Abstract
Objective:
To determine the prognostic significance of histologic subtype in a large series of patients with primary liposarcoma (LS) and to construct a LS-specific postoperative nomogram for disease-specific survival (DSS).
Summary Background Data:
Nomograms, used to define and predict outcome following operative intervention, may contain variables not conventionally used in standard staging systems. A 12-year DSS postoperative nomogram for all sarcomas has already been established.
Methods:
From a single-institution prospective sarcoma database, patients with primary extremity, truncal, or retroperitoneal LS treated between 1982 and 2005 were identified. Histology was reviewed by a sarcoma pathologist and divided into 5 subtypes. A nomogram predictive of 5- and 12-year DSS was developed.
Results:
Of 801 patients with primary LS resected with curative intent, 369 (46%) presented with well-differentiated, 143 (18%) dedifferentiated, 144 (18%) myxoid, 81 (10%) round cell, and 64 (8%) pleomorphic histology. The median tumor burden was 15 cm (range, 1–139 cm). At last follow-up, 560 patients were alive with a median follow-up time of 45 months (range, 1–264 months) and 51 months for surviving patients. The 5- and 12-year DSS rates were 83% (95% confidence interval [CI], 80%–86%) and 72% (95% CI, 67%–77%), respectively. The nomogram was drawn on the basis of a Cox regression model. The independent predictors of DSS were age, presentation status, histologic variant, primary site, tumor burden, and gross margin status. The nomogram was internally validated using bootstrapping and shown to have excellent calibration. The concordance index was 0.827 compared with 0.776 for the general sarcoma postoperative nomogram for 12-year DSS.
Conclusion:
The LS-specific nomogram based on histologic subtype provides more accurate survival predictions for patients with primary LS than the previously established generic sarcoma nomogram. DSS nomograms aid in more accurate counseling of patients, identification of patients appropriate for adjuvant therapy, and stratification of patients for clinical trials and molecular analysis.
The liposarcoma-specific nomogram based on histologic subtype provides more accurate prediction for disease-specific survival (DSS) for patients with primary liposarcoma than the previously established sarcoma nomogram. The independent predictors of DSS were age, presentation status, histologic subtype, tumor burden, location, and gross margin status.
Liposarcoma (LS) is the most common soft tissue sarcoma (STS), accounting for 20% of all sarcomas in adults;1 10,000 cases and 3500 deaths attributable to STS are expected in the United States in 2006.2 Mortality rates for patients with liposarcoma range from 1% to 90%, and recurrence rates range from 5% to 83% depending on the histologic subtype and location.3–10 For appropriate clinical decision making and patient education of expected outcome, it has become increasingly important to improve the prognostic accuracy in patients with LS. With more exact prediction of outcome, a patient at low risk for disease-specific death (DSS) may be reassured, whereas a patient at high risk may be considered for neoadjuvant/adjuvant treatment or novel clinical trials.
While myxoid, round cell, and pleomorphic LS have a predilection for the extremities, well-differentiated and dedifferentiated subtypes occur predominantly in the retroperitoneum. Histologic grade, reflected in the extent of differentiation, remains the most important prognostic factor regarding clinical course and prognosis. Low-grade myxoid and well-differentiated variants each have a 5-year survival of 90%.3–5,7 Conversely, high-grade variants, such as round cell (defined by >5% round cell component), pleomorphic, and dedifferentiated tumors, have 5-year survival rates of 60%,7 30% to 50%,8 and 75%,6 respectively.
Recent work from our group at Memorial Sloan-Kettering Cancer Center (MSKCC) found that the histologic subtype, margin of resection, contiguous organ resection (except for nephrectomy), and age are prognostic for survival in primary retroperitoneal LS.9 For extremity STS, significant independent adverse factors prognostic factors for DSS include size, grade, depth, recurrent disease at presentation, microscopically positive margins, and lower extremity site.11 In a recent study of 126 patients with primary extremity LS, a multivariate analysis revealed that size, histologic subtype, and treatment with ifosfamide-based chemotherapy were independently associated with DSS.12
Nomograms are being increasingly more readily accepted as models in which identified prognostic factors can be combined and used to predict risk of DSS.13–16 Our group has previously published a postoperative nomogram for 12-year DSS for all sarcomas, which we have found useful for patient counseling, follow-up scheduling, and clinical trial eligibility determination.13 These statistically based tools not only use the factors included in a clinical staging system but may also incorporate additional factors suspected to have an impact on outcome.
The previously described sarcoma nomogram accounts for LS subtype only as it is reflected in grade, high versus low. The purpose of this study was to develop a subtype-specific nomogram for patients with LS that integrates various prognostic factors and predicts disease-specific death so as to provide an individualized prognosis for each patient. To accomplish this, we used a large database of LS patients treated at a single institution, carefully assessed for histologic subtype and followed prospectively.
METHODS
From July 1, 1982 through June 30, 2005, 910 adult patients underwent surgical resection for primary liposarcoma at MSKCC. These patients were prospectively entered and followed continuously in a computerized database. Primary LS of the extremity, trunk, or retroperitoneum was classified into 5 histologic types based on the World Heath Organization criteria17,18 as 1) well-differentiated, 2) dedifferentiated, 3) myxoid (<5% round cell), 4) round cell (≥5% round cell), and 5) pleomorphic. After histologic review and reclassification by a single sarcoma pathologist (C.R.A.), there were 801 patients with confirmed primary LS who had undergone surgical resection at MSKCC and had complete clinicopathologic data for correlation to outcome. None of the patients had distant metastasis at the time of presentation. Approval was obtained from the MSKCC IRB for this study. The primary endpoint was DSS.
