Abstract
Objective:
The proper role of endovascular abdominal aortic aneurysm repair (EVAR) remains controversial, largely due to uncertain late results. We reviewed a 12-year experience with EVAR to document late outcomes.
Methods:
During the interval January 7, 1994 through December 31, 2005, 873 patients underwent EVAR utilizing 10 different stent graft devices. Primary outcomes examined included operative mortality, aneurysm rupture, aneurysm-related mortality, open surgical conversion, and late survival rates. The incidence of endoleak, migration, aneurysm enlargement, and graft patency was also determined. Finally, the need for reintervention and success of such secondary procedures were evaluated. Kaplan-Meier and multivariate methodology were used for analysis.
Results:
Mean patient age was 75.7 years (range, 49–99 years); 81.4% were male. Mean follow-up was 27 months; 39.3% of patients had 2 or more major comorbidities, and 19.5% would be categorized as unfit for open repair. On an intent-to-treat basis, device deployment was successful in 99.3%. Thirty-day mortality was 1.8%. By Kaplan-Meier analysis, freedom from AAA rupture was 97.6% at 5 years and 94% at 9 years. Significant risk factors for late AAA rupture included female gender (odds ratio OR, 6.9; P = 0.004) and device-related endoleak (OR, 16.06; P = 0.009). Aneurysm-related death was avoided in 96.1% of patients, with the need for any reintervention (OR, 5.7 P = 0.006), family history of aneurysmal disease (OR, 9.5; P = 0.075), and renal insufficiency (OR, 7.1; P = 0.003) among its most important predictors. 87 (10%) patients required reintervention, with 92% of such procedures being catheter-based and a success rate of 84%. Significant predictors of reintervention included use of first-generation devices (OR, 1.2; P < 0.01) and late onset endoleak (OR, 64; P < 0.001). Current generation stent grafts correlated with significantly improved outcomes. Cumulative freedom from conversion to open repair was 93.3% at 5 through 9 years, with the need for prior reintervention (OR, 16.7; P = 0.001) its most important predictor. Cumulative survival was 52% at 5 years.
Conclusions:
EVAR using contemporary devices is a safe, effective, and durable method to prevent AAA rupture and aneurysm-related death. Assuming suitable AAA anatomy, these data justify a broad application of EVAR across a wide spectrum of patients.
This study examines late outcomes of a 12-year experience with EVAR to determine long-term durability and effectiveness of this method of AAA repair. Assuming suitable AAA anatomy, good late results justify broad application of EVAR across a wide spectrum of patients.
Since Parodi et al1 first reported initial experience with endovascular aortic aneurysm repair (EVAR) 15 years ago, this treatment option has gained widespread acceptance and application as a less-invasive alternative to conventional open surgery for repair of abdominal aortic aneurysms (AAA). Results from multicenter Investigation Device Exemption clinical trials in the United States,2–7 as well as multiple other reports of worldwide experience in the literature,8–11 have conclusively documented the early safety and efficacy of EVAR. In addition, these reports have clearly demonstrated numerous early benefits of EVAR as compared with standard surgical repair, including less blood loss and transfusion requirement, shorter procedure times, diminished ICU utilization, reduced length of hospital stay, markedly lower rates of major adverse events, and dramatically quicker recovery. More recently, 2 important randomized controlled trials12,13 have, for the first time, firmly established a significantly lower perioperative mortality rate for EVAR as compared with open surgery, an observation that has been confirmed by several other recently published population-based observational studies using large statewide or national databases.14–18
While EVAR was initially proposed as an alternative to open repair for older, high-risk patients, such favorable early results have prompted increased utilization of this technology in a broader patient population, including younger patients and those in suitable health for standard open surgical repair. However, several reports of midterm experience with EVAR have described a somewhat disturbing incidence of problems and complications related to device failures, endoleaks, and other potential limitations and shortcomings of endoluminal treatment.19–22 Such reports have led some authors to urge caution, or even pessimism, in regard to more widespread application of EVAR.23,24 It is clear that reliable long-term outcome data are essential to determine whether the early benefits of EVAR are sustained and to clarify the proper role of this treatment method.25,26 The current controversy and lack of consensus as to the spectrum of patients in which EVAR should be used reflect this uncertainty regarding the long-term effectiveness and durability of EVAR.27–29
To document late outcome data that would contribute to such decision-making, we reviewed our 12-year experience with EVAR at a large tertiary academic medical center that has been actively involved in EVAR since the initiation of the first FDA controlled clinical trial in the United States in 1994.
METHODS
Retrospective review of patients undergoing EVAR for repair of AAA from January 7, 1994 through December 31, 2005 at the Massachusetts General Hospital (MGH) was performed. Stent graft procedures for repair of thoracic aortic aneurysms, isolated iliac aneurysms, anastomotic aneurysms, subclavian aneurysms, vascular trauma, or other vascular lesions were excluded from consideration. A total of 873 patients having elective primary endovascular stent graft repair of infrarenal AAA during this 12-year period were identified and constitute the study group.
During the study period, a variety of endovascular stent grafts were used for endoluminal AAA repair. Types and configurations of these devices are detailed in Table 1. Eight different commercially manufactured aortic endograft devices were used, in addition to a moderate number of “MGH” and “Hybrid” custom-made devices fabricated by our group from available prosthetic graft materials and intravascular stents, as previously described.8,30
TABLE 1. Devices
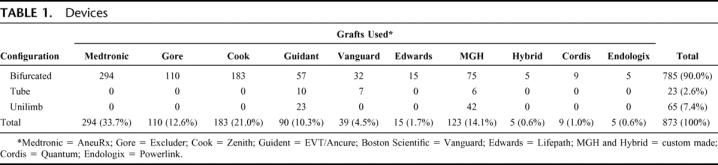
For the purposes of analysis, endografts were classified as “first generation” and “current generation” devices. First generation devices were defined as those used early in our experience, which have been subsequently withdrawn from the commercial market and are no longer available on a commercial basis worldwide. These include the MGH and Hybrid devices, as well as Vanguard and EVT/Ancure endografts. Current generation endografts, generally characterized by modular, bifurcated, fully supported, and lower profile features, are those devices presently in use worldwide. Many of these devices have undergone several iterations and evolution in design features over time but remain in active use.
The primary operating surgeon was responsible for selecting EVAR versus open repair of each patient. Patient selection and decisions regarding devices used, approach, etc, were based on detailed preoperative radiologic imaging with contrast-enhanced fine cut (2.5–3.0 mm) computerized tomography (CT) scans with 3-dimensional reformatting. In earlier years of the experience, patients identified by CT scans as potential candidates for EVAR subsequently underwent multiplanar contrast angiography utilizing a catheter with radio-opaque markers for various length measurements and assessment of pelvic anatomy, particularly in regard to device access. In more recent years, however, preoperative angiography has been largely eliminated due to significant improvements in 3-dimensional reconstruction software, which allows accurate length and “centerline” measurements and other anatomic assessments in addition to axial cross-sectional diameter determinations.31,32
All procedures were performed in the operating room. The patients were prepped and draped for open repair in case this became necessary. Over 90% of patients underwent an epidural or spinal anesthetic. The remaining patients were done under general anesthesia, and a small number with local anesthesia alone. Open common femoral artery exposure by means of a small groin incision was used for access. Radiology imaging was performed in early years of the study with a high-quality portable C-arm fluoroscopic unit with digital imaging and road mapping capability. Since February 2003, procedures were performed in a dedicated endovascular operating room suite with a fixed imaging system. All patients underwent follow-up by contrast-enhanced CT scans either at discharge or within 1 month of EVAR, and then at 6 and 12 months, and subsequently yearly thereafter. All patients had at least a 3-month follow-up.
