused with vesicles compared with those infused with micelles. The type of solution infused had little effect on triglyceride or phospholipid absorbed. It is interesting to note that the intralumenal contents of subjects with inborn errors without BA treatment had markedly reduced subphase or solubilised cholesterol whereas phospholipids in the subphase was less affected and fatty acid solubilisation unaffected by the reduced BA within the lumen. Unlike our findings, Nishioka et al found reduced fatty acid absorption in bile diverted rats whose fatty acids were solubilised with liposomes. There are significant methodological differences between our studies and those of Nishioka et al, including our use of human contents versus their use of liposomes, with attendant differences in fatty acid composition and mode of recovery of triglycerides in serum in their study versus lymph in our studies.39
In conjunction with the rat lymph fistula experiments, it would be attractive to believe that the observed alteration in cholesterol absorption compared with relatively preserved fatty acid and phospholipid absorption may be directly related to the vehicle carrying the solubilised cholesterol (micellar versus vesicular). In the current investigation, cholesterol absorption was markedly reduced when subjects with inborn errors of BA metabolism did not receive BA supplementation. The observed reductions were below the broad range of fractional cholesterol absorption (29–80%) found in a large group (n = 94) of adults aged 17–80 years.40 BA therapy resulted in a significant increase in cholesterol absorption leading to absorption rates similar to healthy control adults in our previous studies (34–83%).14 The observed increase likely underestimates the impact of BA supplementation as the technique used measures fractional absorption and does not take into account the effect of the increased BA pool and biliary secretion with attendant influx of biliary cholesterol associated with it.41
Pathological conditions such as cholestasis may compromise fat and cholesterol absorption because reduced intralumenal BA concentrations are too low to support macroaggregations into micelles; however, direct studies on the effect of cholestasis on cholesterol absorption in humans are limited. In disease states such as cirrhosis, in which the bile salt pools are reduced in proportion to severity of hepatic function impairment, cholesterol absorption correlates positively with the total bile salt pool, with the highest correlation with the CA pool, while CA supplementation increases cholesterol absorption.42 The cholesterol synthetic rate, as assessed by deuterium incorporation, was inversely related to cholesterol absorption. Without treatment, FSR was increased (0.0856 (0.0192)/day) compared with previous studies in adults (0.029 (0.05)/day), and with BA supplements FSR (0.0262 (0.0133)/day) decreased to levels comparable with control adult values.14 Previous studies have demonstrated an inverse relationship between FSR and cholesterol absorption, as might be anticipated; when the amount of exogenous cholesterol, as absorbed dietary cholesterol, is high, the endogenous cholesterol, as synthesised by the liver, is low.43,44 The results of our study provide additional support for the inhibitory effect of increasing absorption of dietary cholesterol on cholesterol synthesis. Concurrent with decreasing FSR with increased cholesterol absorption, there was a decrease in mononuclear HMG CoA reductase mRNA, which is believed to be a reliable reflection of liver HMG CoA reductase mRNA.45
With reduced cholesterol absorption, serum total and LDL‐cholesterol are reduced.46 In the current investigation, no significant reduction in total serum cholesterol or LDL‐cholesterol was observed when not receiving BA supplements. The small sample size may have precluded our ability to show previously observed changes. Interestingly, NPC1L1 knockout mice, with associated reduced cholesterol absorption, do not have reduced plasma cholesterol compared with wild‐type animals.38,46
To summarise, this study demonstrated markedly reduced cholesterol absorption rates in children with inborn errors of BA synthesis with concomitant increases in cholesterol synthetic rates when untreated. With BA treatment, cholesterol absorption increases and cholesterol synthesis rates decrease. Reduced cholesterol absorption was associated with reduced intralumenal aqueous phase cholesterol and cholesterol in micelles. In a series of translational studies we have clearly demonstrated that cholesterol absorption from human intestinal contents is dependent on the cholesterol being incorporated into mixed micelles while other more polar lipids such as fatty acids and phospholipids appear well absorbed when solubilised in either vesicles or micelles.
