Abstract
Objectives:
Although recent studies suggest that physician age is inversely related to clinical performance in primary care, relationships between surgeon age and patient outcomes have not been examined systematically.
Methods:
Using national Medicare files, we examined operative mortality in approximately 461,000 patients undergoing 1 of 8 procedures between 1998 and 1999. We used multiple logistic regression to assess relationships between surgeon age (≤40 years, 41–50 years, 51–60 years, and >60 years) and operative mortality (in-hospital or within 30 days), adjusting for patient characteristics, surgeon procedure volume, and hospital attributes.
Results:
Although older surgeons had slightly lower procedure volumes than younger surgeons for some procedures, there were few clinically important differences in patient characteristics by surgeon age. Compared with surgeons aged 41 to 50 years, surgeons over 60 years had higher mortality rates with pancreatectomy (adjusted odds ratio [OR], 1.67; 95% confidence interval [CI], 1.12–2.49), coronary artery bypass grafting (OR, 1.17; 95% CI, 1.05–1.29), and carotid endarterectomy (OR, 1.21; 95% CI, 1.04–1.40). The effect of surgeon age was largely restricted to those surgeons with low procedure volumes and was unrelated to mortality for esophagectomy, cystectomy, lung resection, aortic valve replacement, or aortic aneurysm repair. Less experienced surgeons (≤40 years of age) had comparable mortality rates to surgeons aged 41 to 50 years for all procedures.
Conclusions:
For some complex procedures, surgeons older than 60 years, particularly those with low procedure volumes, have higher operative mortality rates than their younger counterparts. For most procedures, however, surgeon age is not an important predictor of operative risk.
In this study, we assess the relationship between surgeon age and operative mortality among Medicare patients in the United States undergoing 1 of 8 complex surgical procedures between 1998 and 1999. For 5 of the 8 procedures, surgeon age did not predict operative mortality. Although surgeons over 60 years of age did have higher mortality rates with coronary artery bypass grafting, carotid endarterectomy, and pancreatectomy, this age effect was restricted primarily to low-volume surgeons.
Recent studies focusing on primary care have suggested an inverse relationship between surgeon age and clinical performance.1 Older physicians are less likely to incorporate new treatment strategies into their practice and less likely to prescribe appropriate medications.2,3 Relative to their younger counterparts, older physicians have poorer performance on recertification examinations and are less likely to have a current knowledge base.4,5 Such data have prompted calls for professional organizations to initiate more rigorous processes for performance assessment and credentialing throughout a physician's career.6
Whether physician age is related to clinical performance in surgery is not well established. Compared with primary care, the practice of surgery may present different challenges for the older physician. Complex procedures are long and require considerable physical and mental stamina. Previous research demonstrates that manual dexterity, strength, and visuospatial ability decrease with age, along with cognitive skills and abilities to sustain attention.7–11 Nonetheless, it has not been established whether such factors imply worse outcomes for patients. Two studies have suggested increased mortality rates for older surgeons with coronary artery bypass grafting and carotid endarterectomy, while others have pointed to surgeon youth and inexperience as more important risk factors.12–14
To examine the potential impact of surgeon age on patient outcomes more systematically, we performed a national study of Medicare patients undergoing a wide range of complex cardiovascular and cancer procedures. We hypothesized higher mortality rates at the extremes of surgeon age, as a result of either inexperience (for younger surgeons) or diminished physical and mental skills (for older surgeons). In examining relationships between surgeon age and mortality, we adjusted for potentially confounding variables, including patient characteristics, surgeon procedure volumes, and the attributes of hospitals in which surgeons work.
METHODS
Study Population
We used 100% national Inpatient Files from the Center for Medicare and Medicaid Services for years 1998 and 1999. These files contain hospital discharge abstracts for all fee-for-service, acute care hospitalizations for Medicare patients hospitalized in the United States. The Medicare eligibility file was used to determine vital status after hospital discharge.
Using methods identical to those described previously, we used procedure codes from the International Classification of Diseases, version 9 (ICD-9 codes) to identify all patients 65 to 99 years of age undergoing one of 8 surgical procedures: coronary artery bypass grafting (CABG), elective abdominal aortic aneurysm (AAA) repair, aortic valve replacement (AVR), carotid endarterectomy (CEA), pancreatectomy, esophagectomy, lung resection, and cystectomy.15 For the 4 cancer resections, patients were excluded from the database if they did not carry a diagnosis code for the malignancy corresponding to the procedure. Patients undergoing abdominal aneurysm repair were excluded if they were found to have diagnosis codes reflecting aneurysm rupture, thoracoabdominal aneurysms, or both. Finally, for the CABG cohort, we excluded patients undergoing concurrent valve replacement.