Primary study outcomes examined included operative (≤30 days) mortality, AAA rupture, aneurysm-related mortality, surgical conversion to open repair, and late survival. Aneurysm-related mortality (ARM) is defined as death from any cause within 30 days of the primary EVAR procedure, death ≤30 days of any secondary reintervention or surgical conversion, or any death due to aneurysm rupture or device complication. In addition, secondary outcomes including data related to endoleak, stent-graft migration ≥5 mm, AAA sac maximal diameter enlargement ≥5 mm, and endograft patency were examined. Finally, the frequency of secondary reinterventional procedures was determined, as well as the time, method, and success of such reinterventions.
Clinical success, as defined by the Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery,33 consisted of those patients with technically successful endograft implants as well as freedom from ARM, AAA rupture, type I or III endoleak, open conversion, clinically significant AAA expansion, or graft infection/thrombosis. Primary assisted clinical success was defined as patients requiring additional secondary endovascular reintervention procedures to maintain device integrity and clinical success of EVAR, while secondary clinical success denotes patients requiring additional secondary surgical procedures to maintain clinical success.
Statistical Methods
Analysis of data was performed on an intention-to-treat basis. Subgroup comparisons of demographic data were assessed using 2-tailed t tests for continuous variables, and χ2 tests for categorical data. Late outcomes were assessed using Kaplan-Meier life-table analysis and the log-rank test was used when comparing subgroups. Stepwise logistic regression was performed to identify variables potentially associated with study end-points.
RESULTS
Patient Demographics
As displayed in Table 2, a total of 873 patients underwent primary EVAR procedures during the 12-year study period. During this time interval, the annual number of AAA repairs increased somewhat, but the percentage of repairs done by endoluminal treatment grew substantially (Fig. 1). Indeed, in recent years 60% to 70% of primary infrarenal AAA repairs at MGH have been by EVAR.
TABLE 2. Patient Demographics and Clinical Characteristics
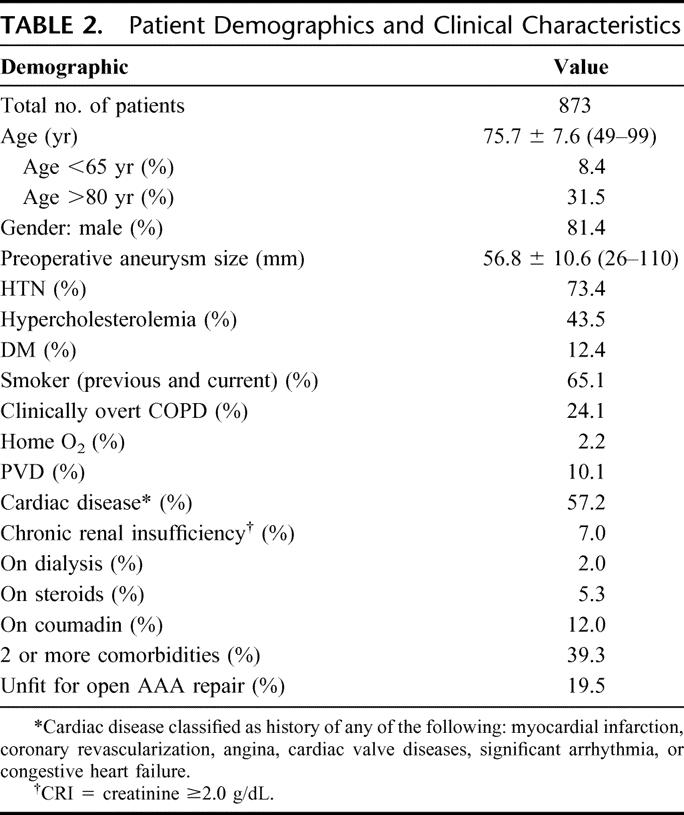
FIGURE 1. Bar graph of AAA by year.
Mean patient age was 75.7 years, with a range of 49 to 99 years. Seventy-three (8.4%) patients were ≤65 years of age and 233 (26.75%) ≥80 years. Males comprised 81.4% of the study group. Mean preoperative maximum AAA diameter was 56.8 mm, with a size range of 26 to 110 mm. Mean follow-up duration for the entire study group was 2.25 years. Follow-up data for 5 or more years were available in 20% of patients.
Significant comorbidities were frequently present in EVAR patients (Table 2). A substantial number of the study group would be considered high-risk patients by virtue of advanced age or extent of clinically significant comorbidities. For example, 39.3% patients had 2 or more major comorbidities, and 19.5% would be classified as “unfit for open surgery” using the classification criteria of the EVAR II randomized controlled clinical trial or the large EUROSTAR registry.34–36 Various manifestations of clinically significant cardiac disease were noted in 57.2% patients. Clinically overt COPD was present in 24% patients, with 2.2% requiring continuous home oxygen usage. The use of epidural anesthesia in the great majority of EVAR patients in our series was felt extremely beneficial in this regard. Chronic renal insufficiency, defined as serum creatinine ≥2.0, was present in 61 (7%) patients, and 17 (2%) patients were on chronic hemodialysis for end-stage renal disease. Because of the presence of significant associated iliac artery aneurysmal disease, 7.3% of patients required preoperative coil embolization of the internal iliac artery, with extension of the limb of the bifurcated endograft to the external iliac artery on that side at the time of EVAR.
Primary Outcomes
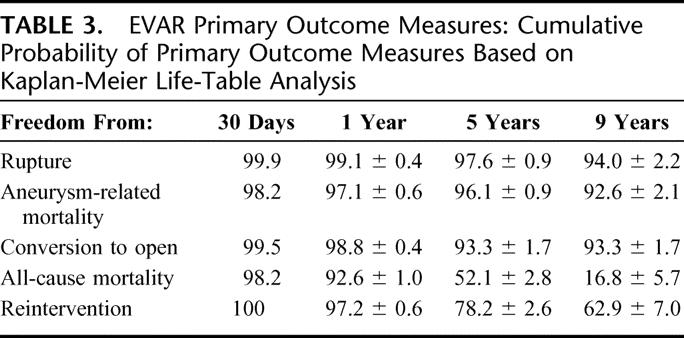
Primary outcome measures are summarized in Table 3.
TABLE 3. EVAR Primary Outcome Measures: Cumulative Probability of Primary Outcome Measures Based on Kaplan-Meier Life-Table Analysis
Perioperative Mortality
Perioperative deaths occurred in 16 of 873 (1.8%) patients. Patients who died within 30 days of the primary procedure were somewhat older (80 years, P = 0.01), and all high-risk patients. All had 2 or more significant comorbidities, and 69% would be classified as unfit for open procedure by EVAR II trial or EUROSTAR criteria. Perioperative deaths included 3 patients on chronic dialysis for end-stage renal disease, and 2 patients with chronic renal insufficiency, emphasizing the power of renal dysfunction as a predictor of mortality risk (odds ratio OR, 18.4; P = 0.003). All patients who died during the perioperative period had large AAA, with mean diameter of 66 mm. It is interesting to note that 6 perioperative deaths occurred at home following discharge after initially uncomplicated postoperative courses, or during readmission within the first 30 days following EVAR for medical or surgical complications.