Acknowledgements
The authors wish to thank the research subjects, Julie McConihay, Christopher Vanstone, the nursing and bionutrition staff of the GCRC, and particularly, Suzanne Gilley, RD, Cindy Deeks, RD, Victoria Henize, RD, and Shanthi Rajan, for their help on completing this research project. We would also like to acknowledge the Interventional Radiology staff at CCHMC, including Lane Donnelly, MD, John Racadio, MD, and Neil Johnson, MD, for their assistance in completing these studies. This work was supported by National Institutes of Health awards P01 DK‐54504 (LA Woollett, P Tso, and JE Heubi), 5U24DK059630 (P Tso), HD‐39419 (LA Woollett), DK‐68463, and National Center for Research Resources grant #M01 RR08084 (JE Heubi).
Abbreviations
FSR - fractional synthetic rate
BA - bile acids
3‐HSD - 3β hydroxy‐C27‐steroid dehydrogenase/isomerase
5β reductase - Δ4‐3‐oxosteroid 5β reductase
racemase - 2‐methylacyl CoA racemase
CMC - critical micellar concentration
CA - cholic acid (3α,7α 12α‐trihydroxy‐5β‐cholanoic acid)
CDCA - chenodeoxycholic acid (3α,7α‐dihydroxy‐5β‐cholanoic acid)
UDCA - ursodeoxycholic acid (3α,7β‐dihydroxy‐5β‐cholanoic acid)
GCA - glycocholic acid
RBC - red blood cells
LDL - low density lipoprotein
HDL - high density lipoprotein
ALT - alanine aminotransferase
GC - gas chromatography
AUC - areas under the curve
Footnotes
Conflict of interest: None declared.
References
- 1.Russell D W, Setchell K D R. Bile acid biosynthesis. Biochemistry 1992314737–4749. [DOI] [PubMed] [Google Scholar]
- 2.Grundy S M. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr 1983371–96. [DOI] [PubMed] [Google Scholar]
- 3.Wilson M D, Rudel L L. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J Lipid Res 199435943–955. [PubMed] [Google Scholar]
- 4.Hernell O, Staggers J E, Carey M C. Physical‐chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry 1990292041–2056. [DOI] [PubMed] [Google Scholar]
- 5.Staggers J E, Hernell O, Stafford R J.et al Physical‐chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry 1990292028–2040. [DOI] [PubMed] [Google Scholar]
- 6.Siperstein M D, Chaikoff I L, Reinhardt W O. C14‐Cholesterol. V. Obligatory function of bile in intestinal absorption of cholesterol. J Biol Chem 1952198111–114. [PubMed] [Google Scholar]
- 7.Clayton P T, Leonard J V, Lawson A M.et al Familial giant cell hepatitis associated with synthesis of 3b,7a‐dihydroxy‐ and 3b,7a,12a‐trihydroxy‐5‐cholenoic acids. J Clin Invest 1987791031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton P T, Verrips A, Sistermans E.et al Mutations in the sterol 27‐hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J Inherit Metab Dis 200225501–513. [DOI] [PubMed] [Google Scholar]
- 9.Setchell K D R, Kritchevsky D, Nair P P.The bile acids: chemistry, physiology and metabolism. New York/London: Plenum Press, 1988
- 10.Setchell K D R, Suchy F J, Welsh M B.et al Delta 4‐3‐oxosteroid 5‐beta reductase deficiency described in identical twins with neonatal hepatitis—a new inborn error of bile acid synthesis. J Clin Invest 1988822148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Setchell K D R, Schwarz M, O'Connell N C.et al Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7a‐hydroxylase gene causes severe neonatal liver disease. J Clin Invest 19981021690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daugherty C C, Setchell K D, Heubi J E.et al Resolution of liver biopsy alterations in three siblings with bile acid treatment of an inborn error of bile acid metabolism (delta 4‐3‐oxosteroid 5‐beta reductase deficiency). Hepatology 1993181096–1101. [PubMed] [Google Scholar]