Characterization of Surgeons
As described in more detail elsewhere, we identified the primary surgeon performing each procedure using the unique provider identification number listed in the “primary operator” field of the inpatient files. The reliability of this approach has been demonstrated in previous studies.15,16 Only physicians self-designated as surgeons were included. For CABG and aortic valve replacement procedures, only physicians self-designated as cardiothoracic surgeons were included.
We determined surgeon age by linking surgeon unique provider identification numbers to date of birth information contained in the Medicare provider file. Information about selected hospital characteristics was obtained by linking hospital identifiers contained in each discharge abstract to files from the American Hospital Association.
As described previously, we determined procedure volume by assessing the average number of procedures performed by each hospital and each surgeon on Medicare patients during the study period 1998 and 1999.15,17 Patients were sorted into 3 evenly sized groups (low-, medium-, and high-volume) according to the procedure volumes of the hospitals or surgeons performing their procedures.
Statistical Analysis
Our primary outcome was operative mortality, defined as death before hospital discharge or within 30 days of the operative procedure. Our main exposure variable was surgeon age, which was examined both as a continuous variable and as a categorical variable as follows: 40 years or younger, 41 to 50 years, 51 to 60 years, and 61 years and older. For analyses assessing mortality at the extremes of surgeon age, surgeons 41 to 50 years of age were selected prospectively as the referent group, based on previous studies examining mortality rates for CABG.13
We used multiple logistic regression analyses to examine the relationship between surgeon age and operative mortality, adjusting for both patient and provider characteristics. Using methods previously described, we adjusted for the following patient factors: admission acuity (elective, nonelective), patient age, patient race (black versus nonblack), gender, and preexisting conditions (based on the Charlson comorbidity scores with published weights18). We then estimated predicted mortality for patients from these characteristics using multiple logistic regression models. To assess the effect of surgeon age on operative mortality, we again used multiple logistic regression adjusting for patient characteristics, surgeon procedure volume (the annual number of a given procedure in Medicare patients), hospital volume, and teaching status.19 All risk adjustment models were developed separately for each procedure and accounted for the effect of clustering of patients within surgeons. Adjusted mortality rates were determined by back-transforming logistic regression using the average values of the characteristics of the patients and surgeons for each procedure. All tests used were 2-sided, and a P value of less than 5% was considered to be statistically significant. All statistical analyses were conducted using STATA 9.0 (Statacorp, College Station, TX).