AAA Rupture
Determination of AAA rupture may be difficult to verify accurately unless information from attempted operative repair, definitive radiologic imaging, or autopsy is available. Retrospective review of our data employing rather conservative judgment parameters suggested that rupture following the primary EVAR procedure occurred in a total of 13 (1.5%) patients. Rupture occurred at a mean interval of 30 months following stent graft placement, but a wide time interval to occurrence (2 days to 5.6 years) was observed. Twelve of the 13 ruptures occurred in patients with endoleaks. Late-onset type I proximal attachment leak developed in 2 patients who experienced rupture, while late distal attachment leaks were responsible in another 2 patients. Device structural failure with late onset type III endoleak was the causative factor in 4 patients with rupture: 3 attributable to modular endograft component separation and 1 to a fabric defect resulting from friction and erosion of the graft wall by an adjacent stent strut. Persistent type II leaks associated with enlarging aneurysms were present in another 3 patients with rupture.
Of the 13 patients experiencing rupture, 9 (69.2%) died. Two patients refused operation, 4 died suddenly at home before transport to a hospital, and 3 underwent emergent surgical repair but died postoperatively. Four patients with rupture survived. One patient with a new onset type III endoleak as the cause of rupture was successfully treated by catheter-based endovascular reintervention, with insertion of a new graft limb to bridge the separation defect of the original device. Another patient survived conversion to standard open graft repair. Interestingly, another 2 patients with ruptures associated with persistent type II lumbar endoleaks and AAA sac enlargement were repaired by opening the AAA sac and suturing the back-bleeding lumbar branches. As the endograft and its proximal and distal attachments were observed to be intact and hemostatic, the AAA sac was simply resutured around the indwelling endograft to complete the repair. Both patients continue to do well at 6 months and 1-year follow-up, respectively. Overall, of 7 patients undergoing emergent repair of post-EVAR ruptured AAA, 4 (57%) survived.
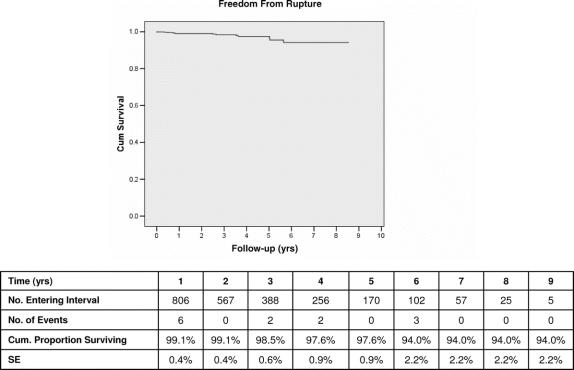
By Kaplan-Meier analysis, freedom from rupture was 99% at 1 year, 98% at 5 years, and 94% at 9 years (Fig. 2). Multivariate analysis revealed independent predictors of late rupture post-EVAR to include late endoleak, early generation endografts, and large (≥5.5 cm) aneurysms (Table 4). Late rupture was also more frequent in women (OR, 6.9; P < 0.004). Adverse anatomy, with short, wide, and/or angulated infrarenal renal necks, was also a common feature in patients experiencing late rupture. Such unfavorable anatomic characteristics may presumably be related to increased rupture risk by leading to a higher incidence of migration and/or late type I attachment leaks.
FIGURE 2. Kaplan-Meier freedom from rupture.
TABLE 4. Multivariate Logistic Regression for Rupture
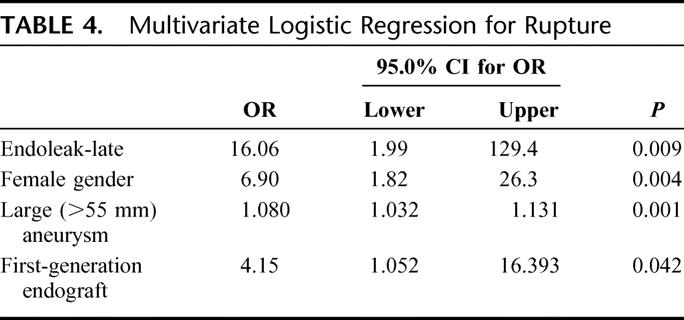
Aneurysm-Related Mortality (ARM)
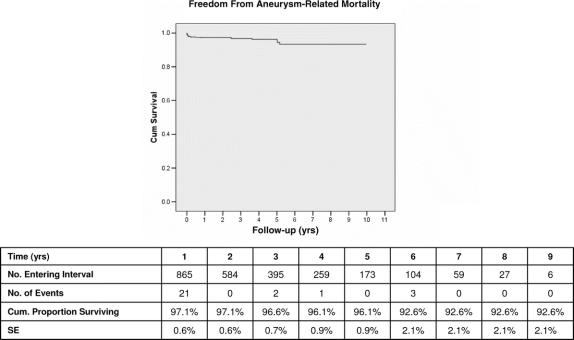
Over the course of the 12-year study period, 27 (3.1%) patients sustained aneurysm-related death. ARM was attributable to perioperative mortality following the initial EVAR procedure in 16 (1.8%) patients, death after late rupture in 9 (1.0%) patients, and 2 (0.3%) patients died of complications following secondary reinterventions. By Kaplan-Meier analysis, freedom from ARM was 97% at 1 year, 96% at 5 years, and 93% at 9 years (Fig. 3). Twenty-one of the AAA related deaths occurred within the first postimplant year, and only 6 subsequent AAA-related deaths were observed 1 to 10 years following EVAR (Fig. 3). Multivariate analysis demonstrated that the need for any reintervention (OR, 5.7), large preoperative AAA size (OR, 1.1), family history of aneurysmal disease (OR, 9.5), and renal insufficiency (OR, 7.1), were the most important predictors of ARM.
FIGURE 3. Kaplan-Meier freedom from aneurysm-related mortality.
Conversion to Open Repair
Conversion to open surgical repair was required in a total of 20 (2.3%) patients over the 12-year experience. Five (0.6%) patients underwent acute intraoperative conversion due to access difficulties or intraoperative vessel injuries. These were all in our earlier experience and largely reflect the combined impact of larger caliber, less flexible early generation devices, as well as likely inappropriate patient selection and poor operator judgment and experience. No early conversions have occurred in the most recent 4 years of our experience, with usage of lower profile, current generation devices, and presumably more appropriate preoperative patient selection. The importance of proper patient selection is emphasized by perioperative death following 2 (40%) of these 5 acute intraprocedural conversions. For the entire series, technical success of endograft implantation was 99.3%.
Fifteen (1.7%) patients required late conversion to open repair, from 6 months to 8 years (mean, 31 months) following initial endoluminal repair for a variety of indications. Five (0.6%) patients underwent emergency conversion for rupture, while 10 patients had elective conversion for migration (2), endograft infection (2), or progressive AAA enlargement post-EVAR (6). In this latter category, 4 patients having conversion for AAA sac growth had associated endoleaks, while 2 with AAA enlargement had no demonstrable endoleak (endotension). Cumulative freedom from conversion to open repair was 99% at 1 year, 93% at 5 years, and 93% at 9 years.
The perioperative mortality risk of conversion was naturally related to the indication and circumstances of the procedure. Mortality of open conversion for rupture was 50%, while only 1 (10%) of the 10 patients undergoing elective conversion died within 30 days of the procedure. Nonetheless, open conversions were clearly more extensive procedures than standard primary open repair, frequently requiring a thoracoabdominal approach and suprarenal clamping for explantation of the device.
