Abstract
Objective:
To identify patterns of errors contributing to inpatient trauma deaths.
Methods:
All inpatient trauma deaths at a high-volume level I trauma center from 1996 to 2004 inclusive were audited. Data were collected with daily trauma registry chart abstraction, weekly morbidity and mortality reports, hospital quality assurance reports, and annual trauma registry analyses of risk of death using TRISS and HARM methodology. Deaths that met criteria for low to medium probability of mortality or those with quality of care concerns were analyzed for errors and then subjected to 3-stage peer review at weekly departmental, monthly hospital, and annual regional forums. Patterns of errors were constructed from the compiled longitudinal data.
Results:
In 9 years, there were 44,401 trauma patient admissions and 2594 deaths (5.8%), of which 601 met low to medium mortality risks. Sixty-four patients (0.14% admissions, 2.47% deaths) had recognized errors in care that contributed to their death. Important error patterns included: failure to successfully intubate, secure or protect an airway (16%), delayed operative or angiographic control of acute abdominal/pelvic hemorrhage (16%), delayed intervention for ongoing intrathoracic hemorrhage (9%), inadequate DVT or gastrointestinal prophylaxis (9%), lengthy initial operative procedures rather than damage control surgery in unstable patients (8%), over-resuscitation with fluids (5%), and complications of feeding tubes (5%). Resulting data-directed institutional and regional trauma system policy changes have demonstrably reduced the incidence of associated error-related deaths.
Conclusions:
Preventable deaths will occur even in mature trauma systems. This review has identified error patterns that are likely common in all trauma systems, and for which policy interventions can be effectively targeted.
Patterns of errors contributing to trauma deaths over 9 years at a high-volume center were identified and described. Institutional protocols demonstrably reduced error-associated deaths. The clinical error categories and error minimization strategies emphasized may have wide applicability.
Trauma care creates a “perfect storm” for medical errors: unstable patients, incomplete histories, time-critical decisions, concurrent tasks, involvement of many disciplines, and often junior personnel working after-hours in busy emergency departments. Studies in several countries have identified adverse events, including death, that occur in trauma and emergency care.1–4
In 1955, Robert M. Zollinger wrote in the Archives of Surgery about the “preventability” of deaths following motor vehicle crashes.5 In the Journal of the American Medical Association, 30 years later, Donald Trunkey reviewed 29 studies of preventable trauma deaths,6 and more have been published since.7–11 These studies supported the development of regionalized trauma care. They also provided insights into the nature of preventable deaths, including the significance of failure to evaluate the abdomen, delays to treatment, and critical care errors. However, estimates of preventable death rates were wide in Trunkey's review, ranging from 2% to 50%, indicating the variability in care provided and the need for standardized approaches to its analysis that minimized potential variability due to definitions, the methods used to detect events, and the type of reviewers making the final determination.12,13
These studies also showed that trauma surgeons were pioneers in error reduction and quality improvement long before interest in medical errors and patient safety became widespread. More recently, much interest and interdisciplinary expertise have been brought to standardizing error detection and classification,14–16 to understanding of predisposing structural and systemic factors17 and the defective information processing18,19 associated with error, and to the development of effective patient safety and error mitigating strategies.20,21
In trauma, as in all fields, it is likely that recognizable clinical situations create predictable vulnerability to human error, and the erroneous decision-making that occurs in response to these situations can be forecast to some degree.22 To reduce errors, institutions need effective means of identifying errors and error-associated deaths. This is all the more difficult in trauma given high baseline mortality rates, often complicated in-hospital care, and the relative paucity of widely applicable management protocols, especially beyond the “Golden Hour” of initial resuscitation, to which Advanced Trauma Life Support (ATLS) protocols apply. Furthermore, errors that result in death may be relatively infrequent; therefore, opportunities to learn from them may be limited by infrequent attention and lack of “institutional memory.” In this study, we aimed to identify errors that had contributed to the death of trauma patients at a specific high-volume regional trauma center over a 9-year period and determine any apparent patterns of occurrence. We also aimed to examine the effect of introduction of local institutional policies on reducing error incidence.
METHODS
Study Population and Identification of Cases
Harborview Medical Center (HMC) in Seattle, WA, is a level I regional trauma center that annually receives over 6000 trauma admissions, 1500 with an Injury Severity Score greater than 15. It serves as the only level I trauma center for 4 states: Washington, Alaska, Idaho, and Montana. The Washington State Department of Health is the verifying and designating legal authority for trauma center designation, and requirements are very similar to that of the American College of Surgeons Committee on Trauma Resources for Optimal Care of the Injured Patient.23 HMC is also the local safety-net hospital for King County, Washington, which incorporates all of urban Seattle's population of approximately 1.8 million. King County is further served by an additional total of 7 (3 level III and 4 level IV) trauma centers which, by regional plan, refer seriously injured patients to HMC.
At HMC, the general surgeon is the captain of the trauma team. A chief resident or fellow in general surgery evaluates all seriously injured patients in the emergency department, and an attending general surgeon is called to all trauma team activations. Patients with multisystem injuries are admitted to the general surgery service, as are unstable patients with isolated injuries for resuscitation and stabilization before transfer to other services. An in-house attending anesthesiologist and a full-time operating room staff are present 24 hours per day, 7 days per week. The CT scanner and the angiography suite are adjacent and easily accessible to the emergency department.
Data on all trauma admissions are included in a hospital trauma registry and in the Washington State Trauma Registry. All trauma deaths are discussed at weekly surgical morbidity and mortality (M&M) meetings. Those identified as being associated with possible or definite errors in care are subsequently reviewed by departmental and hospital quality assurance (QA) officers, and significant cases are presented for discussion at monthly multidisciplinary hospital trauma council meetings. Annual trauma-registry based analyses of the risk of death at the time of admission to the trauma service are also conducted.