RESULTS
A total of 460,738 Medicare patients underwent 1 of the 8 selected procedures between 1998 and 1999. Overall, there were few clinically significant differences in patient characteristics by surgeon age (Table 1). As a result, predicted mortality did not vary considerably by surgeon age. Surgeons older than 60 years of age performing esophageal resections tended to operate on slightly higher-risk patients (predicted mortality, 15.7%) than younger surgeons (predicted mortality, 14.3%). There were no clinically meaningful differences in predicted risk for the other procedures.
TABLE 1. Characteristics of Patients, According to Surgeon Age
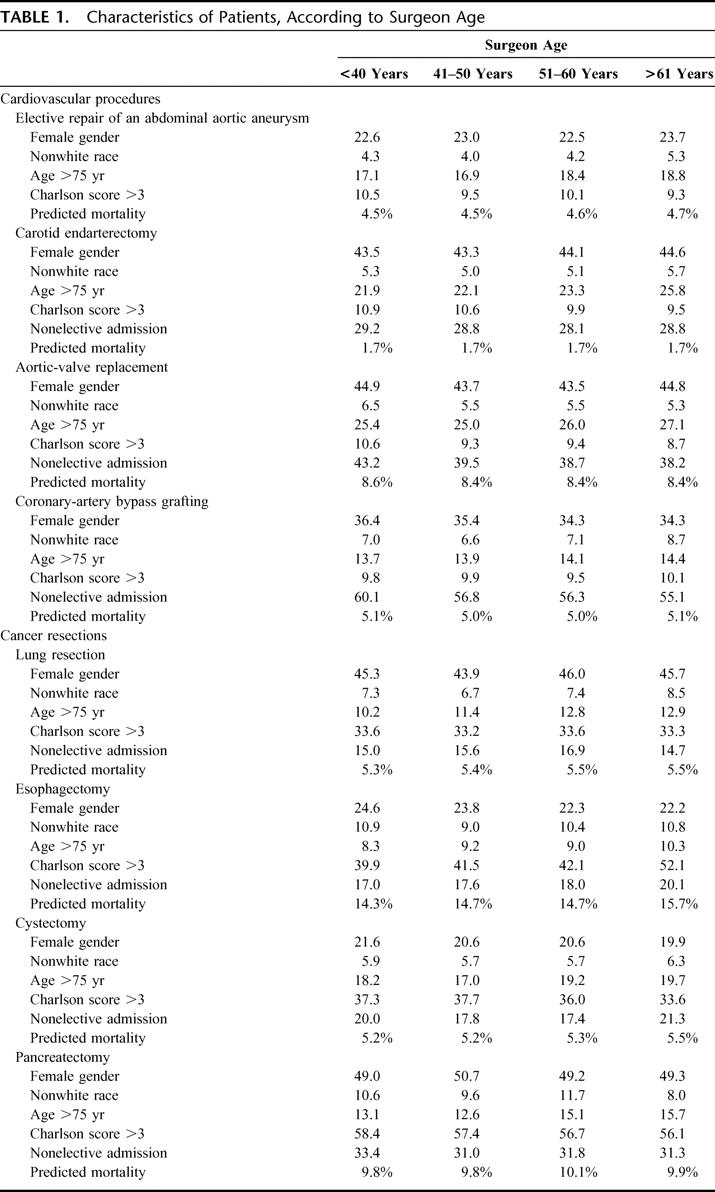
In general, the settings in which surgeons practice did not vary considerably by age. For pancreatectomy, CABG, and AVR, older surgeons were somewhat more likely to practice in teaching facilities, whereas younger surgeons were more likely to practice at such hospitals for CEA and pneumonectomy. Older surgeons performing elective AAA repair, CABG, and cystectomy were more likely to be lower volume surgeons, but procedure volumes did not vary considerably by surgeon age for the other 5 procedures (Table 2).
TABLE 2. Characteristics of Patients by Practice Setting, According to Surgeon Age
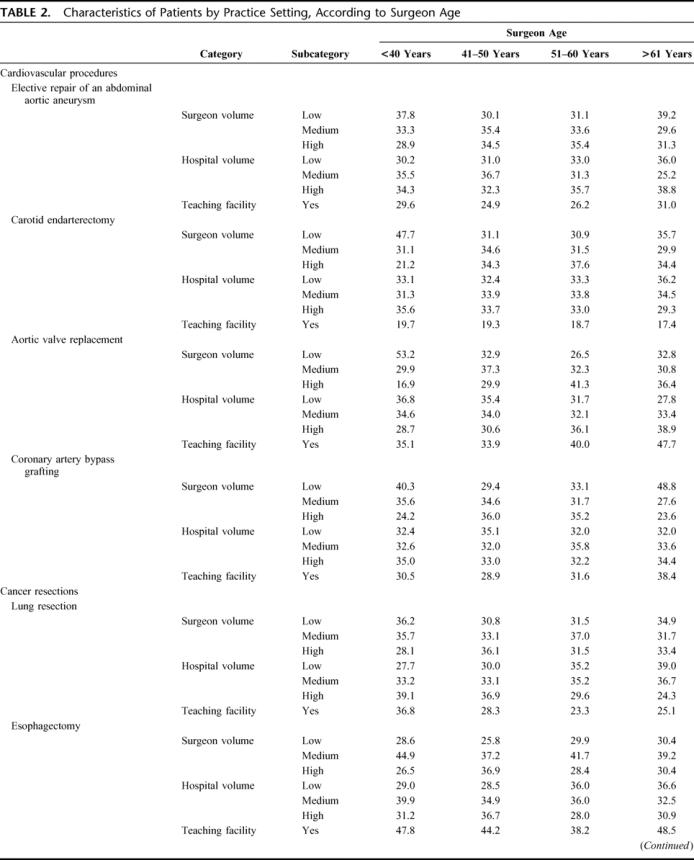
TABLE 2. (Continued)
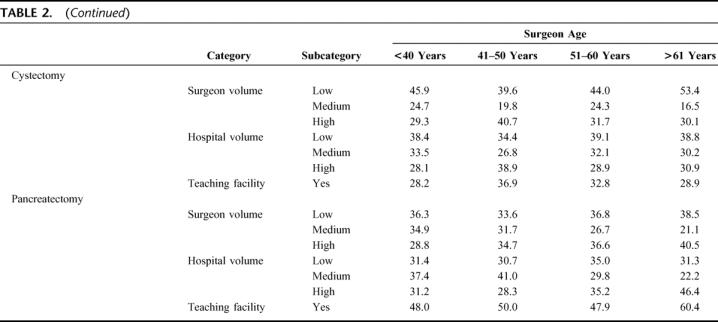
Table 3 summarizes associations between surgeon age and operative mortality, after adjusting for patient and provider characteristics. Surgeons 40 years and younger had higher adjusted odds of death for CEA than surgeons 41 to 50 years of age (adjusted OR, 1.21; 95% confidence interval [CI], 1.07–1.39) after adjusting for patient characteristics alone, but this effect was no longer significant after accounting for provider characteristics. Surgeons older than 60 years performing CEA had higher adjusted odds of operative death compared with surgeons 41 to 50 years of age after adjusting for both patient and provider characteristics (OR, 1.21; 95% CI, 1.04–1.41). Similarly, surgeons over 60 years of age performing CABG had a higher adjusted odds of operative death compared with surgeons 41 to 50 years of age after adjusting for patient and provider characteristics (OR, 1.17; 95% CI, 1.05–1.29). Finally, surgeons over 60 years of age performing pancreatectomy had higher odds of operative mortality compared with surgeons 41 to 50 years of age after adjusting for patient and provider characteristics (OR, 1.67; 95% CI, 1.12–2.49). Surgeons over 60 years of age had operative mortality rates similar to surgeons 41 to 50 years of age for 5 procedures: esophagectomy, cystectomy, lung resection, aortic valve replacement, and aortic aneurysm repair.