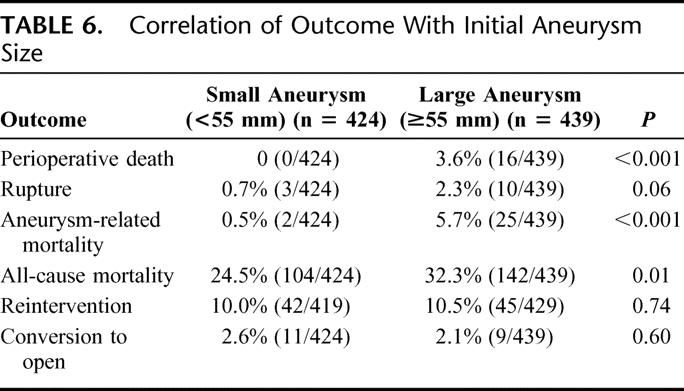
Multivariate analysis revealed significant predictors of the need for late conversion to include early generation devices (OR, 5.3), persistent or late endoleak (OR, 8.8), need for any reintervention (OR, 16.7), and female gender (Table 6).
TABLE 6. Correlation of Outcome With Initial Aneurysm Size
Late Survival
All-cause mortality occurred in 246 (28.2%) patients over the course of the study period. Only 27 (11.0%) of these 246 deaths were attributable to the AAA or its treatment. As would be anticipated, most late deaths were caused by cardiac disease or cancer.
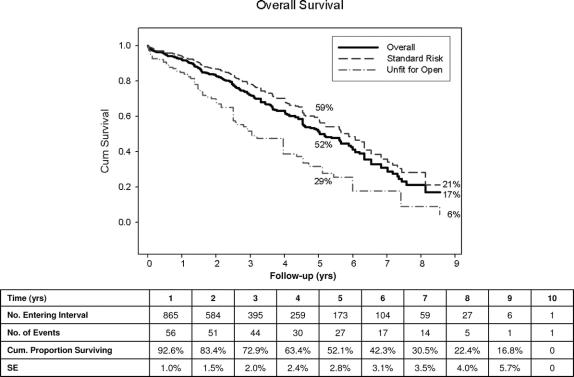
Kaplan-Meier estimates of freedom from all-cause death was 52% at 5 years and 17% at 9 years (Fig. 4). As indicated, late survival was considerably better in standard risk patients as opposed to those deemed unfit for surgery. Significant predictors of all-cause mortality included increased age (OR, 1.1), 2 or more major comorbidities, especially renal dysfunction (OR, 14.1), and large preoperative aneurysm size (OR, 1.1).
FIGURE 4. Kaplan-Meier freedom from all-cause mortality.
Reinterventions
A variety of problems and graft related complications were identified at various intervals during clinical and radiologic postimplant EVAR follow-up surveillance. These included persistent primary endoleaks, (generally type II), late-onset endoleaks (generally type I and type III), instances of graft migration, kinking, or thrombosis, progressive AAA enlargement, and other issues that were thought to threaten endoluminal repair and expose the patient to possible conversion, rupture, or both. For all of these reasons, reintervention was thought necessary. In some instances, those graft-related problems were related to changes in AAA sac and iliac artery morphology as sac shrinkage occurred as a result of effective aneurysm exclusion, a phenomenon we have termed the “paradox of success.”29
Over the 12-year series, a total of 87 (10%) patients required a total of 102 secondary procedures, at a mean interval of 25.5 months following the primary EVAR procedure. Most (92%) of these were catheter-based reinterventions, including balloon angioplasty, stenting, proximal or distal extensions of the original stent graft device, placement of a new stent graft, embolization of branch vessels or the AAA sac itself, thrombolytic therapy, or similar procedures. Twelve patients required 2 reintervention procedures and 1 patient underwent 3 secondary procedures. These endovascular reinterventions were judged clinically effective in correcting or eliminating the problem requiring reintervention in 84% of cases.
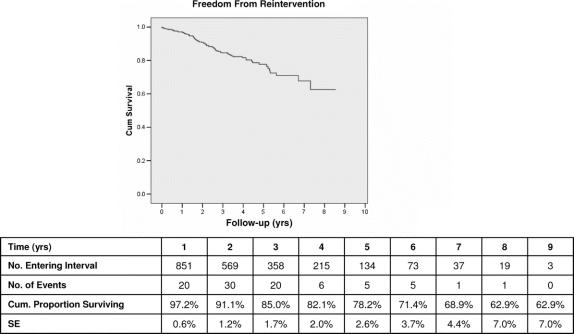
Freedom from the need for reintervention was 97% at 1 year, 78% at 5 years, and 63% at 9 years (Fig. 5). Significant predictors of the need for reintervention included type I or III endoleak (OR, 63.7), persistent or late-onset type II endoleak (OR, 13.5), AAA sac enlargement (OR, 8.6), and use of early generation devices (OR, 1.5).
FIGURE 5. Kaplan-Meier freedom from reintervention.
Secondary Outcomes
Endoleak of any type was detected on CT scan at some point during the follow-up period in a total of 228 (26.1%) patients. The majority (161) of these were early type II branch leaks noted on initial surveillance scans, 131 (79.8%) of which demonstrated spontaneous resolution within 6 to 12 months of endograft implantation. Because of the high number of spontaneous resolutions of such endoleaks, patients with an early type II endoleak were not found to be at an increased risk of reintervention in our series. Most type I26 or type III13 endoleaks were late occurrences, and a significant contributor to late adverse outcomes. The majority of these were associated with aneurysm enlargement or rupture, conversion to open repair, or reintervention. Overall, all late-onset endoleaks, whether type I, II, or III, were a significant risk factor for aneurysm rupture (OR, 16.1; P = 0.009).
Clinically significant migration was detected in 25 (2.9%) patients. This observed migration rate must be viewed with the caveat that lesser amounts of migration likely went undetected, as often only migration resulting in clinically detectible adverse events such as type I endoleak, AAA sac enlargement, or AAA rupture were evident. Graft kinking sufficient to require reintervention or leading to graft thrombosis occurred in 22 (2.5%) patients.
AAA Enlargement
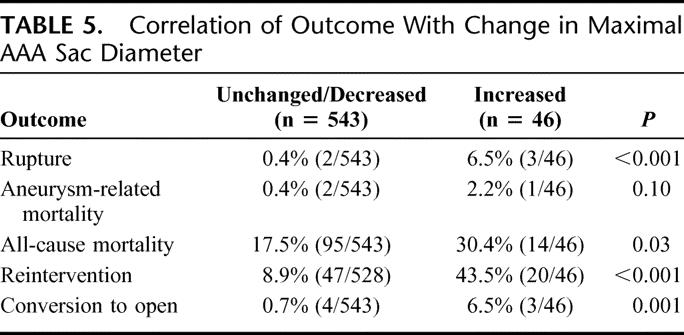
In the series, 589 patients had 2 or more CT scans, which allowed determination of change in maximal AAA sac diameter. Follow-up CT imaging revealed that 46 (7.8%) of patients had AAA sac enlargement ≥5 mm despite endoluminal repair. Overall, AAA size diminished in 49% of patients and remained unchanged in 43% cases. The majority of patients with enlargement had detectable endoleaks, but sac growth occurred in 16 of 46 (35%) patients without identified leaks and represent likely cases of so-called endotension. Our review did not identify any significant correlation between incidence of sac enlargement due to presumed endotension and commercial brand of endograft. AAA sac enlargement following EVAR was a significant marker of adverse outcome. As shown in Table 5, AAA rupture, need for reintervention, and conversion to open repair were all significantly higher in patients with aneurysm enlargement.