The variables considered for the basis of the nomogram were age at diagnosis, gender, histologic variant (well-differentiated, dedifferentiated, myxoid, round cell, pleomorphic), presentation status (biopsy, no prior treatment, prior excision), primary site (lower extremity, upper extremity, trunk, retroperitoneum with or without contiguous organ resection), primary depth (superficial, deep) and primary tumor burden, and margins (negative, microscopically positive, grossly positive).
The following definitions were used. For histologic variant, the well-differentiated, dedifferentiated, and pleomorphic subtypes are represented by categorical variables. The extent of the myxoid/round cell component is generally considered to be a continuum, and for nomogram construction this continuum was divided into 3 categories on the basis of percent of round cell (RC) component: 1) myxoid (<5% RC), 2) round cell 5% to 25% (RC, 5%–25%), and 3) round cell >25% (RC>25%). A tumor was considered to be in the upper extremity if it was at or beyond the shoulder joint and in the lower extremity if it was in the groin, thigh, or leg. Retroperitoneal tumors were classified as with or without contiguous organ resection (eg, kidney, colon, small bowel, pancreas, spleen, bladder, uterus) and ascertained from the operating surgeon's assessment as documented in the operative report. The anatomic depth of tumors was evaluated relative to the superficial investing fascia of the extremity. Tumor burden was defined as the sum of the maximum diameters of the primary tumors as reported at the time of initial surgical resection. Margins were categorized as clear, microscopically positive, or grossly positive. A clear margin indicated that there was no tumor at least 1 mm or more from the edge of the inked specimen; a microscopically positive margin indicated microscopically discernible extension of tumor to within <1 mm of the edge of the inked specimen.
Because adjuvant/neoadjuvant chemotherapy or radiation treatment was not prospectively randomized but included both patients randomized in trials and those given standard of care based on prognosis, these treatment variables were omitted from modeling.
Patients were observed in our STS program at approximately 4-month intervals during the first 3 years and 6-month intervals thereafter. Information obtained during follow-up included status of disease (alive with or without evidence of disease, dead of disease or as a result of sarcoma treatment, dead of other causes without evidence of recurrent disease, dead of unknown causes).
Statistical Methods
The primary end point of the analysis was DSS (defined as time from date of surgery to date of death as a result of disease or complication). DSS was assessed with respect to age (continuous variable), gender, histologic subtype, presentation status, primary site, tumor burden (continuous variable), and margins. DSS was constructed by the Kaplan-Meier method.19 For DSS, death from LS or a treatment complication was considered an event. Five- and 12-year estimates of DSS and the corresponding 95% confidence intervals are reported.
Multivariate analysis was conducted with Cox proportional hazards regression. All decisions with respect to the grouping of the categorical variables were made prior to modeling, as making these decisions afterward can have deleterious effects on the predictive ability of the model.20 Those variables significant at the 0.05 level univariately were all used for nomogram development and were then entered into a model to identify independent predictors of DSS. Hazard ratios and corresponding 95% confidence intervals are reported.
This Cox model was the basis for the nomogram, and our modeling and validation procedure is similar to that used previously.21,22 The model accomplishes tailored prediction by making adjustments for each patient's predictor variable values. A patient's predicted probability of DSS is assumed to be a function of 2 components: the baseline hazard function shared by all patients and a linear combination of the individual patient's predictor variable values.
The nomogram was validated in 2 ways. First, the nomogram was subjected to bootstrapping (with 200 resamples) to calculate an unbiased measure of its ability to discriminate among patients; this was quantified by the concordance index.20 The concordance index is similar to the area under the receiver operating characteristic curve and ranges from 0.5 (no discrimination) to 1.0 (perfect discrimination).20,23,24 In other words, given a randomly selected pair of patients, the concordance index is the probability that the patient who dies first had the higher predicted probability of death. The second validation component consists of comparing the predicted probability of survival and actual survival, known as nomogram calibration on the entire cohort. This is done by using 200 bootstrapping resamples to reduce overfit bias, which would overstate the accuracy of the nomogram. All analyses were performed using S-Plus (Version 2000 Professional, Redmond, WA) with the Design25 and Hmisc25 libraries.
The dataset was then applied to the previously established general postoperative nomogram.13 DSS was assessed with respect to age (continuous variable), tumor size (≤5 cm, 5–10 cm, or >10 cm), histologic grade (high or low), histology (liposarcoma), depth (superficial or deep), and site (lower extremity, upper extremity, visceral, thoracic or trunk, retro-intraabdominal, or head and neck). The concordance index was calculated as previously described.20
RESULTS
Patient and Tumor Characteristics
A total of 801 patients with primary LS were treated by surgical resection at MSKCC. Descriptive characteristics for the patients with LS are listed in Table 1.