TABLE 3. Adjusted Odds Ratio for Operative Death, According to Surgeon Age
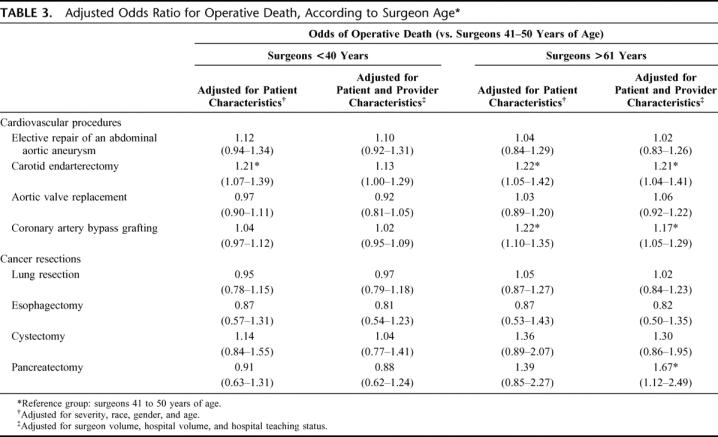
Figure 1 describes adjusted operative mortality rates for the 8 procedures by surgeon age. Across the majority of procedures, the magnitude of difference in adjusted operative mortality rate was small by surgeon age. For the 3 procedures in which advanced surgeon age conferred a significantly higher odds of operative mortality, absolute differences in mortality by surgeon age varied from 0.4% for carotid endarterectomy (1.9% for surgeons >60 years vs. 1.5% for surgeons 41–50 years) to 4.6% for pancreatic resection (14.8% vs. 10.2%).
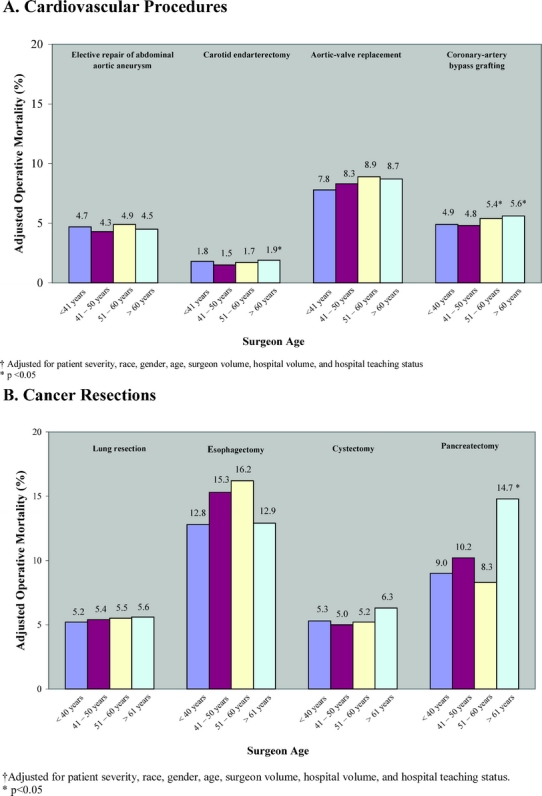
FIGURE 1. Adjusted operative mortality among Medicare patients in 1998 and 1999, according to surgeon age, for 4 cardiovascular procedures (A) and 4 cancer resections (B).