TABLE 5. Correlation of Outcome With Change in Maximal AAA Sac Diameter
Aneurysm Size
Using a definition of large AAA as those ≥55 mm, a comparison of outcome based upon preoperative AAA size reveals some important differences (Table 6). Patients undergoing EVAR for large AAA were somewhat older (76.4 vs. 74.9 years) and had significantly increased comorbidities. Patients with large and small AAA were otherwise comparable with regard to other baseline parameters. Outcomes for EVAR in patients with large AAA were worse, with dramatic differences in perioperative mortality and aneurysm-related mortality. No perioperative deaths occurred in 424 patients with AAA ≤55 mm (vs. 3.6% perioperative mortality in patients with large AAA), and aneurysm related mortality was only 0.5% for those patients with small AAA (vs.5.7% for large AAA).
Clinical Success
Primary clinical success, as defined by the Vascular Societies Ad Hoc Committee on Reporting Standards, was 87% (760 of 873). With appropriate reinterventions, primary assisted clinical success increased to 94.5% and secondary clinical success to 95.2%.
DISCUSSION
The possibility of AAA repair by a less invasive method of treatment, which can reduce risks, allow much quicker patient recovery, and provide other patient benefits, has tremendous appeal to patients and physicians alike and has generated considerable enthusiasm in the development and utilization of such technology. Since 1999 in the United States, the FDA has approved 5 commercial endograft devices, and several others have completed clinical trials and are awaiting approval. Nationally, the percentage of AAA repairs performed with endografts has increased rapidly, with 40% to 50% of all elective AAA being treated by EVAR in the current time frame.14,15,18 In our experience, 65% to 70% of infrarenal AAA are currently managed by EVAR (Fig. 1).
As with open surgical repair, the goal of EVAR is prevention of AAA rupture and avoidance of death due to rupture or AAA treatment itself. While the early mortality and morbidity risk reduction, as well as other early benefits of EVAR as compared with conventional surgical repair, have been firmly established and generally acknowledged, uncertainty persists as to whether these early advantages of EVAR will be sustained over longer follow-up intervals. Concerns remain that problems unique to EVAR, such as endoleak, migration, device structural failure, and related issues may result in ongoing rupture risk, offset any early advantages, and potentially compromise late outcomes.25–29
The primary outcome measures of this study are closely related to examination of this issue of whether or not long-term results of EVAR have effectively achieved the goals of AAA treatment. Late outcome is traditionally defined by results at 5 or more years of follow-up which are statistically valid, generally denoted by a standard error estimate ≤10%.33 Our 12-year experience provides such data and indicates that EVAR has, in fact, succeeded very well in preventing AAA rupture and aneurysm-related death. This confirms findings of other reports of midterm experience with EVAR.37–41 By Kaplan-Meier analysis, our data demonstrate that freedom from AAA rupture is 98% at 5 years and 94% at 9 years. Protection from aneurysm-related death, which may be the most meaningful primary endpoint,42 was achieved in 96% of patients at 5 years and 93% at 9 years. Open operation was avoided by 94% of patients up to 9 years. Maximal AAA sac diameter at 5 years, often considered a proxy for effective isolation of the AAA from the circulation, remained stable or actually decreased in 92.2% of patients. Highly satisfactory durability of EVAR using current generation devices in the contemporary era is documented by relatively stable rupture, ARM, and open conversion rates beyond 5 years. All of these late outcomes must be viewed in the context of the anticipated natural history and behavior of AAA in the 5- to 6-cm size range typically treated in our series. It is well documented that untreated AAA of this diameter may be expected to have an annual rupture risk of 11%, and to expand approximately 10% per year, with correspondingly increasing cumulative rupture risk.27,29,43
Our perioperative mortality rate following EVAR was 1.8%. This is in close agreement with results of other recently published results of randomized trials or other large observational data banks.12–18 Although slightly higher than previously described results of some FDA Investigation Device Exemption clinical trials,41 it must be recognized that many such studies required patients to be candidates for either open repair or EVAR to enroll. Hence, many patients in these studies were usually younger and lower risk. In contrast, our patient population included a large number of elderly high-risk patients, 39% of whom had 2 or more major comorbidities and nearly 20% of whom would be classified as unfit for open surgery by commonly accepted clinical criteria. In our view, the modest risk of perioperative death in such a high-risk patient cohort is testament to the less invasive advantages of EVAR.
The durability and late effectiveness of EVAR have long been a major concern, particularly in regard to possible use in a broader patient population including younger better-risk patients.44 Much of this concern stems from prior reports of device failure and other adverse outcomes. It must be emphasized that results of many of these earlier series were strongly influenced by a predominance of first-generation endograft devices. Our experience and analysis clearly document much improved long-term outcomes with current generation devices, an observation that has been noted by other investigators.45–47 Such improved results are an anticipated consequence of growing recognition and understanding of various modes of device failure, which have influenced the evolution of later generations and iterations of endograft technology. Such alterations in device design, manufacture, and testing have overcome many earlier problems and limitations of the technique. One can only expect that such advances and improvements are likely to continue.
Examination of all-cause mortality reveals survival of 52% at 5 years by life-table analysis. While in general accord with many other series of patients with AAA, such an outcome underscores the observation that truly long-term results may not be of paramount importance in many of these elderly and often medically frail patients with multiple comorbidities. Late procedural and device durability may not, in fact, be the most important objective. Indeed, the recently reported 2-year results of the 2 randomized controlled clinical trials, EVAR I and DREAM, revealed that any early survival advantage had disappeared, but that aneurysm-related mortality remained significantly lower for EVAR patients at 2 years.48,49 It is clear that considerable long-term risks of EVAR will be necessary to offset the significant initial perioperative mortality advantage of endoluminal repair.
Despite improvements in endografts, our experience clearly indicates that problems such as late-onset endoleaks, migration, or aneurysm enlargement may occur and are clearly related to failure to effectively exclude the aneurysm and ongoing rupture risk. For these reasons, ongoing surveillance appears to be of continued importance, despite increased costs and patient inconvenience. Many late problems may not be anticipated and may be undetected until rupture occurs; the best chance for correction prior to a serious adverse event is detection as early as possible by periodic CT scans. Follow-up surveillance is particularly vital in patients with ongoing endoleak or AAA sac enlargement, which are strong predictors of ongoing AAA rupture risk, aneurysm-related mortality, and open conversion. Indeed, in such patients, increased surveillance frequency may well be appropriate.
While the clinical significance of endoleak remains somewhat uncertain, our data document the adverse outcomes related to device-related type I and III endoleaks, and the need for prompt reintervention or conversion in such circumstances. However, it is evident that the majority of early type II leaks seal spontaneously, and that unless AAA sac enlargement occurs, reintervention is not needed for early branch leaks. Similar conclusions have been reached by other investigators.50 Persistent or late-onset type II leaks, however, may have other more significant consequences, and continued surveillance is clearly of importance.51,52
It is evident that secondary procedures will be required by a moderate number of patients over the long-term to maintain clinical success of EVAR. Our data indicate that 10% of patients in our series have needed such procedures. By Kaplan-Meier life-table analysis, freedom from reintervention declines steadily from 97% at 1 year to 74% at 5 years and 59% at 9 years. The great majority (92%) of these reinterventions are catheter-based, however, and 84% successful in correcting the problem that threatened the primary EVAR repair. It is important to recognize that successful reinterventions increased our primary-assisted clinical success rate to 94.5% and did not adversely affect aneurysm-related mortality. The value and success of appropriate reinterventions have been documented by other reports.53–55 Reintervention is clearly increased as compared with open surgery, but a compromise most patients find preferable to more extensive initial conventional open surgical repair. These observations, however, reinforce the importance of patient preference for decision making regarding open repair versus EVAR.21,27 The patient must be properly informed of options and the advantages and disadvantages of each.