TABLE 1. Clinicopathologic and Treatment Characteristics in 801 Patients With Primary Liposarcoma of the Extremity, Trunk, or Retroperitoneum
There were 330 women and 471 men with a median age of 56 years (range, 16–95 years). The histologic subtype was well-differentiated for 369 patients (46%), dedifferentiated for 143 patients (18%), myxoid for 144 patients (18%), round cell for 81 patients (10%), and pleomorphic for 64 patients (8%). With regard to presentation status, 274 patients (34.2%) had undergone a previous biopsy, 331 patients (41.3%) had not had prior surgical treatment, and 196 patients (24.5%) presented with a prior excision. Nearly half of the LS tumors were located in the lower extremity. Retroperitoneal LS was seen in 168 patients (33.5%); half of these patients required resection of at least one contiguous organ. Most patients (n = 728, 91%) had tumors deep to the fascia. The median tumor burden was 15 cm (range, 1–139 cm). Margins were evaluated both grossly and microscopically in 6 dimensions (superior, inferior, medial, lateral, anterior, and posterior). A total of 533 patients (67%) had negative margins, 211 (26%) had microscopically positive margins, and 57 patients (7%) had grossly positive margins.
DSS Analysis
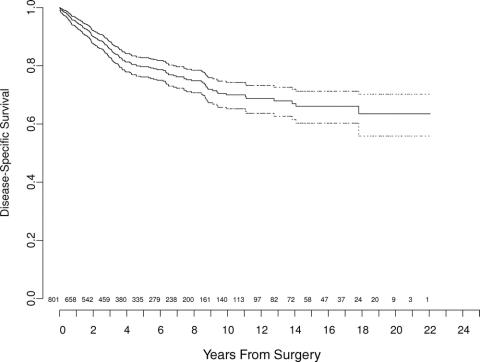
The overall DSS with number of patients at risk over time is illustrated in Figure 1 (n = 801). The median follow-up was 45 months (range, 1–264 months) for all patients and 51 months for survivors. For the entire cohort, 560 patients (70%) remain alive at last follow-up, and 155 (19%) have died of LS (Table 2). Forty-one patients (5%) died of other causes, and 45 patients (6%) died of unknown causes. The 5- and 12-year disease-specific survival probabilities were 83% (95% CI, 80%–86%) and 72% (CI, 67%–77%), respectively.
FIGURE 1. Liposarcoma-specific survival for 801 patients treated at Memorial Sloan-Kettering Cancer Center. Dotted-line bands represent 95% CI. Values at bottom indicate number of patients at risk.
TABLE 2. Patient Disease Status
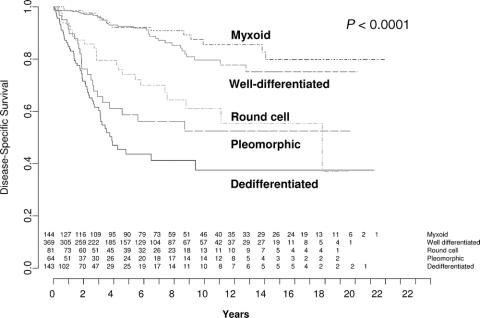
DSS stratified by histologic subtype is demonstrated in Figure 2. The 5-year and 12-year DSS on univariate analysis are also shown in Table 3. The 5-year DSS for low grade lesions, namely well-differentiated and myxoid tumors, were 93% (CI, 88%–95%) and 92% (CI, 85%–96%), respectively. For high grade tumors, the 5-year DSS rates were: dedifferentiated 44% (CI, 33%–54%); round cell 74% (CI, 54%–62%); and pleomorphic 59% (CI, 44%–71%).
FIGURE 2. Liposarcoma-specific survival by histologic subtype. Values at bottom indicate number of patients at risk.
TABLE 3. Univariate Analysis of Histologic Variant in Disease-Specific Survival (DSS)
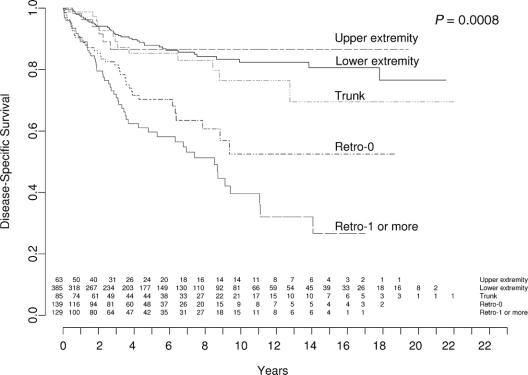
DSS stratified by primary site is illustrated in Figure 3. Extremity lesions (upper extremity, 87% [CI 72%–94%]; lower extremity, 82% [CI, 76%–87%]) enjoyed a higher 12-year DSS compared with truncal LS (77% [CI, 61%–86%]). Conversely, retroperitoneal tumors had a significantly decreased 12-year DSS, with patients requiring resection of one or more contiguous organs having the lowest 12-year DSS at 32% (CI, 19%–46%). Those patients with retroperitoneal tumors which did not require contiguous organ resection had a 12-year DSS of 53% (CI, 37%–66%) (P = 0.0008).
FIGURE 3. Liposarcoma-specific survival by primary location (extremity, trunk, retroperitoneum). Values at bottom indicate number of patients at risk.