- 13.Yao L, Heubi J E, Buckley D D.et al Separation of micelles and vesicles within lumenal aspirates form healthy humans: solubilization after a meal. J Lipid Res 200243654–660. [PubMed] [Google Scholar]
- 14.Woollett L A, Buckley D D, Yao L.et al Effect of ursodeoxycholic acid on cholesterol absorption and metabolism in humans. J Lipid Res 200344935–942. [DOI] [PubMed] [Google Scholar]
- 15.Cohen D E, Carey M C. Rapid (1 hour) high performance gel filtration chromatography resolves coexisting simple micelles, mixed micelles, and vesicles in bile. J Lipid Res 1990312103–2112. [PubMed] [Google Scholar]
- 16.Turley S D, Spady D K, Dietschy J M. Regulation of fecal bile acid excretion in male golden Syrian hamsters fed a cereal‐based diet with and without added cholesterol. Hepatology 199725797–803. [DOI] [PubMed] [Google Scholar]
- 17.Bosner M S, Ostlund R E, Jr, Osofisan O.et al Assessment of percent cholesterol absorption in humans with stable isotopes. J Lipid Res 1993341047–1053. [PubMed] [Google Scholar]
- 18.Jones P J. Use of deuterated water for measurement of short‐term cholesterol synthesis in humans. Can J Physiol Pharmacol 199068955–959. [DOI] [PubMed] [Google Scholar]
- 19.Setchell K D R, Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse‐phase octadecylsilane bonded silica cartridges. Clin Chim Acta 1982125135–144. [DOI] [PubMed] [Google Scholar]
- 20.Setchell K D R. Liquid‐solid extraction, lipophilic gel chromatography and capillary column gas chromatography in the analysis of bile acids from biological samples. In: Barbara L, Dowling RH, Hofmann AF, Roda E, eds. Bile acids in gastroenterology. Lancaster: MTP Press Ltd, 19831–18.
- 21.Bergstedt S, Berstedt J, Fujimoto K.et al Effects of glycerol tripalmitate and glycerol trioleate on intestinal absorption of glycerol tristearate. Am J Physiol 1991261G239–G247. [DOI] [PubMed] [Google Scholar]
- 22.Bollman J, Cain J, Grindlay J. Techniques for the collection of lymph from the liver, small intestine or thoracic duct of the rat. J Lab Clin Med 1949331349–1352. [PubMed] [Google Scholar]
- 23.Tso P, Lee T, Demichele S J. Lymphatic absorption of structured triglycerides vs. physical mix in a rat model for fat malabsorption. Am J Physiol 1999277G333–G340. [DOI] [PubMed] [Google Scholar]
- 24.Heubi J E, Balistreri W F, Partin J C.et al Refractory infantile diarrhea due to primary bile acid malabsorption. J Pediatr 197994546–551. [DOI] [PubMed] [Google Scholar]
- 25.Setchell K D R, O'Connell N C, Squires R H.et al Congenital defects in bile acid synthesis cause a spectrum of diseases manifest as severe cholestasis, neurological disease, and fat‐soluble vitamin malabsorption. In: Northfield TC, Ahmed H, Jazwari R, et al, eds. Bile acids in hepatobiliary disease. Dordrecht: Kluwer Academic Publishers, 199957–65.
- 26.Setchell K D R, Heubi J E, Bove K E.et al Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology 2003124217–232. [DOI] [PubMed] [Google Scholar]
- 27.Mathis R K, Watkins J B, Szczepanik‐Van Leeuwen P.et al Liver in the cerebro‐hepato‐renal syndrome: defective bile acid synthesis and abnormal mitochondria. Gastroenterology 1980791311–1317. [PubMed] [Google Scholar]
- 28.Stieger B, Zhang J, O'Neill B.et al Transport of taurine conjugates of 7alpha‐hydroxy‐3‐oxo‐4‐cholenoic acid and 3beta,7alpha‐dihydroxy‐5‐cholenoic acid in rat liver plasma membrane vesicles. In: Van Berge‐Henegouwen GP, Van Hock B, De Groote J, et al, eds. Cholestatic liver diseases. Dordrecht: Kluwer Academic Press, 199482–87.