A rather striking difference in late outcomes of small versus large AAA is evident from our data, particularly in regard to perioperative mortality and late aneurysm-related death. Other investigators have also noted the influence of preoperative AAA size.56–58 Such findings raise the provocative question as to whether smaller aneurysms should indeed be treated at an earlier stage if endovascular repair is possible, with the goal of improved late-term outcomes. Randomized clinical trials may help answer this controversial question. Our study did not address several issues of potential importance in an assessment of the proper role of EVAR. No evaluation of cost or cost-effectiveness was attempted. Prior studies on this topic have reached conflicting conclusions.59–61 In addition, we did not examine potentially relevant questions as to whether or not EVAR can improve perceived quality of life or lead to a better functional outcome. Functional outcome is clearly of importance, as emphasized by the rather sobering analysis of Williamson et al following open AAA repair.62 Survival alone may be an inadequate outcome measure.
Our experience confirms the well-recognized importance of proper patient selection and careful preoperative evaluation of anatomic suitability for EVAR. The durability of the procedure is strongly influenced by anatomic factors, and injudicious application of EVAR will surely increase the incidence of late adverse outcomes, including endoleak, reinterventions, need for open conversion, and aneurysm-related mortality. However, with appropriate patient selection, our data demonstrate that EVAR can successfully achieve the long-term goals of AAA repair with highly satisfactory early and late mortality and major morbidity, despite a relatively high-risk patient population. While it is clear that reinterventions will be required more frequently as compared with conventional open repair, our findings emphasize that such secondary procedures are usually successful and do not compromise aneurysm-related mortality or overall late outcomes. From our experience, we would conclude that EVAR is clearly the procedure of choice in elderly high-risk patients with appropriate anatomy, as suggested by other authors.63,64 Further, our good long-term outcomes support the possible use of EVAR as a reasonable alternative to standard open surgical repair in a broad range of patients with suitable anatomic findings, including younger and better-risk individuals. Patient preference continues to be of major importance in decision making in these patients.
Discussions
Dr. Gregorio A. Sicard (St. Louis, Missouri): We have just heard an outstanding presentation by Dr. Brewster of a large single-center experience in endoluminal aneurysm repair. His minimally invasive technique introduced by Juan Parodi has gained worldwide acceptance and is quickly becoming the primary form of therapy for elective aneurysm repair. In a similar 10-year experience at Washington University Barnes Jewish Hospital, we have seen a similar decrease in perioperative mortality, hospital stay, and overall safety and effectiveness of this procedure when compared with open aneurysm repair.
One of the major advantages of endoluminal aneurysm repair is the consistent demonstration of a lower perioperative mortality compared with open repair. Publications from single-center experiences, the Society for Vascular Surgery and European Registry data, statewide and national data sets, as well as randomized trials, consistently show a perioperative mortality with EVAR to be less than 2%, which is significantly lower than similar publications for open repair. The MGH experience presented today duplicates this low perioperative mortality despite the use of this technique in 20% of patients deemed unfit for surgical repair, and a large number, 69%, of patients that were very high risk. I have 3 questions for Dr. Brewster.
Your multivariate analysis showed that an independent predictor of late aneurysm rupture was aneurysms that were larger than 5.5 cm. Did you find any correlation between the diameter and length of the infrarenal neck and late aneurysm rupture?
Your data, as well as other retrospective analyses, have demonstrated better perioperative and long-term outcomes in EVAR patients with small aneurysms. Based on your own results, has your group modified the aneurysm size which you offer this technique and is it different than the criteria that you use for open repair?
Lastly, if we are going to expand the indications of endoluminal aneurysm repair to younger and patients with smaller aneurysms, should we consider modifying the follow-up surveillance protocol, which currently includes at least yearly CAT scans? Obviously, this creates an associated potential complication of cumulative radiation exposure and its risk. Do you think that the cardioMEMS intrasac pressure device that has recently been introduced has a future in post-EVAR surveillance and/or is color duplex in vivo ultrasonography sufficient?
I appreciate the opportunity to comment on this landmark contribution to the growing field of minimally treatment of aneurysm repair.
Dr. David C. Brewster (Boston, Massachusetts): In regard to aneurysm size, I think we and other groups have certainly found that size does matter. There is a strong correlation with adverse or unfavorable anatomy and large aneurysms. Remember, of course, that in EVAR anatomy is the driving force. So in small aneurysms, which tend to almost uniformly have more favorable anatomy, clearly the applicability rate is higher, and many groups, including us, have shown much better results of EVAR when done in small AAA.
Your second question really is the provocative one that I touched upon in the manuscript, and that is: should we in fact have a lower treatment threshold if aneurysms are suitable for EVAR? Several years ago, the Society for Vascular Surgery published guidelines on AAA treatment. I was privileged to chair that committee. We concluded at that time that the threshold in fact should not be different for open versus endoluminal repair. This conclusion was based at that time on data which was uncertain in terms of long-term effectiveness and durability. However, I think as our data and other subsequent reports document the long-term durability and effectiveness of EVAR, in my own opinion we probably will lower the threshold. I believe this would be appropriate.
Certainly, if I see a patient with a smaller aneurysm, 5 cm, let's say, and they have very favorable anatomy, I will discuss with them the possibility of endoluminal repair. This is where patient preference really does come into play. But as you well know, many patients don't really like having aneurysms and if you can offer them a highly reliable method of repair at an earlier interval many of them will indeed want to do that.
Finally, your other very good question concerns possible modification of follow-up protocols. Clearly, there is a surveillance burden for patients that have EVAR. It is an expensive proposition for society as well. I think our experience demonstrates to us, however, that we can't abandon surveillance. Many of these important, late problems such as late onset endoleak, migration with late onset attachment leak, and so forth, which are powerful predictors of rupture or other serious problems, often really can't be predicted.
So I think we have to continue surveillance. Whether we can do it, as some groups are urging, by ultrasound, or, as you indicate, with new technology such as a pressure sensor that is put into the aneurysm sac at the time of endograft deployment and which can then be noninvasively monitored to verify exclusion of the aneurysm sac from systemic arterial pressure, which is the goal of stent graft repair after all, remains to be determined. These are certainly important issues in this form of less invasive treatment.
Dr. Victor M. Bernhard (Palisade, Colorado): My concern is maintenance of endograft integrity. Enough time has passed since the inception of this approach to aneurysm therapy to determine the long-term durability of these devices. What has been your experience with endograft deterioration, either the metal components for fixation or the graft wall material?
Dr. David C. Brewster (Boston, Massachusetts): I think graft degeneration or structural failure was a very important problem with so-called first-generation endografts. Indeed, it led to elimination of several commercially available devices that went to trial and so forth. They are not used anymore because of these structural failures. But I think it is our opinion, and this is confirmed by multiple other investigators, that third- and fourth-generation devices that we are currently using have learned a lot from these sort of problems and that they are much less of a problem currently.
Dr. Julie Ann Freischlag (Baltimore, Maryland): As you know, we are doing a large multicenter study in the VA looking at endovascular repair versus open repair and we have randomized about 600 patients. My question to you is, when you look at your outcome from this data do you have similar data in your open repairs during the same time looking at mortality not only in the short term, in the long term.
Your long-term mortality is very high when you look at 5-year survival. In most of our aneurysm studies about 75% of patients will be alive. You have prevented deaths from aneurysm rupture, no question. But they are still dying. Do you know why they died?
We are going to use our data set to try to figure out which patients you shouldn't operate on who are going to die of something else anyway even though their aneurysm is fixed. Because as you know, this is a very expensive procedure and if you could have it just for the patients who needed it, that would be better.
So do you know why they died? And is there a group of patients now that you know you shouldn't do an endovascular repair on because they are going to die anyway in 5 years with a very expensive graft in place.