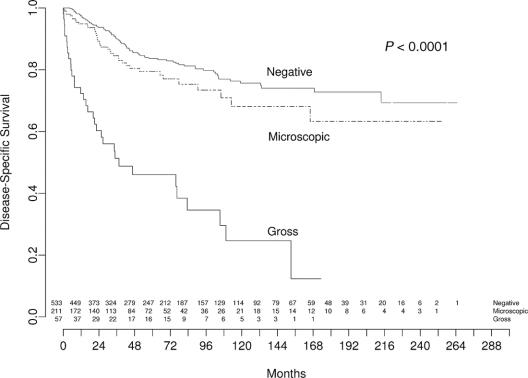
DSS stratified by margin status is depicted in Figure 4. While patients with microscopically negative and positive margins had 12-year DSS rates of 74% (CI, 68%–79%) and 68% (CI, 56%–77%), respectively, those with grossly positive margins had a significantly decreased 12-year DSS of 25% (CI, 11%–42%) (<0.0001).
FIGURE 4. Liposarcoma-specific survival by margin of resection. Values at bottom indicate number of patients at risk.
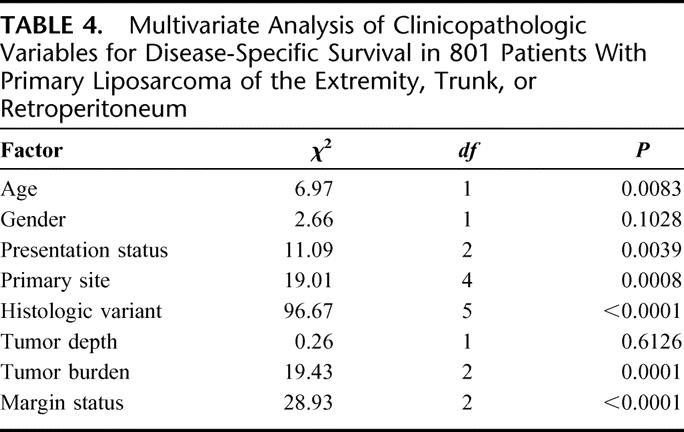
The multivariate analysis of prognostic factors of importance to DSS for 801 patients with primary LS is shown in Table 4. The independent predictors of DSS were age (P = 0.008), presentation status (P = 0.004), primary site (P = 0.0008), histologic variant (P < 0.0001), tumor burden (P = 0.0001), and gross margin status (P < 0.0001).
TABLE 4. Multivariate Analysis of Clinicopathologic Variables for Disease-Specific Survival in 801 Patients With Primary Liposarcoma of the Extremity, Trunk, or Retroperitoneum
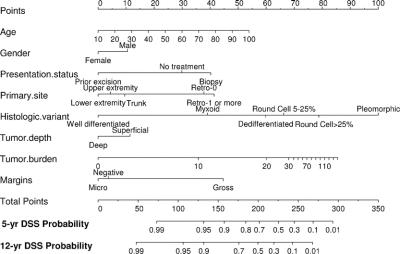
On the basis of the maturity of the data, we felt we were able to reliably predict disease-specific survival to 12 years with reasonably narrow CIs. A nomogram based on the Cox model is illustrated in Figure 5. Each variable in the Cox model was associated with LS-specific survival (P < 0.05) on univariate analysis. The nomogram predicts the probability that the patient will die of LS within 5 and 12 years of his initial surgery, assuming he or she does not die of another cause first. The bootstrapping concordance index was 0.827. Additional bootstrapping of this nomogram and plotting the survival probabilities against the corresponding observed survival probabilities obtained by the Kaplan-Meier method suggests excellent calibration of the nomogram (Fig. 6). Values on the x-axis represent probabilities that were calculated using the nomogram; values on the y-axis represent the observed 12-year DSS for patients in the cohort. The diagonal dashed line represents the performance of an ideal nomogram, for which predicted outcomes would correspond perfectly with actual outcomes. The line containing error bars represents the actual outcomes of the cohort.
FIGURE 5. Nomogram for predicting 5- and 12-year liposarcoma-specific survival probabilities.
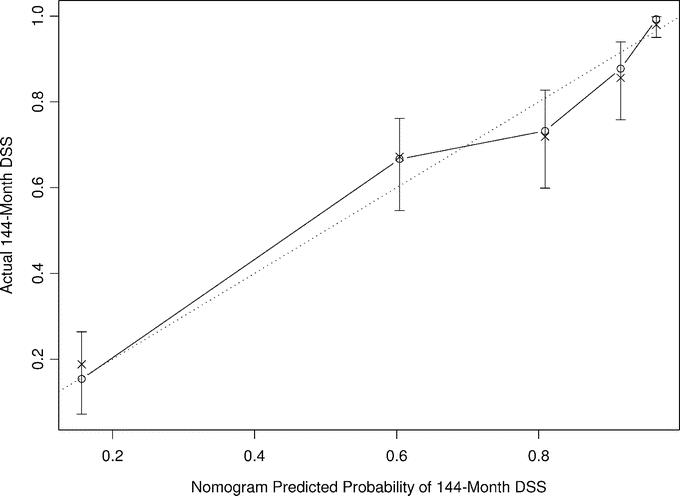
FIGURE 6. Calibration of the liposarcoma-specific survival nomogram at 12 years. Bootstrapping was used to correct for optimistic bias. x-axis is nomogram-predicted probability of survival. y-axis is observed disease-specific survival.
When the dataset was applied to the previously established general postoperative nomogram, the bootstrapping concordance index was 0.776.