All trauma deaths that occurred after arrival in the emergency room and prior to discharge from HMC in the 9 years from January 1, 1996 to December 31, 2004 were eligible for this study. Those deaths identified at M&M meetings as being possibly or definitely associated with errors in care were critically reappraised. Self-reporting of errors and chart review have both been shown to be effective methods of case detection, and the combination is probably better than either one alone.24,25 We therefore also critically appraised all deaths that had less than 50% probability of death at the time of admission, as determined by the Trauma Injury Severity Score (TRISS)26 or the Harborview Adjusted Risk of Mortality (HARM) score.27 Both scoring methods help predict probability of survival based on the anatomy of injury and/or physiologic derangement caused by the injury.
The critical appraisal of cases identified through M&M reviews and the TRISS/HARM methodology was performed annually by a senior trauma fellow who was not associated with any of the cases, in conjunction with the Trauma Medical Director. Data was obtained from M&M reports, hospital quality assurance reports, departmental and trauma council minutes, and the medical record, which included electronic data, pathology and imaging reports, emergency department flow sheets, operating room reports, and outpatient reports. These data, occasionally supplemented by interviews with staff, were appraised in a standardized fashion, and any potential error that contributed to each patient's death was described in detail. Potential errors were identified by examining the cause of death and its antecedent events, and reviewing the process of care for apparent errors in decision-making, timing, conduct of procedures, and nonprocedural mishaps.
Peer Review
Each case of suspected error was subjected to peer review at one or more of the following forums: weekly M&M meetings, monthly trauma council QA meetings, quarterly hospital quality assurance forums, and annual regional QA forums. Each case was therefore reviewed by the staff involved in the case, other surgical staff, representatives of the divisions of nursing, emergency medicine, critical care, operating room, radiology, laboratory services, hospital management, and by the regional level III and IV trauma directors. Those cases in which it was agreed that an error in care likely contributed to the patient's death were retained, while we discarded those in which errors were regarded to be unlikely contributors to death on the basis of the available data.
Error Definition and Classification
We were guided by James Reason's definition of an error as “the failure of a planned action to achieve its desired goal.”28 We concentrated on identifying and defining “active failures,” that is, errors that were performed by the physicians, nurses, or others who had direct care of the patient. Predisposing system-based factors, also known as “latent failures,” were not examined in detail.18
We subsequently classified errors, first according to clinical features, and second in categories consistent with the elements of the National Quality Forum-endorsed JCAHO Patient Safety Net Taxonomy.15,16 This taxonomy comprises 5 aspects of errors, 1 of which considers error prevention strategies and the remainder of which include:
Error Impact, which in our study was “death.”
Error Type, classified as many others have done2,9 as errors in diagnosis, treatment, prevention, or other (equipment failures; communication failures; and errors in transfer).
Error Domain, for which we were most interested in the phase of trauma management when it occurred, and for which the classifications were: initial assessment and resuscitation (including prehospital); secondary survey and tests (eg, CT); interhospital transfers; initial interventions (eg, OR, Angio); ICU; general ward; and rehabilitation. Other domains that might have been relevant, but for which we did not have a mechanism in place to capture, included the person who erred, the time of day, and patients' comorbidities.
-
Error Cause, which refers to the “psychologic” cause, that is, it relates to what was probably going on in the mind of the person who erred. Reason has contributed much to this understanding, and we used an “internal processing classification” adapted from his work (Sir Alfred Cuschieri, personal communication) that included:
· Input error: the input data are incorrectly perceived; therefore, an incorrect intention is formed and the wrong action is performed.
· Intention error: the input data are correctly perceived, but an incorrect intention is formed, and the wrong action is performed.
· Execution error: the input data are correctly perceived and the correct intention is formed, but the wrong action is performed; that is, the action is not what was intended.
Pattern Recognition and Impact of Policies
Broad descriptive categories were developed to represent major groups of error that we identified. We described any apparent error patterns corresponding with particular phases of trauma management, error types, or likely underlying causes. Relationships between these variables were identified using cross-tabulations and graphical representation.
In each error category, we examined whether or not any relevant institutional or trauma system policies had been implemented during the study period. These were identified from institutional policy documents, HMC Trauma Council minutes and relevant local peer-reviewed publications. The occurrence of errors relative to each policy's implementation was then plotted to give an indication of whether or not such policies had been effective in reducing error occurrence. Observations were categorized into whether or not a new policy was implemented during the study period.
RESULTS
In 9 years between 1996 and 2004, inclusive, there were 44,401 trauma patient admissions that resulted in 2594 deaths (5.8% of admissions). Of the deaths, 69% were male, the median age was 46 years, and 74% were due to blunt trauma, 17% due to penetrating trauma, and 9% due to burns and other mechanisms (Table 1). Fifty-three deaths (2.0%) had quality of care concerns discussed at M&M review that may have contributed to the death, and 601 deaths (1.4% admissions, 23.2% deaths) had less than 50% mortality risk at the time of admission, as defined by TRISS and HARM scores (Fig. 1). Over the 9 year period, on average 6 cases from M&M (range, 2–16 cases) and 67 cases identified from the registry were critically appraised annually. After this review, 64 patients (0.14% admissions, 2.5% deaths over the 9-year period) had recognized errors in care that were likely to have contributed to their death.