For these 3 procedures, Figure 2 depicts the relationship between operative mortality and surgeon age, stratified by surgeon volume. For surgeons older than 60 years performing pancreatectomy, CEA, and CABG, significant differences in mortality rates were largely restricted to those with low procedure volumes. Among low-volume surgeons, surgeons over 60 years of age had higher mortality rates for each procedure, differences that were both clinically and statistically significant. Among high-volume surgeons, however, there were no significant differences in mortality rates for CEA and CABG. For pancreatectomy, indeed, there were nonsignificant trends toward lower mortality rates in older surgeons.
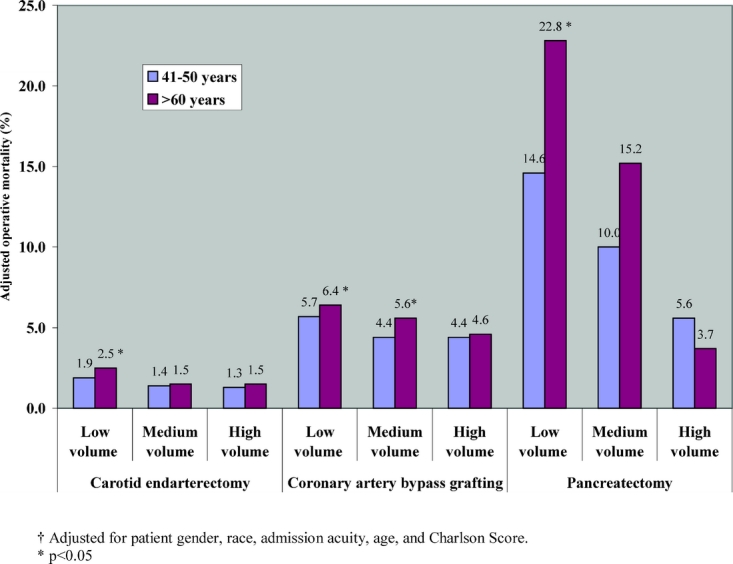
FIGURE 2. Adjusted operative mortality among Medicare patients in 1998 and 1999, according to surgeon age, and stratified by surgeon volume.
DISCUSSION
In this nationally representative sample of Medicare patients in the United States, surgeon age over 60 years was not associated with operative mortality for 5 of the 8 procedures examined. Older surgeons did have higher operative mortality rates for pancreatectomy, CABG, and carotid endarterectomy relative to younger surgeons. However, the overall magnitude of this difference was small, and the effect of surgeon age was largely restricted to surgeons with low procedure volumes. Surgeons younger than 40 years did not have higher operative mortality than older surgeons for any procedure after adjusting for surgeon procedure volume.
Although this study is the most comprehensive to date, other authors have examined the effect of surgeon age on postoperative outcomes. Hartz et al examined operative mortality among 275 surgeons performing coronary artery bypass grafting.13 Consistent with our findings, mortality rates increased with years of experience. Additionally, O'Neill et al revealed that patients of older surgeons performing carotid endarterectomy had higher mortality rates compared with younger surgeons.12 Finally, a recent study of laparoscopic inguinal herniorrhaphy found that older surgeons had higher hernia recurrence rates than younger surgeons.20
Although our study does not address potential mechanisms of the relationship of surgeon age on operative outcomes, several reasons may explain this effect. First, higher mortality may be attributable to diminished fine motor skills and visual acuity necessary for some complex procedures. In this study, the procedures for which surgeon age was related to higher mortality, CABG, CEA, and pancreatectomy, involve multiple, small anastomoses, and require a high degree of precision. Second, older surgeons may be more likely to delegate aspects of the procedure and perioperative care to surgical trainees. However, it is important to note the effect of surgeon age persisted after controlling for hospital teaching status in our analyses. Finally, older surgeons may be less familiar with new operative techniques and technologies. Although this could explain differences in patient outcomes for newer procedures, operations included in this analysis have been well established over recent decades. Although there are many theories regarding the effect of surgeon age on patient outcomes, these remain largely speculative. Explication of the effect of physician aging on operative outcomes must await additional studies.
This study has 2 important limitations. First, this study was restricted to Medicare patients older than 65 years and thus may not be generalizable to younger patients. Although older patients tend to have higher operative risks in general, the effect of surgeon age on operative outcome is unlikely to be restricted to older patients. Second, given limitations with risk adjustment using administrative data, it is possible that the age-related differences in mortality were due to differences in patients’ baseline risk. In our analysis, we found no evidence that patient age, gender, race, admission acuity, or Charlson comorbidity scores varied substantially by surgeon age. Although we cannot rule out confounding by unmeasured variables in our analysis, there is no evidence from other studies that older surgeons systematically operate on higher-risk patients.