Dr. David C. Brewster (Boston, Massachusetts): In our series, only 11% of patients died related to aneurysm-related death. Hence, 89% died of other causes, predominantly, of course, cardiac, their other comorbidities or cancer. We are operating in general on an old, high-risk group. So their long-term survival was somewhat limited, 52% at 5 years by the Kaplan-Meier analysis. That is not too different, though, from many other series, 50% or 60% of patients who undergo open repair. Maybe the Veteran's patients are tougher and younger, I don't know.
But your question is a very important one in terms of to whom we shouldn't offer this, and hopefully your trial and other data from the United Kingdom EVAR II Trial will help us get at the important predictors there.
Dr. K. Craig Kent (New York, New York): That was a great study. What this area is lacking is long-term data, and I think your study will go a long way to fill this void.
My question is: why do 6% of these patients die of aneurysm rupture? You have a monitoring protocol in place that should allow patients with enlargement or endoleaks to be identified and then treated. So why in fact do 6% go on to rupture while being monitored?
Dr. David C. Brewster (Boston, Massachusetts): This is largely due, Dr. Kent, to these late developing problems, late type 1 or type 3 endoleaks that developed in the window between annual CT scans. So that is one reason why we certainly feel strongly that we can't abandon surveillance.
On the other hand, since we only survey them once a year in general after things are all right for a year or so, events are going to happen in that interval. Most of these were migration and late attachment leaks with reperfusion sudden leak profusion of the previously excluded aneurysm sac. We think perhaps that a period of exclusion has weakened the wall or led to atrophy of the sac wall, and then sudden repressurization may indeed lead to rupture.
Footnotes
Supported in part by the Harold and June Geneen Vascular Research Fund.
Reprints: David C. Brewster, MD, Massachusetts General Hospital, One Hawthorne Place, Boston, MA 02114. E-mail: dcbrewster@partners.org.
REFERENCES
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. [DOI] [PubMed] [Google Scholar]
- 2.Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg. 1999;29:292–308. [DOI] [PubMed] [Google Scholar]
- 3.Moore WS, Brewster DC, Bernhard VM. Aorto-uni-iliac endograft for complex aortoiliac aneurysms compared with tube/bifurcation endografts: results of the EVT/Guidant trials. J Vasc Surg. 2001;33(suppl):11–20. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura JS, Brewster DC, Makaroun MS, et al. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003;37:262–271. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg RK, Chuter TAM, Sternberg C, et al. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg. 2004;39:1209–1218. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JP, Anderson WN, Brewster DC, et al. Multicenter pivotal trial results of the Lifepath System for endovascular aortic aneurysm repair. J Vasc Surg. 2004;39:34–43. [DOI] [PubMed] [Google Scholar]
- 7.Criado FJ, Fairman RM, Becker GJ, et al. Talent LPS AAA stent graft: results of a pivotal clinical trial. J Vasc Surg. 2003;37:709–715. [DOI] [PubMed] [Google Scholar]
- 8.Brewster DC, Geller SC, Kaufman JA, et al. Initial experience with endovascular aneurysm repair: comparison of early results with outcomes of conventional open repair. J Vasc Surg. 1997;27:992–1005. [DOI] [PubMed] [Google Scholar]
- 9.May J, White GH, Weiyun Y, et al. Concurrent comparison of endoluminal versus open repair in the treatment of abdominal aortic aneurysms: analysis of 303 patients by life table method. J Vasc Surg. 1998;27:213–221. [DOI] [PubMed] [Google Scholar]
- 10.Adriaensen ME, Bosch JL, Halpern EF, et al. Elective endovascular versus open surgical repair of abdominal aortic aneurysms: systemic review of short-term results. Radiology. 2002;224:739–747. [DOI] [PubMed] [Google Scholar]
- 11.Drury D, Michaels JA, Jones L, et al. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg. 2005;92:937–946. [DOI] [PubMed] [Google Scholar]
- 12.Prinssen M, Verhoeven ELG, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–1618. [DOI] [PubMed] [Google Scholar]
- 13.Greenhalgh RM, Brown LC, Kwong GP, et al. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1): 30 day operative mortality results: randomized controlled trial. Lancet. 2004;364:843–848. [DOI] [PubMed] [Google Scholar]
- 14.Lee WA, Carter JW, Upchurch G, et al. Perioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001. J Vasc Surg. 2004;39:491–496. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PL, Arons RR, Moskowitz AJ, et al. A statewide experience with endovascular abdominal aortic aneurysm repair: rapid diffusion with excellent early results. J Vasc Surg. 2004;39:10–19. [DOI] [PubMed] [Google Scholar]
- 16.Hua HT, Cambria RP, Chuang SK, et al. Early outcomes of endovascular versus open abdominal aortic aneurysm repair in the National Surgical Quality Improvement Program–Private Sector (NSQIP-PS). J Vasc Surg. 2005;41:382–389. [DOI] [PubMed] [Google Scholar]
- 17.Leon LR, Labropoulos N, Laredo J, et al. To what extent has endovascular aneurysm repair influenced abdominal aortic aneurysm management in the state of Illinois? J Vasc Surg. 2005;41:568–574. [DOI] [PubMed] [Google Scholar]
- 18.Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446–452. [DOI] [PubMed] [Google Scholar]
- 19.Harris PL, Vallbhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the Eurostar experience. J Vasc Surg. 2000;31:739–749. [DOI] [PubMed] [Google Scholar]
- 20.Beebe HG, Cronenwett JL, Katzen BT, et al. Results of an aortic endograft trial: impact of device failure beyond 12 months. J Vasc Surg. 2001;33(suppl):55–63. [DOI] [PubMed] [Google Scholar]
- 21.Dattilo JB, Brewster DC, Fan CM, et al. Clinical failures of endovascular abdominal aortic aneurysm repair: incidence, causes, and management. J Vasc Surg. 2002;35:1137–1144. [DOI] [PubMed] [Google Scholar]
- 22.Bernhard VM, Mitchell RS, Matsumura JS, et al. Ruptured abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2002;35:1155–1162. [DOI] [PubMed] [Google Scholar]
- 23.Ohki T, Veith FJ, Shaw P, et al. Increasing incidence of midterm and long-term complications after endovascular graft repair of abdominal aortic aneurysms: a note of caution based on a 9-year experience. Ann Surg. 2001;234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collin J, Murie JA. Endovascular treatment of abdominal aortic aneurysm: a failed experiment. Br J Surg. 2001;88:1281–1282. [DOI] [PubMed] [Google Scholar]
- 25.Lederle FA. Abdominal aortic aneurysm: open versus endovascular repair. N Engl J Med. 2004;351:1677–1679. [DOI] [PubMed] [Google Scholar]
- 26.Rutherford RB, Krupski WC. Current status of open versus endovascular stent graft repair of abdominal aortic aneurysm. J Vasc Surg. 2004;39:1129–1139. [DOI] [PubMed] [Google Scholar]
- 27.Brewster DC, Cronenwett JL, Hallett JW, et al. Guidelines for the treatment of abdominal aortic aneurysms: report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117. [DOI] [PubMed] [Google Scholar]