DISCUSSION
Liposarcomas are the most common histologic subtype of STS and pose a challenge with regard to diagnosis, prediction of clinical behavior including recurrence or death, and treatment response. Nomograms are useful diagnostic tools that can provide more accurate individualized risk assessment for DSS. This is superior to what can be achieved with Kaplan-Meier analysis or simple counting of individual patient risk factors.13,26 Nomograms effectively integrate numerous prognostic variables to aid in predicting the probability of death and recurrence, in counseling patients, and in planning for surveillance and follow-up. This is profoundly helpful for patients who believe they are at high risk of dying, as they may be comforted from their nomogram prediction. Moreover, patients who are at high risk for DSS or recurrence should be identified so that surveillance and neoadjuvant/adjuvant therapy may be aggressively undertaken.
Based on findings in prospectively-followed adult patients with primary STS, Kattan et al13 developed the general postoperative sarcoma (GPS) nomogram, which generates disease-specific mortality probabilities over 12 years following operation for all soft tissue sarcomas. This prognostic tool relies on age, size, depth, site, grade, and histology. Internally validated and found to have a concordance index of 0.776, this GPS nomogram was validated by an external patient cohort, demonstrating its accuracy and universal applicability.27
We developed a LS-specific nomogram based on histologic subtype/variant to more accurately predict DSS compared with the previously established GPS nomogram. The LS-specific nomogram, with a concordance index of 0.827, discriminates LS-specific death among patients in terms of their mortality risk more accurately than the GPS nomogram. In other words, for a given pair of randomly selected patients, if the patient with the shorter follow-up period died of LS, the nomogram has an 83% chance of yielding a higher LS-specific mortality probability for that patient. Calibration accuracy, demonstrated by the calibration index, revealed that the LS-specific nomogram is also very well calibrated (Fig. 3).
The improved ability of the LS-specific nomogram to predict DSS is related to several features. First, tumor burden (size) in our LS nomogram was modeled as a continuous variable extending to 86 on the points axis as opposed to 3 discreet categorical variables (≤5 cm, 5–10 cm, and >10 cm) in the GPS nomogram, which had a 70-point range. The greater range and more precise discrimination when size is modeled as a continuous variable may account for some of the improved accuracy of the LS nomogram for predicting DSS.9
Second, in LS, grade is determined by histologic subtype. Well-differentiated and myxoid LS subtypes are classified as low-grade; dedifferentiated, round cell, and pleomorphic LS subtypes are considered to be high grade. In our subtype-specific LS nomogram, histologic subtype is the only prognostic variable that uses the entire range of the points axis. Visualization of the histologic subtype axis reveals that myxoid LS has a worse prognosis than well-differentiated LS even though both are considered low grade. For high-grade LS, examination of the subtype axis illustrates the magnitude of worsening prognosis as the subtype changes from dedifferentiated to round cell to pleomorphic. The concordance index of the LS nomogram using a 2-stage grading system instead of histologic subtype was 0.817. The higher concordance index for a LS nomogram that uses histologic subtype demonstrates the improvement in prediction of DSS that is achieved when a model using histologic subtype as opposed to grade is used.
For example, if we have a 50-year-old gentleman with a 10 cm, deep, dedifferentiated liposarcoma of the upper extremity, in the LS-specific nomogram, he would have 170 points. His 12-year sarcoma-specific survival rate would be 78%. In the previously established generic nomogram, in which grade is used instead of histologic subtype, his predicted 12-year sarcoma-specific survival is substantially different at 46%. If we change our patient's histologic subtype to pleomorphic, which is also considered high grade, but keep all of the same clinicopathologic features, his 12-year DSS is 38%, which is lower than the 46% predicted DSS in the generic nomogram. This illustrates how the liposarcoma-specific nomogram discriminates better than the previously established nomogram. In the first example, the GPS nomogram results in a 40% inaccuracy in predictive value: if a dedifferentiated subtype, it underestimates 12-year DSS by 30%, and if a pleomorphic subtype, the old nomogram overestimates 12-year DSS by 10%.
Third, factors used in the original nomogram are given additional strength in our LS-specific nomogram. Retroperitoneal LS are differentiated between those who did or did not require contiguous organ resection, which portends a significantly worse prognosis.9
Our inclusion of additional prognostic factors all of which were significant on univariate analysis (P < 0.05), such as gender, presentation status, and gross margin status, enriches the nomogram and thereby improves our ability to predict DSS compared with the GPS nomogram. Patients with primary liposarcoma who presented with a previous resection/excisional biopsy had a significantly improved DSS, which has been shown previously.28 This most likely reflects selection bias in that LS that underwent marginal resection due to technical reasons prior to referral to our institution were easier to remove completely; thus, reexcision may be associated with a more favorable prognosis than those patients treated with core/incisional biopsy or referred without prior biopsy. Inclusion of this variable improves the concordance index of the model.