The current study has potential implications for surgeons and their professional organizations. There is growing interest in developing strategies for rigorous assessment of surgeon performance with some procedures. For example, the American College of Surgeons has implemented criteria for credentialing and ensuring surgeon competency in bariatric surgery.22 Aiming more broadly, it also recently launched the Accreditation of Education Institutes program for surgeons learning new or very specialized procedures to ensure their proficiency. Similar efforts could be implemented longitudinally over a surgeon's career to measure clinical and technical proficiency with specific procedures. Our findings also have implications for practicing surgeons. Many surgeons approach retirement by gradually tapering off their clinical practices and operative volumes. Our findings suggest that, for some procedures, this strategy may not be optimal from the perspective of patient safety.
From the perspective of patients facing complex surgery, this study suggests that surgeon age should not be a primary factor in choosing a surgeon. Surgeon age is a relatively weak predictor of operative mortality in aggregate and certainly much worse for discriminating performance among individual surgeons. Instead, other surgeon characteristics may be more important, including risk-adjusted outcome measures when available and reliable, hospital and surgeon volume for selected procedures, and, perhaps, other less quantitative factors, such as surgeons’ local reputations.
Discussions
Dr. Andrew L. Warshaw (Boston, Massachusetts): This paper is actually quite reassuring to many of us in the audience in showing little or no relation between surgeon age and surgical mortality. And I presume, Dr. Pellegrini, that I have been asked to discuss it because I do qualify for the old guys’ group, I still do pancreatic resections, and I can also make it all the way to the microphone!
We older surgeons potentially have significant handicaps. We may have lost some of our physical skills, failed to acquire relevant new knowledge, and chosen not to or can't adapt to new technology such as minimally invasive and endovascular approaches. Many of us also begin to restrict our practices to those operations we know and remain confident that we still can do. We may also subliminally, despite the fact that it didn't turn up in your database, avoid the sicker, more complex patient with comorbidities, referring them to our younger colleagues to take on, and that becomes not so subtle cherry picking. These practice modifications may, in fact, be in the patient's best interest but may account in part for the failure to show differences in the outcomes across age groups.
As you have also pointed out, we have to differentiate between knowledge and technical skill. Your outcome measured mortality probably most reflects technical skill. The literature (mainly in the primary care area) does show that age and experience, gray hair, does not guarantee familiarity with and use of contemporary medical knowledge. The hotshot surgeon of 30 years ago may be mired back there.
And it is not just the “cognitive physicians” who are at risk. George Cruft, in his study of the American Board of Surgery recertification exams, showed an inverse relationship between age and exam performance. Not surprisingly, Neumayer and her colleagues, as cited in your paper, recently showed that older surgeons using newer techniques, in that case laparoscopic inguinal herniorrhaphy, were more likely to incur recurrences. Thus, my 2 questions:
First, Dr. Birkmeyer, I don't understand why there are different findings among your 8 index operations, even the ones where there was a significant difference. To my way of thinking at least, pancreatectomy and esophagectomy seem comparable in their complexity and demands. Yet one has a significantly higher mortality in the hands of older surgeons and the other does not. Why the difference?
Second, your endpoint was mortality. But today we can rescue most patients who have a complication and an initial rocky postoperative course. Perhaps that ability to keep most everyone alive confounds the mortality statistics that you were able to come up with, especially in an environment of residents. And although only one third of your study population were in a resident environment, those do tend to correlate with the high-volume practices as well: so the 2 may not be separable. I recognize the limitations of a Medicare administrative database, but do you know anything about the relationship between surgeon's age and the likelihood of postoperative complications, not mortality?
Congratulations on a valuable study and for reassuring my future patients.
Dr. John D. Birkmeyer (Ann Arbor, Michigan): Thank you very much, Dr. Warshaw, for taking the time to give our paper such a thoughtful reading. You ask several interesting questions.
First, why did surgeon age seem to matter for some operations but not others? The short answer is, “I don't know.” Our database obviously isn't suited to getting at many of the potential mechanisms underlying differences in mortality, either by surgeon age or by other factors. I have shared our data with several surgeons, and a few had theories as to why we found age effects with carotid endarterectomy, CABG, and pancreatectomy. One surgical resident cited the 5-0 Prolene theory, meaning that older surgeons shouldn't do any operation that requires a 5-0 suture or smaller. I'm sure there are other possible explanations, but that is the best I am going to be able to do for you now.
I agree with you that esophagectomy is just as complicated as pancreatectomy, and many would have expected a surgeon age effect with that procedure. It is important, however, to avoid overreading positive or null effects in subgroups for those 2 operations. Both are relatively esoteric operations, and the number of surgeons doing them overall is small. The number of surgeons doing it who are over 60 is really, really small. In fact, Dr. Warshaw, you are one of only 10 high-volume surgeons for pancreatectomy who is over 60.