TABLE 1. Characteristics of Trauma Presentations and Deaths
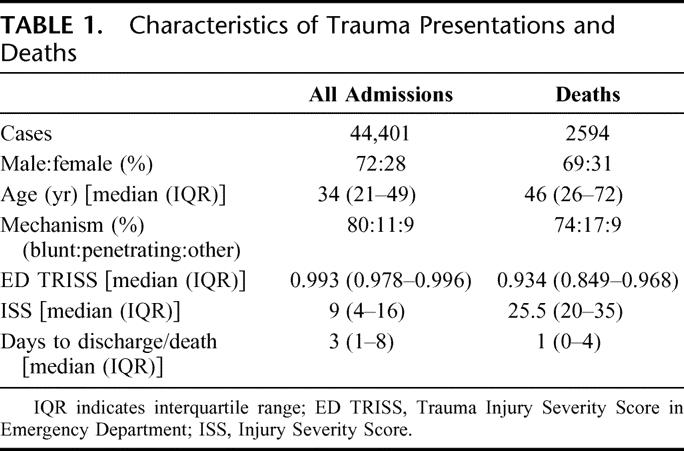
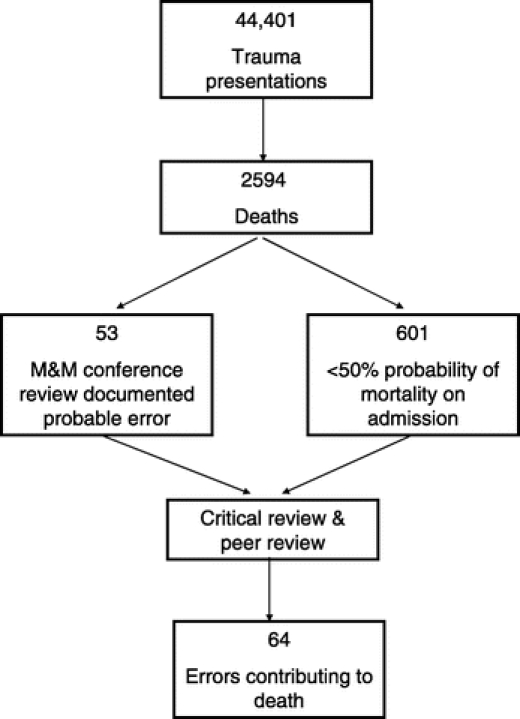
FIGURE 1. Assessment of errors contributing to death.
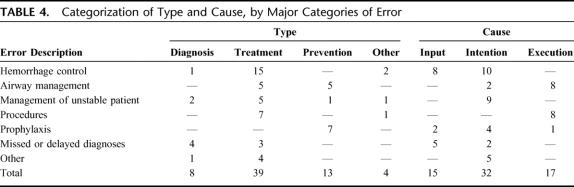
The major clinical groupings of errors are shown in Table 2 and included hemorrhage control (28%), airway management (16%), inappropriate management of unstable patients (14%), complications of procedures (12%), inadequate prophylaxis (11%), missed or delayed diagnoses (11%), over-resuscitation with fluids (5%), and other poor management decisions (3%).
TABLE 2. Major Patterns of Errors Contributing to Trauma Mortality

Delayed control of abdominal or pelvic hemorrhage using operative or angiographic methods was usually due to delays in the emergency department (ED) assessment of a patient in shock, and occasionally involved the performance of other nonurgent tests or procedures. Delayed control of intrathoracic hemorrhage was most often a delay in the diagnosis of massive hemothorax with inadequate evacuation of blood from the chest, or inadequate recognition of the volume that had already been evacuated. Half of all fatal airway errors involved unsuccessful attempts at endotracheal intubation and failure to adequately gain or regain control using simple maneuvers or a surgical airway. The other airway errors were failure to adequately protect the airway from aspiration during subsequent phases of care.
In the operating room, damage-control principles dictate that control of hemorrhage and control of contamination should be prioritized, deferring prolonged surgical interventions and reconstruction until correction of hypothermia, acidosis, and coagulopathy is accomplished in the ICU.29 We found 5 cases in which these principles were not followed, and the patient subsequently progressed to death due to exsanguination or multiorgan failure.
We found 7 cases in which a missed injury or delayed diagnosis led to death. In 4 cases, there was a positive test result that failed to be acted on (a head CT showing subdural hemorrhage, a pericardial ultrasound showing a tamponade, a positive blood culture, and hyperkalemia on blood chemistry). Over-resuscitation is a consequence of aggressive fluid management in the face of hypotension, often due to primary pump failure. The pulmonary consequences of over-resuscitation were fatal in 3 patients.
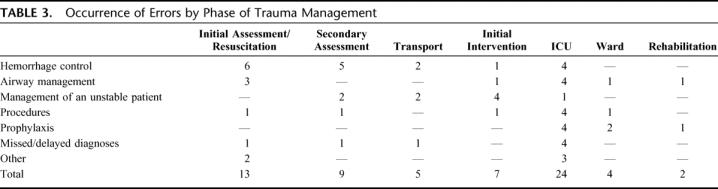
By phase of trauma management, 34% of errors occurred in the ED (20% during initial assessment and resuscitation, 14% during the secondary survey and initial diagnostic tests), 8% during stabilization and interhospital transport, 11% during initial interventions (surgery and/or angiography), 37% during the intensive care unit stay, and 9% during the general or rehabilitation ward inpatient stay (Table 3).
TABLE 3. Occurrence of Errors by Phase of Trauma Management
Error types and causes are shown in Table 4. By type of error, 61% were errors of treatment, 20% were errors of prophylaxis, 13% were errors of diagnosis, 5% were errors associated with transfer, and only 1 was a result of equipment failure. By the internal processing classification of cause, 23% were input errors, 50% were intention errors, and 27% were execution errors.
TABLE 4. Categorization of Type and Cause, by Major Categories of Error
Figures 2 and 3 show when in the phases of trauma management errors occurred, by type and cause, respectively. Errors of treatment predominated; however, diagnostic errors were particularly evident in the secondary survey and ICU phases, and errors of prophylaxis were most evident after the initial management was completed, in the ICU and post-ICU phases. While half of all errors were intention errors and occurred throughout the hospital stay, input errors and exe-cution errors were particularly prominent in the ICU phase of care, and to a lesser extent, the initial assessment and resuscitation phase.