Inspection of the margin of resection axis reveals that a grossly positive margin is associated with a significantly worse prognosis compared with negative or microscopically positive margins. Incomplete resection is a particularly important prognostic factor in primary retroperitoneal LS with a hazard ratio of 3.8 for DSS.9 However, on multivariate analysis, the microscopic versus negative margins was not a significant predictor of DSS for retroperitoneal LS. On the margin axis, negative and microscopically positive margins are relatively close (approximately 3 points apart) with negative margins having a slightly worse prognosis than microscopically positive margins. This small difference on the margin axis may well be correlated with a change in other axes of the nomogram, suggesting a potential interaction with a different prognostic variable. One example is the correlation with site. In extremity LS, margins may make a difference in outcome, but in retroperitoneal LS, complete gross resection is the dominant factor.9
The nomogram is useful for visualizing the associations between each predictor variable and sarcoma-specific death. The nomogram illustrates the worsening prognosis as the age increases. In addition, tumor burden also extends across the full range of the points axis. Patients with tumors of the extremities fare better than those located in other sites. Retroperitoneal LS have the worst prognosis, and contiguous organ resection portends an even less favorable outcome.9 With regard to histologic subtype, one can see the full spectrum from well-differentiated to pleomorphic, as well as the continuum from myxoid to round cell. For nomogram stratification, the round cell histologic subtype was divided into 2 groups (5%–25% RC and >25% RC component) to see if the degree of round cell differentiation was predictive of DSS. Although round cell >25% lies to the right of round cell 5% to 25% on the nomogram axis, in truth, their 5-year DSS rates are equal on univariate analysis, and the observed difference on the nomogram axis may be related to interactions with other prognostic variables.
When examining the associations of the nomogram predictors, one can understand that these factors are correlated; moving a patient on one axis may move him on another axis as well. This may explain the discrepancies between what one thinks is intuitive compared with the nomogram itself. With regard to tumor depth, although patients with superficial tumors appear to do worse than those with deep LS on the nomogram axis, the difference is small (approximately 10 on the points axis). Patients with deep tumors will fare worse on other nomogram variables. For example, 23% of superficial tumors were high grade, and 65% of deep tumors were low grade. Thus, the deep versus superficial axis is essentially a correction factor because it is artificial to fix all of the other axes and to only move the patient on the depth axis.
Similar reasoning applies to margin status. Although in the DSS nomogram, patients with microscopically positive margins appear to do better than those with negative margins, the difference is slight and insignificant. Typically, the patient will move on other axes when he moves on the margin status axis. Thus, the microscopically positive versus negative margin axis is a correction factor which improves the concordance of the model when used with other variables.
There are weaknesses in the LS-specific DSS nomogram. Despite including more patients and tumor variables and having a higher concordance index compared with the original MSKCC Sarcoma Nomogram, the nomogram is not perfectly accurate. With a bootstrap-corrected concordance index of 0.827, the nomogram has an 83% chance of predicting LS-specific death. However, this LS-specific nomogram may provide the most accurate predictions presently available. In addition, if a patient acquires <50 points or >275, his 12-year DDS can only be predicted at >99% or <1%, respectively.
Another limitation of the nomogram is that it relies on postoperative variables, making it an inadequate preoperative counseling tool. It also does not take into consideration adjuvant treatment, such as chemotherapy or radiotherapy. Although traditional adjuvant doxorubicin-based therapies have improved local recurrence rates, they have had little to no impact on survival.29–31 A recent study from MSKCC and ULCA found that ifosfamide-based chemotherapy was associated with improved survival in patients with high-risk, primary, extremity LS.12 Ifosfamide-based chemotherapy was not introduced until 1990 and was not uniformly applied or prospectively randomized to LS patients treated at MSKCC. Thus, chemotherapy and radiotherapy treatment variables were omitted from the nomogram model.
Developing a nomogram requires an extensive database with long-term follow-up. While our database is powerful as it contains patients who have been followed more than 20 years, the LS nomogram will only predict LS-specific death to a maximum of 12 years; this allows comparison of our LS-specific nomogram to the previously established GPS nomogram. Patients can still die of liposarcoma after 12 years; however, of the 86 patients at risk, only 4 patients have died of LS, emphasizing a relatively low risk of death after 12 years. This nomogram is a tool that can be adapted to desktop and handheld software. We plan to provide this service, free of charge, as we do with our other software, at http://www.mskcc.org/predictiontools.
The LS-specific postoperative nomogram discriminates DSS better than the previously established GPS nomogram. A LS nomogram model that uses histologic subtype as opposed to grade improves prediction of DSS. Understanding prognostic variables is important for counseling patients, selecting patients for aggressive adjuvant therapy, stratifying patients for clinical trials, and setting goals for patient treatment.32 In this way, one can improve the patient's psychologic state and potentially modify recurrence and disease-specific death. In the future, improved understanding of molecular markers may allow their inclusion in these nomograms to further enhance their predictive power.
ACKNOWLEDGMENTS
The authors thank Ruby Chang, research study assistant for the MSKCC sarcoma database, for her assistance in data collection.
Discussions
Dr. William G. Cance (Gainesville, Florida): This is an excellent study based on almost a quarter century of following sarcoma patients in a prospective database at Memorial Sloan-Kettering. This illustrates the value of these databases provided they are maintained and updated like the Sloan-Kettering databases have been done.
This shows the continued refinement of the prognostic variables for sarcoma. We have come a long way from the original variables of size, grade, and depth to a much more detailed discrimination of prognostic variables. So, it seems like we are moving from lumping these sarcomas together as a group of diverse but somewhat related tumors to being able to have a much more detailed understanding of prognostic variables, as you have shown quite nicely with the different subtypes.