Second, you ask why the surgeon age effect is smaller than many might have guessed. It could be, as you point out, that older surgeons are able to compensate for minor atrophies in their technical skill by being smarter and avoiding the wrong (ie, high-risk) patients. If true, I would view that as a good thing, not “cherry picking.”
More broadly, very little is known about mechanisms underlying variation in surgeon performance. Little is known about whether surgeons with high mortality rates have those higher rates because 1) they have a higher incidence rate of complications or 2) they are less skilled at managing those complications once they have occurred, so called failure to rescue. We are very interested in this particular research topic. Our group at the University of Michigan is working with the American College of Surgeons on addressing just that question in the field of cancer surgery, and I hope to give you a better answer in about 5 years.
Dr. Richard J. Shemin (Boston, Massachusetts): This is a very interesting and well-done study with findings that requires further conformation. I have 3 questions.
First, in the CABG group, what were the cut points between low, medium, and high volume? Second, since coronary bypass surgery is the most studied and measured operation in the history of medicine, we have learned from many studies that administrative data and risk adjustment models are flawed when compared to a more robust clinical database (eg, the Society of Thoracic Surgeons [STS] Database). Therefore, I would encourage confirming the results for CABG operations in this study with surgeon age as a risk factor with a more robust clinical data set such as the STS database. Third, in this era of transparency and public reporting of outcomes, if I were approaching you from the media or press, how would you explain the implications of this study and its potential impact on credentialing of surgeons in performing various procedures?
Dr. John D. Birkmeyer (Ann Arbor, Michigan): I can't give you volume cut points for CABG off the top of my head, but they are published in our 2003 paper on surgeon volume in the New England Journal of Medicine. Give or take, a low-volume surgeon does about 50 cases or fewer per year, a medium-volume surgeon does 50 to 100, a high-volume surgeon does about 100 or higher.
We don't have time to get into all of the nuances of risk adjustment with claims data versus clinical data. I think claims data do a terrible job in capturing illness severity and baseline risk, particularly when you are dealing with individual surgeons and small numbers. However, when you aggregate large groups of surgeons, such differences tend to average out. So I don't think this is a likely explanation for differences in mortality rates by surgeon age noted in this study.
Finally, how should the public and/or media interpret these data? Overall, I view our findings as good news rather than bad news. With few exceptions, they indicate that differences in mortality by surgeon age are very small and that older surgeons can maintain satisfactory performance by staying busy.
Dr. Palmer Q. Bessey (New York, New York): I enjoyed your paper, Dr. Birkmeyer. Thank you for bringing it to us. And please congratulate Dr. Waljee. I have 2 questions.
First, as you know, as you add variables to a logistics regression model, you can often get statistical significance, ie, positive odds ratio. If you use a little bit more stringent criteria like the AIC or the Schwartz criterion, would the age still be a significant predictor?
The second question has to do with the potential theory also to explain this. And that is as a role of specialization. Since most of the surgeons over 60 at the time of your study would have finished their training by the early 1970s, I wonder if the more recent surgeons now become much more specialized and focused right from the beginning, including fellowship training and so forth, and whether that might have a role also.
Dr. John D. Birkmeyer (Ann Arbor, Michigan): It is true when you work with databases where sample sizes run into the hundreds of thousands, you can find very small P values attached to very small mortality differences. Ultimately, what is “significant” is more a clinical judgment than a statistical one. For the 3 operations for which older surgeons had higher risks, the odds ratios ranged between 1.2 and 1.6. The excess mortality rates ranged between 0.4% and 4.6%.
Whether such differences are significant is a matter of perspective. If you are a patient or you are an individual surgeon, you can't tell the difference between 4.6% mortality and 5.4% mortality for CABG. From a public health perspective, however, there are 300,000 CABGs done each year in the United States. A mortality difference is 0.8%, which means 2400 excess deaths every year. Those judgments I leave to you.
Whether surgeon age functions mainly as a proxy for specialty training or specialization is a good question. Unfortunately, we do not have the data to answer your question directly.
Dr. Leigh A. Neumayer (Salt Lake City, Utah): It is always great to have another study that confirms our prior findings of this interaction between surgeon age and volume. I am reminded of my childrens’ karate creeds: “perfect practice makes perfect,” not just “practice makes perfect.” In this large administrative database, is there any way to use some kind of surrogate for whether or not the surgeons in the system are looking at their outcomes, as the Hawthorne effect (a good thing) may be playing a role.
Dr. John D. Birkmeyer (Ann Arbor, Michigan): Although we do not have the data to explore it directly, that is a very interesting hypothesis.