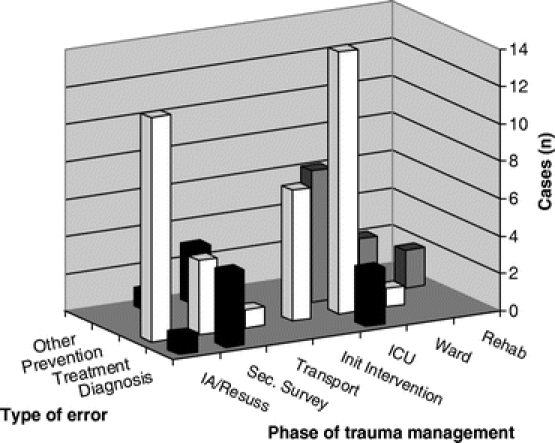
FIGURE 2. Type of error by phase of trauma management.
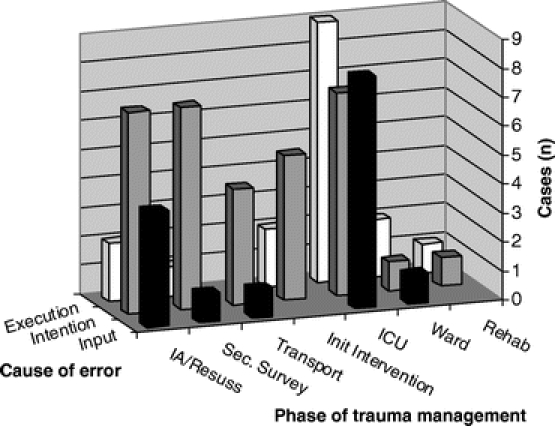
FIGURE 3. Internal processing cause of error, by phase of trauma management.
Intention errors of treatment predominated, as shown in Figure 4. Treatment errors were also caused, to a lesser extent, by input and execution errors. Diagnostic errors were equally contributed to by input and intention errors, and prevention errors were most often either intention or execution errors.
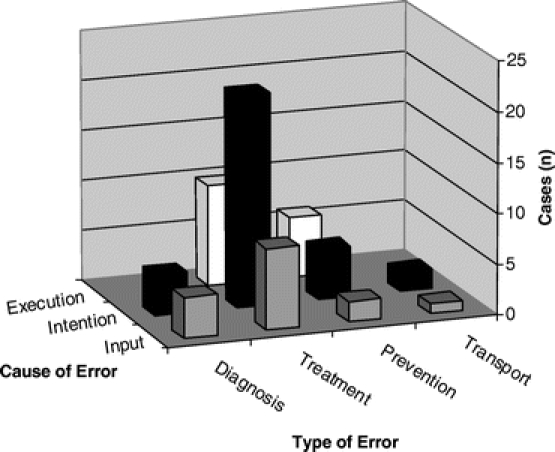
FIGURE 4. Relationship between error cause and error type. (NB Excludes 1 equipment based error.)
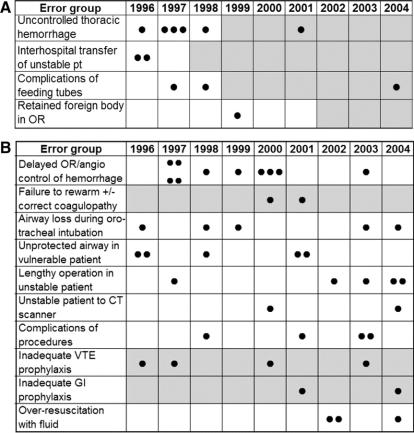
The effect of policy initiatives on error reduction is shown in Figure 5. Institutional policies were implemented in 4 of the 14 categories during the study period: prevention of acute retained hemothorax by insertion of a second ipsilateral chest tube in patients who drain more than 10 mL/kg of blood through their first chest tube; establishment of a transfer center to coordinate all transfers to HMC and to ensure communication with a surgical attending prior to transfer; surgical consult prior to radiologic placement of percutaneous feeding tubes in patients with previous abdominal surgery and contraindication of jejunostomy feeding tubes in patients with open abdomens;30 and mandatory plain abdominal x-ray at the end of every laparotomy to identify retained instruments or packs. The incidence of error-related deaths was reduced after each new policy implementation. Three additional error groups had management policies that existed throughout the study period (active rewarming of hypothermic patients,31 and DVT and gastrointestinal stress ulcer prophylaxis32) but which did not prevent all errors. A new policy to prevent overaggressive resuscitation was instituted at the end of this study period, and there has been inadequate time to determine the effect of this intervention. No specific institutional policies had been developed in the remaining 5 categories, in which occasional sporadic error-related deaths occurred during the study period.
FIGURE 5. A, Effect of institutional policies on error-associated deaths: new policy implemented during study period. Dot represents occurrence of an error-associated death. Gray squares indicate time when a relevant institutional protocol was in place. B, Effect of institutional policies on error-associated deaths: no new policy implemented during study period. Dots represent occurence of an error-associated death. Gray squares indicate time when a relevant institutional protocol was in place.
DISCUSSION
This study addressed the types and nature of errors that contribute to trauma deaths and the integration of error detection into an institutional patient safety program. We have demonstrated that, even in mature trauma systems, errors still occasionally lead to patients' deaths. Among 44,401 admissions and 2594 deaths over 9 years, 2.47% of deaths at our institution were contributed to by errors. This is among the lowest reported preventable death rate in trauma patients. Despite increasing numbers and increasing complexity of cases at our institution, our findings compare favorably with the 23% pedestrian and bicycle fatalities regarded as potentially preventable 18 years ago.10 As others have suggested, a 2% to 3% error-related death rate may be an absolute baseline in complex trauma systems.