I think that one of the most significant findings in this paper is your ability to integrate all these prognostic variables and take the Cox regression analyses and extend them to a nomogram. That is a major step forward that your group has led and is now refining. In addition, it helps us significantly, as you described, when we counsel our patients and when we stratify for clinical trials.
I have 3 questions. You excluded the patients that were on ifosfamide and received chemotherapy. What are your plans for being able to incorporate the chemotherapy patients into your nomograms? That was a prognostic variable in other studies. Are you going to be able to put that in eventually, and how will that factor in for the patients that receive that in an adjuvant setting?
For the primary liposarcomas that have had a previous resection, they had a better prognosis. Does that apply to the retroperitoneal sarcomas as well, particularly those that have had an incomplete resection? The patients that are the most challenging to the tertiary referral centers are the retroperitoneal sarcomas that have had an incomplete resection with positive margins. How do those patients fit onto your nomogram when they come in to see you?
Finally, I think that your 83% accuracy is phenomenal. Why do you need molecular markers as you mention in the paper? We all know how difficult it is to correlate molecular markers with prognosis. Shouldn't you just use molecular markers to define the targets and use it more on a therapeutic basis?
Dr. Samuel Singer (New York, New York): In terms of ifosfamide-based chemotherapy, we have previously done a study in combination with UCLA, and so the majority of those patients who had ifosfamide from that study were treated at UCLA. We had about 12% at Memorial who were treated with ifosfamide-based adjuvant therapy. So most of the patients that were treated at Memorial did not have these adjuvant therapies. And because these were not generally treated in a uniform fashion either for radiation or chemotherapy, we elected not to model those as part of this study.
But clearly, this type of nomogram approach could be used to look at the effect of treatment such as chemotherapy in the future if we were looking at a prospective trial. It would also help us to more accurately develop how to run that prospective trial based on careful breakdown of patients between the different subtypes so we would make sure we would have equal balance in the clinical trial.
In terms of incomplete resection and then the patient coming in for another resection, in this model the majority of patients that had that approach were extremities, and we didn't go down and break it down into how the patients who were retroperitoneal who had incomplete resection did. I think in this nomogram most of the patients who had the prior resection, I think that there was selection bias in that they had an improved outcome in that if it was easy to do on the outside then they had that performed. But we didn't break it down, so I can't answer your question on the retroperitoneal specifically in this subset.
And then finally, your third question about why add in molecular features. I think the concordance index for this is very good. It is not perfect. I think it remains to be determined whether we can then take this nomogram and then look, add in molecular predictors. I have shown from our microarray work that we had excellent correlations between gene expression analysis and subtypes. So clearly, the gene expression profile very carefully adjusts for subtypes. We need to determine, even if we adjust for subtypes, are there genes that still hold predictive value, for example, for metastasis and in turn influence survival?
So I think this nomogram serves as a basis now where we can then add to it information from gene (genomes) and try and see if we can do better with a genomic analysis as well as these clinical variables in combination. So we plan to take this Cox regression model, combine it with our molecular analysis, and see if we can improve it. My own bias is I think there is still room for improvement. But we will see.
Dr. Miguel Angel Mercado (Mexico City, Mexico): In this type of tumors, survival, did they die because of tumor recurrence after 15 years, all the patients have died are tumor related? Because it seems after 15 or 20 years, survival is related to perhaps, or some other types. In the very well-differentiated tumors, the survival approaches that of the normal population. Do they die because of recurrence of the tumor?
Dr. Samuel Singer (New York, New York): We presented here disease-specific survival, disease as it relates to liposarcoma. We only had 4 deaths after 12 years, and 86 patients were at risk. So not many patients die after 12 years. The typical patient who would recur late would be a retroperitoneal tumor where the curves continue to deteriorate with time. So those would be the ones that would be at greatest risk for late death.
Dr. James E. Goodnight, Jr. (Sacramento, California): Dr. Singer, a beautiful and very sophisticated study. Traditionally, we have found that limb sarcomas have a better prognosis than retroperitoneal, presumably because of where they present the ability to get local control. As you look at where these histologic subtypes present, is that beginning to dominate over the anatomic presentation of the sarcoma in terms of prognosis?
Dr. Samuel Singer (New York, New York): The extremity tumors, the subtypes that predominate, allow myxoid, round cell, pleomorphic with some well-differentiated liposarcomas mixed in. But it is mainly myxoid round cell, pleomorphic. In the retroperitoneum, it is the well-differentiated that predominates, there are no pleomorphic tumors. So although, for example, pleomorphic and round cell have a higher distant metastasis rate and they occur in the extremity and patient survival has allowed to you determine by distant metastasis, those survival curves flatten right out.
In the retroperitoneum, on the other hand, with well-differentiated and dedifferentiated tumors, the curves, although initially they have a lower rate of distant metastasis, they largely die of local control effect because the dedifferentiated tumors don't metastasize to other sites as frequently. So a lot of times you will get later deaths in that location. So it is a combination of both effects, subtype and site.
Dr. Harold J. Wanebo (Providence, Rhode Island): I would like to compliment Dr. Singer and his group on the development of prognostic nomogram and on redefining our understanding of sarcoma from the perspective of tumor type. Clinical classification schemes have changed over the years. We were initially splitters focusing on tumor type, but became lumpers just looking at grade and tumor size. It looks like we have come full circle with your detailed prognostic nomogram of a major tumor type, liposarcoma.
