Intractable diarrhoea of infancy (IDI) can often become life threatening and still poses a major diagnostic challenge. It embraces a diverse group of disorders1 and in a significant number of infants the underlying cause cannot be identified. We describe a child who presented with IDI where routine investigations were unhelpful, but more detailed tests of neutrophil function revealed a congenital defect of neutrophil specific granule formation.
A three week old baby girl of Pakistani origin of non‐consanguineous parents living in the UK was referred because of pyrexia of unknown origin, as well as vomiting and severe watery diarrhoea associated with failure to thrive. Rhodococcus equi isolated after repeated blood culture responded to treatment with teicoplanin and Co‐trimoxazole. The umbilical cord finally separated at five weeks of age. No pathogens were isolated from repeated stool cultures. Oesophagogastroduodenoscopy and colonoscopy were unremarkable. Small bowel and colonic mucosal biopsies showed villous atrophy, a moderate non‐specific inflammatory infiltrate, but no granulomas (fig 1A, B). Microvillous inclusion disease was ruled out by electron microscopy. Antienterocyte and goblet cell antibodies were negative. Standard cellular and humoral immune tests was normal.
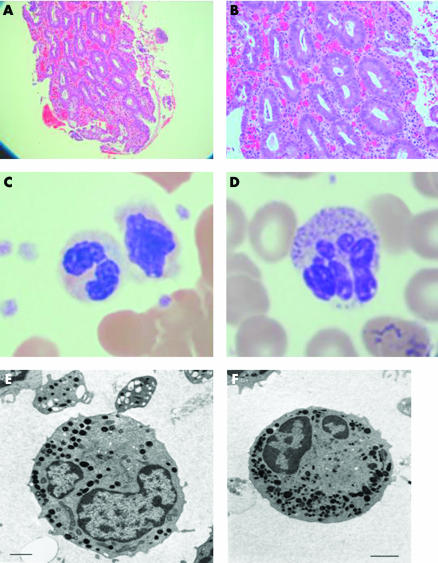
Figure 1 Gut histology and neutrophil morphology in our patient with neutrophil specific granule deficiency. (A) 100× and (B) 200× haematoxylin‐eosin section of duodenum showing villous atropy, a non‐specific lymphocytic infiltrate, and no granuloma. (C) Haematoxylin‐eosin staining of the patient's neutrophils in peripheral blood showing reduced granule staining compared with a control (D). Electron microscopy of the patient's neutrophils (E) compared with a control (F) showing primary but not secondary granules. Scale bar = 1 μm.
The child's diarrhoea persisted and she was unable to gain weight without parenteral nutrition. Over the following months she developed a number of pyogenic subcutaneous abscesses as well as septicaemia. Because of the constellation of clinical features, further investigations were performed. A blood film was re‐examined and showed markedly reduced granules (fig 1C). Electron microscopy showed that although neutrophils had normal numbers of primary granules there were reduced numbers of specific granules (fig 1E). A neutrophil oxidative burst test was abnormal with a reduced stimulation index of 4–14 (normal >50). Neutrophil granule proteins were assayed by ELISA, as previously described,2,3,4,5,6 and proteins classically found in specific granules were found to be absent (table 1). No mutations were found in the myeloid specific transcription factor CCAAT/enhancer binding protein‐epsilon gene, known to be associated with some cases of neutrophil specific granule deficiency.7,8
Table 1 Neutrophil granule protein concentrations.
| Patient | Control | |
|---|---|---|
| Myeloperoxidase (ng/ml) | 9744 | 21312 |
| Defensins (ng/ml) | 0 | 3808 |
| Human cationic antimicrobial protein (hCAP18) (ng/ml) | 87 | 51200 |
| Lactoferrin (ng/ml) | 0 | 3920 |
| Neutrophil gelatinase associated lipocalin (NGAL) (ng/ml) | 7 | 2575 |
| Gelatinase (ng/ml) | 537 | 7478 |
Because of the severity of the child's illness, she underwent unrelated donor stem cell transplantation at 13 months of age using busulfan, cyclophosphamide, and Alemtuzumab as conditioning. Granulocyte transfusions were given during the two week period of neutropenia prior to engraftment. The post‐transplant course was complicated by a Candida glabrate chest infection, skin graft versus host disease, and mild veno‐occlusive disease. The child is now well at 2½ years old, with full donor cell engraftment post‐transplantation, on full oral feeds, and off all immunosuppressive medication. Gastrointestinal symptoms and recurrent infections have settled completely.
Neutrophil specific granule deficiency is a rare primary immunodeficiency disease associated with frequent and severe bacterial infections. Specific granules contain lactoferrin, lysozyme, and defensins and are important in the extracellular mobilisation of inflammatory mediators.9 Mutations in the CEBPE gene cause some cases of specific granule deficiency but no mutations were detected in this case. The clinical clues to the diagnosis of a primary neutrophil disorder in this case were the delayed separation of the umbilical cord after birth and the episode of neonatal Rhodococcus septicaemia, which has previously been described predominantly in patients with immunodeficiency, particularly AIDS. The enteropathy in this child is likely to have been caused by suboptimal containment of bacterial commensals within the gut lumen leading to a chronic inflammatory mucosal reaction. Intermittent release of gut bacteria into the bloodstream may have led to episodes of septicaemia and recurrent abscesses. This is not only the first report linking IDI to this primary immunodeficiency disease but also the first published case of bone marrow transplantation as a definitive curative treatment for this condition.
Footnotes
Conflict of interest: None declared.
References
- 1.Sherman P M, Mitchell D J, Cutz E. Neonatal enteropathies: defining the causes of protracted diarrhea of infancy. J Pediatr Gastroenterol Nutr 20043816–26. [DOI] [PubMed] [Google Scholar]
- 2.Sengelov H, Kjeldsen L, Diamond M S.et al Subcellular localization and dynamics of Mac‐1 (alpha m beta 2) in human neutrophils. J Clin Invest 1993921467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faurschou M, Sorensen O E, Johnsen A H.et al Defensin‐rich granules of human neutrophils: characterization of secretory properties. Biochim Biophys Acta 2002159129–35. [DOI] [PubMed] [Google Scholar]
- 4.Kjeldsen L, Bjerrum O W, Hovgaard D.et al Human neutrophil gelatinase: a marker for circulating blood neutrophils. Purification and quantitation by enzyme linked immunosorbent assay. Eur J Haematol 199249180–191. [DOI] [PubMed] [Google Scholar]
- 5.Kjeldsen L, Bainton D F, Sengelov H.et al Identification of neutrophil gelatinase‐associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 199483799–807. [PubMed] [Google Scholar]
- 6.Sorensen O, Cowland J B, Askaa J.et al An ELISA for hCAP‐18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods 199720653–59. [DOI] [PubMed] [Google Scholar]
- 7.Chumakov A M, Grillier I, Chumakova E.et al Cloning of the novel human myeloid‐cell‐specific C/EBP‐epsilon transcription factor. Mol Cell Biol 1997171375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lekstrom‐Himes J A, Dorman S E, Kopar P.et al Neutrophil‐specific granule deficiency results from a novel mutation with loss of function of the transcription factor CCAAT/enhancer binding protein epsilon. J Exp Med 19991891847–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallin J I. Neutrophil specific granule deficiency. Ann Rev Med 198536263–274. [DOI] [PubMed] [Google Scholar]