Like many studies, we have shown that the initial assessment, resuscitation, and initial intervention phases are particularly error prone. Similar to other studies, we have demonstrated a considerable number of errors in the critical care phase as well. Therefore, error-reduction strategies must address decision making in both the ED and ICU.
This study is likely to assist error reduction in 3 important ways. The first is through identification of specific categories of errors that may be targeted. The most commonly identified groups related to airway and hemorrhage control, the “ABCs” of acute severe trauma management. Several other major error categories were also relatively specific to trauma: inappropriate management of an unstable patient, missed or delayed diagnoses, management of feeding tubes, and over-resuscitation with fluids. We also identified procedure-related errors and inadequate prophylaxis that are not specific to trauma.
The second way this study may help is through considering the type and underlying psychologic cause, which may provide insights into the most useful error-reduction strategies. For example, execution errors are addressed through technical training, and ensuring that those performing the tasks are technically competent and appropriately credentialed. Input errors require clinicians to be aware of potential problems and appropriately use and interpret diagnostic tests. Attention to detail, checklists, and supervision can be effective in reducing both input and execution errors.
The majority of error-associated deaths, however, were intention errors affecting treatment. Indeed, some errors that seemed at first to be execution errors, such as failure to achieve orotracheal intubation leading to anoxic brain injury, may be more appropriately viewed as intention errors, in that one should always be ready to perform a surgical airway in the event of a challenging intubation. Intention errors are greatly reduced by protocols and algorithms that simplify or serve as reminders for particularly complex or time-critical management decisions. The ATLS protocols for early management of severe trauma are an excellent example of effective guidelines. This study raises important questions of what other institutional policies or protocols might be helpful.
The third way in which this study supports error reduction is by demonstrating the likely effectiveness of such evidence-based institutional protocols. In our comparison of major error categories and institutional policies, we instituted new policies that related directly to 4 of the 14 error categories. All 4 policies had emerged after recognition that errors were occurring and that a policy was needed to address them. In all 4, error-associated deaths were less frequent after institution of the policy. Although the numbers of deaths were small, why the 3 other relevant policies that were in effect throughout the study period (venous thromboembolism prophylaxis, gastrointestinal stress ulceration prophylaxis, and active rewarming of hypothermic trauma patients) were not 100% effective at preventing deaths may relate to the disease in question or to the effectiveness of the policies or the care they prescribe. It is important to realize that deaths, while the most serious outcome, are just the tip of the iceberg of morbidity associated with errors, and for every prevented death there are probably many patients for whom nonfatal single or multiple organ failure is averted.
Error reduction cannot be solely attributed to the implementation of policies or protocols, however. Changes in staffing, training, equipment, supervision, and any number of other reasons are likely to also affect error occurrence. Of course, the effect of looking repeatedly for recurrent problems or errors may itself contribute to a reduction in errors through a Hawthorne-type effect of enhanced awareness. Yet by examining the types of errors that occur with some frequency as to constitute a repetitive pattern, we are forced to consider what protocol and policy options might affect real improvement. We have recently recognized overly aggressive resuscitation as a problem causing 3 deaths in the past 3 years. To minimize the risk of overresuscitation, in 2005 a new protocol was instituted that required early invasive central venous monitoring in the ED, and clear guidelines to limiting fluids, beginning inotropic agents, and obtaining rapid control of bleeding. Challenges emerging from this study that are yet to be addressed include reducing deaths associated with delays to the operating room or angiography for abdominopelvic hemorrhage, employing surgical or other airway maneuvers when faced with a challenging airway, and ensuring damage control principles are followed when necessary.
Furthermore, it is generally regarded that protocols alone are insufficient to consistently change behavior. Protocols and clinical guidelines are most effectively implemented when interactive and multifaceted approaches are used to facilitate learning through rehearsal of decision-making strategies.33 This is achieved in the ATLS program through combinations of lectures, skills stations, and moulage scenarios. Others have used video-taped trauma resuscitations as effective teaching tools.34,35 This study therefore also raises questions about how protocols can be best implemented and what sustained and ongoing strategies will be most effective in ensuring protocol adherence in the dynamic, real-time stresses of an academic trauma center.
Our strategy for identifying error-associated deaths combined standard weekly M&M review, trauma registry data predicting survivability, and multiple levels of independent peer review; however, it was not a comprehensive survey of all errors in hospital. Instead, it focused on errors that were associated with the most serious consequence, death. Its design and methodology are likely to have influenced the errors we detected. We did not find large numbers of technical errors in the operating room, large numbers of missed diagnoses, or many drug-related deaths, for example. It is possible that these were few in number or that they didn't lead to death. It is also possible that our strategy was not particularly sensitive for their detection. Like any approach, it is unlikely to have identified all errors in care associated with deaths.
Just as Dr. Hiram Polk reminded us in his 2004 American Surgical Association Presidential Address of the importance of transparency and trust in patient safety,20 we have tried to learn from errors, develop error-reducing environments and systems, and avoid “punitive” approaches to error reduction. Focusing on events with serious outcomes, detecting cases by both self-report and routine review of registry data, using a longitudinal approach to detect patterns over time, and subjecting potential errors to multiple levels of peer review have been important parts of our strategy. Tracking the effects of corrective interventions completes the loop and supports the continued resources required.
This study combines contemporary understanding of error causation, classification, and remediation with an institution specific process that is sensitive to institutional structure and operations. The process uses existing systems and is goal-oriented in seeking out patterns of errors, which can then be targeted. The process is likely to be relevant to other institutions and may be as applicable in other surgical disciplines as it is in trauma.