Dr. Murray F. Brennan (New York, New York): Dr. Birkmeyer, you took my emotions on an absolute rollercoaster in that talk. You told me that as a surgeon doing pancreatectomy my mortality was worse than all my younger colleagues. You then rescued me by saying if I was a high-volume surgeon, not only rescuing me but John Cameron, Andy Warshaw, and a number of others. And then you dashed me by telling me that it was better to burn out than fade away, which is what I planned on. And then you gave me this tantalizing little lift that if I just stop using 5-0 suture for the anastomosis, I would be okay. But I did that 3 or 4 years ago! So I am conflicted. I cannot slow down without flaming out. So could you just give me one other helpful thought that would allow some of us in this room not to flame out by Friday? Thank you.
Dr. John D. Birkmeyer (Ann Arbor, Michigan): Thank you, Dr. Brennan. In the middle of the controversy over hospital and surgeon volume, I was always somewhat spared by being a low-volume surgeon for most of the operations we were analyzing. And, as a “younger surgeon,” that is exactly why I didn't want to do this presentation. I asked Dr. Mulholland if Dr. Greenfield could give the talk, but he said, “No, I want you to do it.”
I am not sure that I have any additional pearls for you. I was only kidding that older surgeons should burn out and not fade away. However, they might take an all-or-nothing approach when it comes to a small subset of higher-risk operations, rather than a more gradual withdrawal.
Footnotes
Reprints: Jennifer Filip Waljee, MD, MPH, University of Michigan, MI. E-mail: filip@med.umich.edu.
REFERENCES
- 1.Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142:260–273. [DOI] [PubMed] [Google Scholar]
- 2.Stolley PD, Becker MH, Lasagna L, et al. The relationship between physician characteristics and prescribing appropriateness. Med Care. 1972;10:17–28. [DOI] [PubMed] [Google Scholar]
- 3.Rhee SO. Factors determining the quality of physician performance in patient care. Med Care. 1976;14:733–750. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey PG, Carline JD, Inui TS, et al. Changes over time in the knowledge base of practicing internists. JAMA. 1991;266:1103–1107. [PubMed] [Google Scholar]
- 5.Cruft GE, Humphreys JW Jr, Hermann RE, et al. Recertification in surgery, 1980. Arch Surg. 1981;116:1093–1096. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger SE, Duffy FD, Cassel CK. ‘Practice makes perfect’ or does it? Ann Intern Med. 2005;142:302–303. [DOI] [PubMed] [Google Scholar]
- 7.Jackson GR, Owsley C. Visual dysfunction, neurodegenerative diseases, and aging. Neurol Clin. 2003;21:709–728. [DOI] [PubMed] [Google Scholar]
- 8.Jackson GR, Owsley C, Cordle EP, et al. Aging and scotopic sensitivity. Vision Res. 1998;38:3655–3662. [DOI] [PubMed] [Google Scholar]
- 9.Mani TM, Bedwell JS, Miller LS. Age-related decrements in performance on a brief continuous performance test. Arch Clin Neuropsychol. 2005;20:575–586. [DOI] [PubMed] [Google Scholar]
- 10.Peisah C, Wilhelm K. The impaired ageing doctor. Intern Med J. 2002;32:457–459. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield LJ. Farewell to surgery. J Vasc Surg. 1994;19:6–14. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill L, Lanska DJ, Hartz A. Surgeon characteristics associated with mortality and morbidity following carotid endarterectomy. Neurology. 2000;55:773–781. [DOI] [PubMed] [Google Scholar]
- 13.Hartz AJ, Kuhn EM, Pulido J. Prestige of training programs and experience of bypass surgeons as factors in adjusted patient mortality rates. Med Care. 1999;37:93–103. [DOI] [PubMed] [Google Scholar]
- 14.Prystowsky JB. Are young surgeons competent to perform alimentary tract surgery? Arch Surg. 2005;140:495–500; discussion 502. [DOI] [PubMed]
- 15.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 16.Miller ME, Welch WP, Welch HG. The impact of practicing in multiple hospitals on physician profiles. Med Care. 1996;34:455–462. [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DH Jr, Whellan DJ, Sloan FA. Effects of admission to a teaching hospital on the cost and quality of care for Medicare beneficiaries. N Engl J Med. 1999;340:293–299. [DOI] [PubMed] [Google Scholar]
- 20.Neumayer LA, Gawande AA, Wang J, et al. Proficiency of surgeons in inguinal hernia repair: effect of experience and age. Ann Surg. 2005;242:344–348; discussion 348–352. [DOI] [PMC free article] [PubMed]
- 21.D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32:382–387. [PubMed] [Google Scholar]
- 22.Surgeons ACo, Network ACoSBSC, Care ACoSDoRaOP. Accreditation Program Manual. 2006:18.