My question is related to the issue that the definition of the various subtypes appears highly dependent on the experience and thinking of the pathologists involved. So I would think that your nomogram, which looks very interesting, would have to be validated by other centers that have experience with sarcomas regarding the use of the subtypes.
My other question is a practical one. It used to be thought that certain liposarcomas were perhaps more sensitive to radiation than other sarcomas, especially in the retroperitoneum. I don't know if that is true or not, but I just wondered if there are any distinctive relationships to therapies besides surgery that might have treatment application? In other words, are certain tumors, if they are high grade and in the retroperitoneum, more effectively treated using neoadjuvant or adjuvant therapies rather than just standard resection? Can the normogram provide therapeutic insight regarding therapy?
Dr. Samuel Singer (New York, New York): We didn't look at the issue of adjuvant therapy in this study, but in general for the extremity lesions certainly we would recommend radiation. Radiation is really an investigative tool in the retroperitoneal location. In terms of systemic therapy, the round cell and pleomorphic subtypes are particularly sensitive to ifosfamide-based chemotherapy. The dedifferentiated subtype generally tends to be much less sensitive. So there is a subtype-specific sensitivity in terms of chemotherapy that one would approach.
In terms of the grading system for these tumors, it is fairly universal that pathologists use subtypes to define grade in liposarcoma. So this is the criterion they go back to when they define grade. Grade is somewhat redundant in certain histologic types, liposarcoma being one of them. So I think it is more precise to actually look at subtype rather than talking a 2-grade simple system.
Footnotes
Supported by NIH Program Project Grant No. P01CA47179 and the Kristen Ann Carr Fund.
Reprints: Samuel Singer, MD, Department of Surgery, Memorial-Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: singers@mskcc.org.
REFERENCES
- 1.Mack T. Sarcomas and other malignancies of soft tissue, retroperitoneum, peritoneum, pleura, heart, mediastinum, and spleen. Cancer. 1995;75:211–244. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E., et al. Cancer Statistics, 2006. CA Cancer J Clin. 2006;56:106–130. [DOI] [PubMed] [Google Scholar]
- 3.Henricks WH, Chu YC, Goldblum JR, et al. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271–281. [DOI] [PubMed] [Google Scholar]
- 4.Linehan DC, Leung J, Leung D, et al. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol. 2000;18:1637–1643. [DOI] [PubMed] [Google Scholar]
- 5.Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma. Ann Surg. 1998;228:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick D, Mentzel T, Beham A, et al. Dedifferentiated liposarcoma: clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994;18:1213–1223. [DOI] [PubMed] [Google Scholar]
- 7.Antonescu CR, Tschernyavsky SJ, Decuseara R, et al. Prognostic impact of P53 status. TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- 8.Gebhard S, Coindre JM, Michels JJ, et al. Pleomorphic liposarcoma: clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: a study from the French Federation of Cancer Centers Sarcoma Group. Am J Surg Pathol. 2002;26:601–616. [DOI] [PubMed] [Google Scholar]
- 9.Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kooby DA, Antonescu CR, Brennan MF, et al. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Ann Surg Oncol. 2003;11:78–84. [DOI] [PubMed] [Google Scholar]
- 11.Pisters PWT, Leung DHY, Woodruff J, et al. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 12.Eilber FC, Eilber FC, Eckardt J, et al. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004;240:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:627–629. [DOI] [PubMed] [Google Scholar]
- 14.Kattan MW, Heller G, Brennan MF. Competing risks nomogram. Statist Med. 2003;22:3515–3525. [DOI] [PubMed] [Google Scholar]
- 15.Brennan MF, Kattan MW, Klimstra D, et al. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher C, Unni K, Mertens F. Pathology and genetics of tumors of soft tissue and bone. In: Kleihues P, ed. World Health Organization Classification of Tumors, vol. 4. Lyon, France: International Agency for Research on Cancer Press, 2002:427. [Google Scholar]
- 18.Fletcher CM, Akerman P, Cin I, et al. Correlation between clinical pathological features and karyotype in lipomatous tumors. Am J Pathol. 1996;148:623–630. [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Cancer Res Clin Oncol. 1958;53:457–816. [Google Scholar]
- 20.Harrell FE, Lee K, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Eastham J, Stapleton AMF, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. [DOI] [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 25.Harrell FE Jr. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag, 2001:19. [Google Scholar]
- 26.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;175:1499–1507. [DOI] [PubMed] [Google Scholar]
- 27.Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101:2270–2275. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JJ, Leung D, Espat J, et al. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan MF, Alektiar K, Maki RG. Soft tissue sarcoma. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins, 2001:1841–1891. [Google Scholar]
- 30.Eilber FC, Eilber FR. Soft tissue sarcoma. In: JL Cameron, ed. Current Surgical Therapy. St. Louis: Mosby, 2001:1213–1218. [Google Scholar]
- 31.Sarcoma meta-analysis collaboration. Adjuvant chemotherapy for localized resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1993;350:1647–1654. [PubMed] [Google Scholar]
- 32.Grobmyer SR, Brennan MF. Predictive variables detailing the recurrence rate of soft tissue sarcomas. Curr Opin Oncol. 2003;15:319–326. [DOI] [PubMed] [Google Scholar]