ACKNOWLEDGMENTS
The authors thank James Holmes, MD, Ram Nirula, MD, Marie Crandall, MD, Matthew Rosengart, MD, Jason London, MD, and Garth Utter, MD who contributed to the data collection and initial error analysis while trauma fellows at Harborview Medical Center, and Kier Warner who also assisted with data collection.
Discussions
Dr. Hiram C. Polk, Jr. (Louisville, Kentucky): I think the key piece in this presentation was that algorithm that showed 64 preventable deaths among 44,000 patients. And that gets into a problem we have in surgery right now. In fact, while everybody is concerned about quality outcome, most of our care is very, very good and you are going to end up with very small numbers of adverse events, and this issue of studying small numbers and make something out of it is a real problem.
The decision to use surrogates or performance measures, for example, in the SCIP project for Medicare connecting those surrogates to outcomes that you are concerned about is another whole other issue. The tyranny of small numbers shows up here in a fairly significant way. I think you have done the best possible job to focus upon a significant number of cases and try to analyze them in this way.
I thought the comments about the typology of errors, including when and where they occur, is very useful. I don't know what you can say about the policy changes because of the tremendous outgoing Hawthorne effect with that, as you know, and doctors and nurses will pay attention to something and then not sustain the gains made.
This paper is a reminder of things which most good trauma centers know in that: 1) unstable patients are unstable and they seldom need testing in a dark room; 2) preexisting blood loss is always more deceptive than that which occurs in their own shoes, and 3) that A * B * C has the “A” first because it is so easy to mismanage the airway, especially with the epidemic of obesity, a tremendous problem of just technical access to airway there.
I do think people tend to say that perfection is the enemy of good. I would tell you in surgery right now we are a long way from perfection but closing in on it.
The key question is: what do you do about feedback to your level 3 trauma centers, the ERs around your area, and the EMS people which feed you those patients? That is something you want to try not to bruise feelings, and it would probably be a real value to share some of your ideas about that.
This is important work. Congratulations on it. I think it will be widely quoted.
Dr. Russell L. Gruen (Seattle, Washington): Thank you for your insightful comments, Dr. Polk. The Hawthorne effect may be one reason that protocols are effective. To reduce errors, protocols are intended to serve as reminders for what we think is best practice care, especially in stressful situations such as trauma resuscitations, for which the heightened awareness that defines the Hawthorne effect is beneficial.
Feedback following error occurrence is a vital part of closing the quality assurance loop. Our approach is not punitive, it is educational, and it occurs at multiple levels. The responsible committee, such as the Trauma Council or the M&M Committee, first writes to the head of the relevant department or a specific individual to ask them to look into the error. It is important that individuals who erred feel they have learned from their errors, particularly if they are junior with many years of practice ahead. And finally, Harborview Medical Center is actively involved in coordinating educational conferences at institutional and regional level to focus on things that we have identified as being problems. We term these “Lessons Learned” conferences. This is particularly where feedback to the EMS services and to other trauma centers actually takes place.
Dr. Thoralf M. Sundt (Rochester, Minnesota): This is a terrific paper and shows how much progress we can make when we get beyond the issue of fear of using the word “error.” And I congratulate you for that. I have 2 questions.
The first relates to your methodology and the concern over recognized versus unrecognized errors. Mark DeLeval's work with human factors experts in the cardiac surgical operating theater demonstrated how often there are errors that are unrecognized by the practitioners. My question, therefore, relates to your dependence on self-reporting. Are you confident that you really captured all of the errors derived from the mortality and morbidity conferences? For example, when you went back to the practitioners, the front-line individuals, how often were they surprised, for example, that you judged fluid resuscitation to be excessive and they thought, “Gosh, everything went well.” I think that the practicing clinician missing the error is not a rare event.
The second question relates to error management as opposed to error prevention. It is a characteristic of high reliability organizations that errors can be captured and managed. And I just wonder if you have thought about how you might go about tracking near misses that were captured and managed well.
Dr. Russell L. Gruen (Seattle, Washington): Thank you, Dr. Sundt. There is no perfect strategy for uncovering errors, but it is useful to consider 2 broad approaches. One is self-reporting by practitioners who recognize they or a colleague has erred. The other is routine case review, often involving a sample of cases over a period of time. We have tried to do both in this study to give us maximum likelihood of detecting errors. Nonetheless, there may be categories of errors that are underrepresented in part because of our methodology. For example, we were surprised that there weren't more operating room errors, misdiagnoses, or medication errors, and it is possible that our strategy was not particularly sensitive to them.
For practical reasons, we chose death as the outcome of interest, and then we worked back to detect errors. We felt there could be no argument that the outcome was important, and it suited both the M&M and the case review strategies. Especially when a patient dies, I think it prompts many people involved in their care to think about whether anything could have been done differently. Even if feedback was not available immediately, early and constructive feedback was often given at the weekly M&M conference, where the error could be discussed in the context of the whole case, and peer review sought.
We did not examine “near misses” because case findings would have been considerably more difficult and potentially imprecise, and analysis of potential errors much more complex. I'd expect that detecting near-misses would rely more on self-report, unless routine data collection were very comprehensive and very sophisticated methods of case review were undertaken. Yet I suspect the messages in this study are as relevant to near misses as they are to deaths, particularly as far as implementing protocols is concerned.
Dr. David B. Hoyt (San Diego, California): Nice presentation, Dr. Gruen. Some of these had to be attributed potentially to attendings, some to residents. What is your spin on your results based on going forward in terms of in-house surgeons, in-house attendings to supervise the ICU and resuscitation phase? Also, was there any impact over this period of time in the 80-hour workweek?