- 29.Swell L, Trout E C, Jr, Hopper J R.et al Specific function of bile salts in cholesterol absorption. Proc Soc Exp Biol Med 195898174–176. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann A F, Borgstom B. Physico‐chemical state of lipids in intestinal content during their digestion and absorption. Fed Proc 19642143–50. [PubMed] [Google Scholar]
- 31.Hofmann A F, Borgstrom B. The intraluminal phase of fat digestion in man: the lipid content of the micellar and oil phases of intestinal content obtained during fat digestion and absorption. J Clin Invest 196443247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgstrom B. The micellar hypothesis of fat absorption: must it be revisited? Scand J Gastroent 198520389–394. [DOI] [PubMed] [Google Scholar]
- 33.Stafford R J, Donovan J M, Benedek G B.et al Phyisical‐chemical characteristics of aqueous duodenal content after a fatty meal. (Abstr) Gastroenterology 1980801291 [Google Scholar]
- 34.Swell L, Flick D F, Field J H.et al Influence of dietary bile salts on blood cholesterol levels. Proc Soc Exp Biol Med 195384428–431. [DOI] [PubMed] [Google Scholar]
- 35.Gallo‐Torres H E, Miller O N, Hamilton J G. Further studies on the role of bile salts in cholesterol esterification and absorption from the gut. Arch Biochem Biophys 197114322–36. [DOI] [PubMed] [Google Scholar]
- 36.Watt S M, Simmonds W J. The specificity of bile salts in the intestinal absorption of micellar cholesterol in the rat. Clin Exp Pharmacol Physiol 19763305–322. [DOI] [PubMed] [Google Scholar]
- 37.Lee M H, Lu K, Hazard S.et al Identification of a gene, abcg5, important in the regulation of dietary cholesterol absorption. Nat Genet 2001179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altmann S W, Davis H R, Zhu L‐J Y X.et al Niemann‐Pick C1 like L1 protein is critical for intestinal cholesterol absorption. Science 20043031201–1204. [DOI] [PubMed] [Google Scholar]
- 39.Nishioka T H R, Tazuma S, Stellaard F.et al Administration of phosphatidylcholine‐cholesterol liposomes partially reconstitutes fat absorption in chronically bile diverted rats. Biochim Biophys Acta 2004163690–98. [DOI] [PubMed] [Google Scholar]
- 40.Bosner M S, Lange L G, Stenson W F.et al Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res 199940302–308. [PubMed] [Google Scholar]
- 41.Mok H Y, Grundy S. Cholesterol and bile acid absorption during bile acid therapy in obese subjects undergoing weight reduction. Gastroenterology 19807862–67. [PubMed] [Google Scholar]
- 42.Ponz de Leon M, Loria P, Iori R.et al Cholesterol absorption in cirrhosis: the role of total and individual bile acid pool size. Gastroenterology 1981801428–1437. [PubMed] [Google Scholar]
- 43.Vanstone C A, Raeini‐Sarjaz M, Parsons W E.et al Unesterified plant sterols and stanols lower LDL‐cholesterol concentrations equivalently in hypercholesterolemic persons. Am J Clin Nutr 2002761272–1278. [DOI] [PubMed] [Google Scholar]
- 44.Jones P J, Raeini‐Sarjaz M, Ntanios F Y.et al Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res 200041698–705. [PubMed] [Google Scholar]
- 45.Powell E E, Kroon P A. Low density lipoprotein receptor and 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase gene expression in human mononuclear leukocytes is regulated coordinately and parallels gene expression in human liver. J Clin Invest 1994932168–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis H R, Jr, Zhu L ‐ J, Hoos L M.et al Niemann‐Pick C1 Like 1 (NPC 1L1) is the intestinal phytosterol and choesterol transporter and a key modulator of whole‐body cholesterol homeostasis. J Biol Chem 200427933586–33592. [DOI] [PubMed] [Google Scholar]