- 28.Brewster DC. Do current results of endovascular abdominal aortic aneurysm repair justify more widespread use? Surgery. 2002;131:363–367. [DOI] [PubMed] [Google Scholar]
- 29.Brewster DC. Presidential address: What would you do if it was your father? Reflections on endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2001;33:1139–1147. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J, Brewster DC, Geller S, et al. Custom bifurcated stent-graft for abdominal aortic aneurysms: initial experience. J Vasc Interv Radiol. 1999;10:1099–1106. [DOI] [PubMed] [Google Scholar]
- 31.Wyers MC, Fillinger MF, Schermerhorn ML, et al. Endovascular repair of abdominal aortic aneurysm without preoperative arteriography. J Vasc Surg. 2003;38:730–738. [DOI] [PubMed] [Google Scholar]
- 32.Parker MV, O'Donnell SD, Chang AS, et al. What imaging studies are necessary for abdominal aortic endograft sizing? A prospective blinded study using conventional computed tomography, aortography, and three-dimensional computed tomography. J Vasc Surg. 2005;41:199–205. [DOI] [PubMed] [Google Scholar]
- 33.Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Sur. 2002;35:1048–1060. [DOI] [PubMed] [Google Scholar]
- 34.Brown LC, Epstein D, Manca A, et al. The UK Endovascular Aneurysm Repair (EVAR) Trials: design, methodology and progress. Eur J Vasc Endovasc Surg. 2004;27:372–381. [DOI] [PubMed] [Google Scholar]
- 35.Buth J, van Marrewijk CJ, Harris PL, et al. Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions unfit for an open procedure: a report of the EUROSTAR experience. J Vasc Surg. 2002;35:211–221. [DOI] [PubMed] [Google Scholar]
- 36.EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomized controlled trial. Lancet. 2005;365:2187–2192. [DOI] [PubMed] [Google Scholar]
- 37.Moore W, Kashyap V, Vescera C, et al. Abdominal aortic aneurysm: a 6 year comparison of endovascular vs. transabdominal repair. Ann Surg. 1999;230:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AneuRx Clinical Investigators. Zarins CK, The US AneuRx clinical trial: 6-year clinical update 2002. J Vasc Surg. 2003;37:904–908. [DOI] [PubMed]
- 39.Marin ML, Hollier LH, Ellozy SH, et al. Endovascular stent graft repair of abdominal and thoracic aortic aneurysms: a ten-year experience with 817 patients. Ann Surg. 2003;238:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao P, Verzini F, Parlani G, et al. Clinical effect of abdominal aortic aneurysm endografting: 7-year concurrent comparison with open repair. J Vasc Surg. 2004;40:841–848. [DOI] [PubMed] [Google Scholar]
- 41.Lifeline Registry of EVAR Publications Committee. Lifeline registry of endovascular aneurysm repair: long-term primary outcome measures. J Vasc Surg. 2005;42:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Arko FR, Lee A, Hill BB, et al. Aneurysm-related death: primary endpoint analysis for comparison of open and endovascular repair. J Vasc Surg. 2002;36:297–304. [DOI] [PubMed] [Google Scholar]
- 43.Finlayson SRG, Birkmeyer JD, Fillinger MF, et al. Should endovascular surgery lower the threshold for abdominal aortic aneurysm? J Vasc Surg. 1999;29:973–985. [DOI] [PubMed] [Google Scholar]
- 44.Carpenter JP, Baum RA, Barker CF, et al. Durability of benefits of endovascular versus conventional abdominal aortic aneurysm repair. J Vasc Surg. 2002;34:222–228. [DOI] [PubMed] [Google Scholar]
- 45.May J, White GH, Waugh R, et al. Comparison of first-and second-generation prostheses for endoluminal repair of abdominal aortic aneurysm: a six-year study with life table analysis. J Vasc Surg. 2000;32:124–129. [DOI] [PubMed] [Google Scholar]
- 46.May J, White GH, Waugh R, et al. Improved survival after endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: a 5-year concurrent comparison using life-table method. J Vasc Surg. 2001;33(suppl):21–6. [DOI] [PubMed] [Google Scholar]
- 47.Torella F. Effect of improved endograft design on outcome of endovascular aneurysm repair. J Vasc Surg. 2004;40:216–221. [DOI] [PubMed] [Google Scholar]
- 48.Greenhalgh RM, Brown LC, Epstein D, et al. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomized controlled trial. Lancet. 2005;365:2179–2186. [DOI] [PubMed] [Google Scholar]
- 49.Blankensteijn JD, de jong, SECA, Prinssen M, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352:2398–2405. [DOI] [PubMed]
- 50.van Marrewijk C, Buth J, Harris PL, et al. Significance of endoleaks after endovascular repair of abdominal aortic aneurysm: the EUROSTAR experience. J Vasc Surg. 2002;35:461–473. [DOI] [PubMed] [Google Scholar]
- 51.Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029–1035. [DOI] [PubMed] [Google Scholar]
- 52.van Marrewijk CJ, Fransen G, Laheij RJF, et al. Is a Type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. 2004;27:128–137. [DOI] [PubMed] [Google Scholar]
- 53.Laheij RJF, Buth J, Harris PL, et al. Need for secondary interventions after endovascular repair of abdominal aortic aneurysm: intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg. 2000;87:1666–1673. [DOI] [PubMed] [Google Scholar]
- 54.May J, White GH, Waugh R, et al. Life-table analysis of primary and assisted success following endoluminal repair of abdominal aortic aneurysms; the role of supplementary endovascular intervention in improving outcome. Eur J Vasc Endovasc Surg. 2000;19:648–655. [DOI] [PubMed] [Google Scholar]
- 55.Becquemin JP, Kelley L, Zubilewicz T, et al. Outcomes of secondary interventions after abdominal aortic aneurysm endovascular repair. J Vasc Surg. 2004;39:298–305. [DOI] [PubMed] [Google Scholar]
- 56.Peppelenbosch N, Buth J, Harris PL, et al. Diameter of abdominal aortic aneurysm and outcome of endovascular aneurysm repair: does size matter? A report from EUROSTAR. J Vasc Surg. 2004;39:288–297. [DOI] [PubMed] [Google Scholar]
- 57.Ouriel K, Srivastava SD, Sarac TP, et al. Disparate outcome after endovascular treatment of small versus large abdominal aortic aneurysm. J Vasc Surg. 2003;37:1206–1212. [DOI] [PubMed] [Google Scholar]
- 58.Zarins CK, Crabtree T, Arko FR, et al. Endovascular repair or surveillance of patients with small AAA. Eur J Vasc Endovasc Surg. 2005;29:496–503. [DOI] [PubMed] [Google Scholar]
- 59.Patel ST, Haser PB, Bush HL, et al. The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: a decision analysis model. J Vasc Surg. 1999;29:958–972. [DOI] [PubMed] [Google Scholar]
- 60.Sternbergh WC III, Money SR. Hospital cost of endovascular versus open repair of abdominal aortic aneurysms: a multicenter study. J Vasc Surg. 2000;31:237–244. [DOI] [PubMed] [Google Scholar]
- 61.Clair DG, Gray B, O'Hara PJ, et al. An evaluation of the costs to health care institutions of endovascular aortic aneurysm repair. J Vasc Surg. 2000;32:148–152. [DOI] [PubMed] [Google Scholar]
- 62.Williamson WK, Nicoloff AD, Taylor LM Jr, et al. Functional outcome after open repair of abdominal aortic aneurysm. J Vasc Surg. 2001;33:913–920. [DOI] [PubMed] [Google Scholar]
- 63.Sicard GA, Rubin BG, Sanchez LA, et al. Endoluminal graft repair for abdominal aortic aneurysms in high-risk patients and octogenarians: is it better than open repair? Ann Surg. 2001;234:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lange C, Leurs LJ, Buth J, et al. Endovascular repair of abdominal aortic aneurysm in octogenarians: an analysis based on EUROSTAR data. J Vasc Surg. 2005;42:624–630. [DOI] [PubMed] [Google Scholar]