We were interested in the paper by Mutoh and colleagues1 showing the development of epithelial intestinal metaplasia and mesenchymal proliferation in human stomach resections and Cdx2 transgenic mice. The authors used α smooth muscle actin (α‐SMA) staining to mark periglandular fibroblasts and failed to show any α‐SMA positive cells surrounding the en face glands of normal mouse and human stomach mucosa. In metaplastic tissue however, the periglandular fibroblast sheath was easily discernible, and the authors concluded that the fibroblast sheath was generated from the intestinal submucosa, possibly through expression of Cdx2.
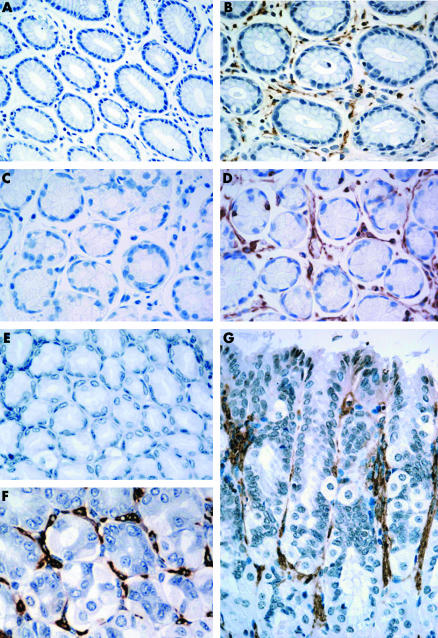
Mesenchymal cells such as intestinal subepithelial myofibroblasts (ISEMF) are widely distributed. They are important coordinating cells that possess significant influence on their environment by virtue of their receptor profile and the signals they produce. Characteristically, ISEMF form a protective fenestrated sheath around the stem cell compartment, creating the stem cell niche—the optimal microenvironment for stem cells to give rise to differentiated progeny.2 The stem cell niche is situated in the isthmus/neck region of the gastric gland. ISEMF regulate stem cell behaviour via paracrine secretion of growth factors and cytokines, and perform vital functions in the growth, differentiation, and development of the embryological stomach. They participate in mucosal wound healing and the response to inflammatory stimuli in the adult gastrointestinal tract.3 As these are vital homeostatic roles, it seems unlikely that the periglandular fibroblast sheath is only generated in abnormal metaplastic tissue. We immunostained for ISEMF in paraffin embedded normal mouse and human stomach specimens. To identify ISEMF, we stained for α‐SMA in three mouse gastric specimens, and α‐SMA and vimentin in six sets of human gastric biopsies. Sections for vimentin staining underwent 10 minutes of microwave treatment in citrate buffer for antigen retrieval. Immunostaining was completed using the same antibodies and methods described in detail by Direkze and colleagues.5 Antibody binding was detected by 1,3‐diaminobenzidine (DAB; Sigma, St Louis, Missouri, USA). ISEMF were identified on the basis of their morphology and positive immunoreactivity for α‐SMA in mouse tissue, and α‐SMA and vimentin in human tissue. They were clearly and consistently seen surrounding the stomach glands in normal mouse and human stomach sections, both in the en face and cross sectional plane (see fig 1). There was little variation in staining intensity from sample to sample in the three mouse and six human subjects studied.
Figure 1 The normal stomach periglandular fibroblast sheath (PGFS). (A) Human α smooth muscle actin (α‐SMA) control. (C) Human vimentin control. Normal human gastric glands counterstained with haematoxylin (40× magnification). (B) α‐SMA immunostaining. (D) Vimentin immunostaining. Normal human gastric glands with surrounding PGFS (brown stain) embracing the epithelial cells of the gastric glands (40× magnification). (E) Mouse α‐SMA control. Normal mouse gastric glands counterstained with haematoxylin (40× magnification). (F, G) α‐SMA immunostaining. Normal mouse gastric glands in en face (F) and cross sectional (G) orientation showing the close association of the PGFS (brown stain) surrounding and enveloping the normal stomach glands (60× magnification).
ISEMF are involved in the response to damage or disease in the stomach. After epithelial injury, ISEMF contraction limits the exposed area of the wound while secreted growth factors such as transforming growth factors α and β, epidermal growth factor, and fibroblast growth factor promote epithelial cell migration and proliferation.3 In intestinal‐type gastric cancer, myofibroblasts appear not only at the edge of the tumour, contributing to a desmoplastic reaction, but also within the tumour stroma.4 The presence of increased inter‐tubular reticulin, the histological hallmark of increased extracellular matrix deposition, is regarded as among the first signs of chronic atrophic gastritis, and likely to be caused by elevated numbers or activity of ISEMF. The source of these cells is very interesting—it is more likely that these cells are recruited from circulating precursor cells rather than being generated by metaplastic mucosa. Direkze et al have shown a large contribution of bone marrow donor derived myofibroblasts, making up to 64% of the periglandular fibroblast sheath in mouse stomach after total body irradiation and bone marrow transplant,5 and Nakayama et al hypothesise that engraftment is responsible for myofibroblast presence in tumours.4 The mechanisms initiating this engraftment are unclear but it may relate to the release of growth factors, such as transforming growth factor β,6 released from inflammatory cells and the existing periglandular fibroblast sheath in response to gastritis induced damage.
We conclude that the periglandular myofibroblast sheath in normal stomach is very much a reality and likely to be pivotal in modulating epithelial cell behaviour.
Footnotes
Grant support was provided by the Medical Research Council Clinical Research Fellowship.
Conflict of interest: None declared.
References
- 1.Mutoh H, Sakurai S, Satoh K.et al Pericryptal fibroblast sheath in intestinal metaplasia and gastric carcinoma. Gut 20055433–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spradling A, Drummond‐Barbosa D, Kai T. Stem cells find their niche. Nature 200141498–104. [DOI] [PubMed] [Google Scholar]
- 3.Powell D W, Mifflin R C, Valentich J D.et al Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999277(1 Pt 1)C1–C9. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama H, Enzan H, Miyazaki E.et al Alpha smooth muscle actin positive stromal cells in gastric carcinoma. J Clin Pathol 200255741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Direkze N C, Forbes S J, Brittan M.et al Multiple organ engraftment by bone‐marrow‐derived myofibroblasts and fibroblasts in bone‐marrow‐transplanted mice. Stem Cells 200321514–520. [DOI] [PubMed] [Google Scholar]
- 6.Abe R, Donnelly S C, Peng T.et al Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 20011667556–7562. [DOI] [PubMed] [Google Scholar]