Dr. Russell L. Gruen (Seattle, Washington): Thank you, Dr. Hoyt, especially given that your team in San Diego has been a leader in this area of research. In this study, and as a general policy, we did not attempt to assign error to individuals, just find solutions to prevent future error occurrence. We did not have data to examine issues of supervision or the impact (positive or negative) of work hour limitations.
Dr. Philip S. Barie (New York, New York): Given that, error analysis, root cause analysis, shows more errors actually to be system errors than human errors. But among the human errors, one thing that we haven't heard discussed yet is failure of communication, which is actually prominent in the airline industry when fatal errors occur. So how did you parse your human errors as to skill deficit, knowledge deficit, for the very important but as yet unmentioned factor of communication failure?
Dr. Russell L. Gruen (Seattle, Washington): That is an excellent question, Dr. Barie, and one that we wrestled with for some time. We also felt that communication errors were important, including inadequate or erroneous communication of patient care issues. The American College of Surgeons, too, has recognized this and has included communication as a separate category of error in its monograph on patient safety. In our analysis, we looked for communication errors, but clearly our strategy, and the data we used, was not very sensitive for their detection. Communication could have been part of the problem in many errors, but they also could be defined in other ways that seemed more robust from the data. Therefore, they ended up all being categorized into other groups.
Dr. A. Brent Eastman (San Diego, California): Congratulations to Dr. Gruen and his colleagues from Harborview once again for teaching us all about trauma systems: in this case, preventable deaths. In San Diego, we saw preventable deaths rate fall from 22% to zero to 1% beginning in 1983 incorporating much of what you have done.
I think part of the significance of this paper is the fact that we will be moving toward regionalization of not only trauma surgery but emergency surgery in this country. Given the nocturnal phenomena of “specialty-penia,” that is, the difficulty of getting consultations at night, this is going to be critical. And again, the Harborview people have been very, very instrumental in leading that, and I congratulate them.
I have one question. Does your medical examiner attend your systemwide quality studies? They often have the last word on preventable deaths.
Dr. Russell L. Gruen (Seattle, Washington): Thank you, Dr. Eastman, for your question and all your previous work in this area. When an autopsy has been done, the medical examiners' reports are always available and routinely searched for in death reviews, but the medical examiner infrequently attends the actual meetings.
Dr. Anna M. Ledgerwood (Detroit, Michigan): Just to follow up on that question. On this study of deaths, I never heard the word “autopsy.” What percent of your patients had autopsy including toxicology? Were there any errors with medications or errors identified in the area of pain management?
Dr. Russell L. Gruen (Seattle, Washington): Thank you, Dr. Ledgerwood. In terms of the autopsy rates, Seattle is no different to most other cities in that we are having to deal with declining autopsy rates, from 67% in 1995 to 26% in 2004 among trauma deaths at our institution. It is largely an issue of funding. Obviously, this impacts on death reviews in some cases where the actual circumstances or cause of death is not clear.
We, too, were particularly interested in medication errors, and yet the only ones we found that clearly caused death were a few errors in prophylaxis of DVT or stress ulceration. The small number of medication error-associated deaths suggests that these errors were uncommon and infrequently fatal, which may in part be due to the fact that the ward pharmacist attends ICU rounds daily, and consults on medication use.
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): Historically, the injury to admission interval has had an important role in survival of injured patients. Were you able to identify a similar relationship in your study?
Secondly, you mentioned the ATLS course. Could errors be associated with having taken or not taken the ATLS course?
You mentioned that 1 of 20 deaths were due to exceed fluid and that you have taken steps to change that. Just how has that been changed? Lastly, when you do give less fluid, what is your index for adequacy of resuscitation?
Dr. Russell L. Gruen (Seattle, Washington): Thank you, Dr. Pruitt. We demonstrated that errors were associated with interhospital transfers, particularly unstable patients who died during or soon after transport. Obviously, a level I trauma center's role is to receive unwell patients from other centers, and it appears that the introduction of a “transfer center” to coordinate these arrivals has helped to reduce deaths in transit.
The ATLS program is a program I have had the privilege to be involved with for a long time. We have a very active ATLS program at Harborview Medical Center, and we believe ATLS protocols help reduce both anxiety and subsequent error in acute trauma scenarios. The work of surgeons such as Jameel Ali shows the benefit of ATLS on trauma management and trauma outcomes, although none of us has focused on errors and ATLS explicitly.
Lastly, we guide early resuscitation and avoid over-hydration with central venous pressure monitoring initially, using a protocol from the Large Scale Collaborative Research Program (Glue Grant) Guidelines for Shock Resuscitation. In hypotensive patients or those with a base-deficit of 6 or more, we insert a central venous line in the ER and infuse crystalloid or blood to push the central venous pressure up to 15. If the patient is still in shock, evident by hypotension or persistent base deficit, we assess myocardial function and rule out tamponade with an echo or a pulmonary artery catheter, and then provide additional cardiovascular support with vasopressors or inotropes.
Footnotes
Reprints: Gregory J. Jurkovich, MD, Department of Surgery, Harborview Medical Center, Box 359796, 325 Ninth Avenue, Seattle, WA 98104. E-mail: jerryj@u.washington.edu.
REFERENCES
- 1.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–376. [DOI] [PubMed] [Google Scholar]
- 2.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients: results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RM, Runciman WB, Gibberd RW, et al. The quality in Australian Health Care Study. Med J Aust. 1995;163:458–471. [DOI] [PubMed] [Google Scholar]
- 4.Famularo G, Salvini P, Terranova A, et al. Clinical errors in emergency medicine: experience at the emergency department of an Italian Teaching Hospital. Acad Emerg Med. 2000;7:1278–1281. [DOI] [PubMed] [Google Scholar]
- 5.Zollinger R. Traffic injuries: a surgical problem. AMA Arch Surg. 1955;70:694–800. [DOI] [PubMed] [Google Scholar]
- 6.Cales RH, Trunkey DD. Preventable trauma deaths: a review of trauma care systems development. JAMA. 1985;254:1059–1063. [DOI] [PubMed] [Google Scholar]
- 7.Shackford SR, Hollingworth-Fridlund P, Cooper GF, et al. The effect of regionalization upon the quality of trauma care as assessed by concurrent audit before and after institution. J Trauma. 1986;26:812–820. [DOI] [PubMed] [Google Scholar]
- 8.Shackford SR, Mackersie RC, Hoyt DB, et al. Impact of a trauma system on outcome of severely injured patients. Arch Surg. 1987;122:523–527. [DOI] [PubMed] [Google Scholar]
- 9.Shackford SR, Hollingsworth-Fridlund P, McArdle M, et al. Assuring quality in a trauma system—the Medical Audit Committee: composition, cost, and results. J Trauma. 1987;27:866–875. [PubMed] [Google Scholar]
- 10.Rivara FP, Maier RV, Mueller BA, et al. Evaluation of potentially preventable deaths among pedestrian and bicyclist fatalities. JAMA. 1989;261:566–570. [PubMed] [Google Scholar]
- 11.Davis JW, Hoyt DB, McArdle MS, et al. An analysis of errors causing morbidity and mortality in a trauma system: a guide for quality improvement. J Trauma. 1992;32:660–666. [DOI] [PubMed] [Google Scholar]
- 12.Michel P, Quenon JL, de Sarasqueta AM, et al. Comparison of three methods for estimating rates of adverse events and rates of preventable adverse events in acute care hospitals. BMJ. 2004;328:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walshe K. Adverse events in health care: issues in measurement. Qual Health Care. 2000;9:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington DC: National Academy Press, 2000. [PubMed] [Google Scholar]
- 15.Chang A, Schyve PM, Croteau RJ, et al. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17:95–105. [DOI] [PubMed] [Google Scholar]
- 16.National Quality Forum. Standardizing a Patient Safety Taxonomy. Washington, DC: National Quality Forum, 2006. [Google Scholar]
- 17.Helmreich RL, Musson DM, Sexton JB. Human factors and safety in surgery. In: Manuel BM, Nora P, eds. Surgical Patient Safety: Essential Information for Surgeons in Today's Environment. Chicago: American College of Surgeons, 2004:5–18. [Google Scholar]
- 18.Reason J. Human error: models and management. BMJ. 2000;320:768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reason J. Safety in the operating theatre. Part 2: Human error and organisational failure. Qual Saf Health Care. 2005;14:56–60. [PMC free article] [PubMed] [Google Scholar]
- 20.Polk H. Quality, safety, and transparency. Ann Surg. 2005;242:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polk H, Birkmeyer JD, Hunt D, et al. Quality and safety in surgical care. Ann Surg. 2006;243:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackersie RC, Rhodes M. Patient safety in trauma care. In: Manuel BM, Nora P, eds. Surgical Patient Safety: Essential Information for Surgeons in Today's Environment. Chicago: American College of Surgeons, 2004:61–78. [Google Scholar]
- 23.Committee on Trauma of the American College of Surgeons. Resources for Optimal Care of the Injured Patient. Chicago: American College of Surgeons, 1999. [Google Scholar]
- 24.Thomas EJ, Lipsitz SR, Studdert DM, et al. The reliability of medical record review for estimating adverse event rates. Ann Intern Med. 2002;136:812–816. [DOI] [PubMed] [Google Scholar]
- 25.O'Neil AC, Petersen LA, Cook EF, et al. Physician reporting compared with medical-record review to identify adverse medical events. Ann Intern Med. 1993;119:370–376. [DOI] [PubMed] [Google Scholar]
- 26.Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method. Trauma Score and the Injury Severity Score. J Trauma. 1987;27:370–378. [PubMed] [Google Scholar]
- 27.West TA, Rivara FP, Cummings P, et al. Harborview assessment for risk of mortality: an improved measure of injury severity on the basis of ICD-9-CM. J Trauma. 2000;49:530–541. [DOI] [PubMed] [Google Scholar]
- 28.Reason JT. Understanding adverse events: the human factor. In: Vincent C, ed. Clinical Risk Management: Enhancing Patient Safety. London: BMJ Books, 2001:6–30. [Google Scholar]
- 29.Feliciano D, Moore E, Mattox K. Trauma damage control. In: Moore EE, Feliciano D, Mattox KL, eds. Trauma. New York: McGraw-Hill, 2004:877–900. [Google Scholar]
- 30.Holmes JH, Brundage SI, Yuen P, et al. Complications of surgical feeding jejunostomy in trauma patients. J Trauma. 1999;47:1009–1012. [DOI] [PubMed] [Google Scholar]
- 31.Gentilello LM, Cobean RA, Offner PJ, et al. Continuous arteriovenous rewarming: rapid reversal of hypothermia in critically ill patients. J Trauma. 1992;32:316–317. [DOI] [PubMed] [Google Scholar]
- 32.Maier RV, Mitchell D, Gentilello L. Optimal therapy for stress gastritis. Ann Surg. 1994;220:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grimshaw J, Eccles M, Tetroe J. Implementing clinical guidelines: current evidence and future implications. J Contin Educ Health Prof. 2004;24:31–37. [DOI] [PubMed] [Google Scholar]
- 34.Hoyt DB, Shackford SR, Fridland PH, et al. Video recording trauma resuscitations: an effective teaching technique. J Trauma. 1988;28:435–440. [DOI] [PubMed] [Google Scholar]
- 35.Townsend RN, Clark R, Ramenofsky ML, et al. ATLS-based videotape trauma resuscitation review: education and outcome. J Trauma. 1993;34:133–138. [DOI] [PubMed] [Google Scholar]