Abstract
Objective:
To review the outcomes in 130 consecutive remedial explorations for primary hyperparathyroidism.
Summary Background Data:
Remedial surgery for primary hyperparathyroidism is challenging and requires meticulous preoperative evaluation and imaging to expedite a focused surgical exploration that has traditionally been performed under general anesthesia. This prospective series of 130 consecutive remedial operations for primary hyperparathyroidism selectively used minimally invasive techniques and tested the hypothesis that these techniques could improve outcomes.
Methods:
Between 1990 and 2005, 1090 patients were evaluated and explored for primary hyperparathyroidism. Of these, 130 remedial explorations were performed in 128 patients who underwent either conventional exploration under general anesthesia (n = 107) or minimally invasive parathyroidectomy (n = 23) employing cervical block anesthesia, directed exploration, and curative confirmation with the rapid intraoperative parathyroid hormone assay.
Results:
The sensitivity of preoperative imaging were: Sestamibi (79%), ultrasound (74%), MRI (47%), CT (50%), venous localization (93%), and ultrasound guided parathyroid fine needle aspiration (78%). The cure rate in the conventional remedial group (n = 107) was 94% and was associated with a mean length of stay of 1.6 ± 0.2 days. Remedial exploration employing minimally invasive techniques (n = 23) resulted in a cure rate of 96% and a mean length of stay of 0.4 ± 0.1 days. Complications were rare in both remedial groups. These results were almost identical to those achieved in 960 unexplored patients.
Conclusions:
Remedial parathyroid surgery can be accomplished with acceptable cure and complication rates. Minimally invasive techniques can achieve outcomes that are similar to those obtained in unexplored patients.
A total of 130 remedial cervical explorations were performed in 128 patients for primary hyperparathyroidism. The overall success rate was 95%, and minimally invasive remedial surgery was performed in 23 patients.
The first successful operation for primary hyperparathyroidism was performed by Felix Mandl in Vienna in 1925.1 Unfortunately, the patient (Albert J.) developed recurrent disease 6 years postoperatively and ultimately died of recalcitrant hypercalcemia. In the United States, the saga of Captain Charles Martell is well characterized, having undergone 6 unsuccessful parathyroid explorations ultimately to be cured by the 7th procedure that resulted in his postoperative death due to sepsis and hypocalcemia.2,3 Fortunately, modern surgical series, in previously unexplored patients, are characterized by initial success rates exceeding 95% with few complications. Furthermore, in a large number of centers, primary hyperparathyroidism is now successfully treated as an outpatient procedure under local or regional anesthesia.4–7
Despite the success rate enjoyed in the modern era, parathyroid surgeons still encounter 3 groups of patients representing challenging management issues:
Patients with persistent primary hyperparathyroidism having failed initial exploration;
Patients who develop recurrent primary hyperparathyroidism having sustained a period of eucalcemia for greater than 6 months after their initial operation;
Patients who have undergone previous neck explorations, particularly total thyroidectomy, who then develop primary hyperparathyroidism.
In each of these settings, remedial cervical explorations are associated with decreased success rates and increased complication rates.8,9 The normal tissue planes are obliterated in the postoperative neck making identification and preservation of delicate structures including the recurrent laryngeal nerve difficult. Accordingly, patients who require remedial exploration for primary hyperparathyroidism represent a challenging subset of individuals. The data presented represent a single surgeon experience over 15 years in 130 consecutive remedial surgical explorations for primary hyperparathyroidism. Several trends appear to be emerging in the remedial setting, including utilization of the techniques used in minimally invasive parathyroidectomy (MIP).
MATERIALS AND METHODS
Between January 1990 and August 2005, 1090 patients were evaluated and explored for primary hyperparathyroidism. We have previously reported a subset of these patients (n = 656) with primary hyperparathyroidism.4 The current series represents patients who underwent remedial cervical exploration from a larger database. Data were collected prospectively and encompassed demographic information, symptoms and signs, biochemical data, imaging, operative, and pathologic findings as well as follow-up information. All historical information, including biochemical and imaging data, as well as operative and pathologic findings, were carefully reviewed. In all cases, the biochemical diagnosis was confirmed and the indications for surgery were reevaluated. These included measurements of serum parathyroid hormone (PTH), calcium, and creatinine. In most cases, a 24-hour urinary excretion of calcium was measured. A detailed family history was obtained to screen for familial hypocalciuric hypercalcemia (FHH), MEN1, MEN2A, isolated familial hyperparathyroidism, and other rare hypercalcemic syndromes.10 Preoperative imaging was used in all patients who required remedial cervical exploration. All imaging studies were reviewed and were available in the operating room. These patients then underwent either conventional exploration under general anesthesia or more recently, in select patients, MIP, employing cervical block anesthesia, image-based directed exploration with curative confirmation obtained in the operating room employing the intraoperative rapid PTH assay.4 In the majority of remedial cervical explorations, the previous Kocher incision was reincised and a lateral approach was used. This approach mobilizes the plane between the carotid sheath and the strap muscles permitting exposure through operative planes that are generally less scarred than the more typical medial approach. The rapid intraoperative PTH assay was first used in 1998 and immediately became a standard procedure for all parathyroid surgery. The utility of this assay was further enhanced in 2002 when its use was extended to perform ex vivo aspiration of the resected specimens to prove that they represented parathyroid glands, thereby obviating the utility of intraoperative frozen section consultation. In addition, in every case, drawings were performed in the operating room demonstrating the site of the abnormal parathyroid gland(s) and other relevant structures. Postoperative calcium levels were obtained at the first follow-up visit (3–5 days) and long-term follow-up data were requested in all cases.
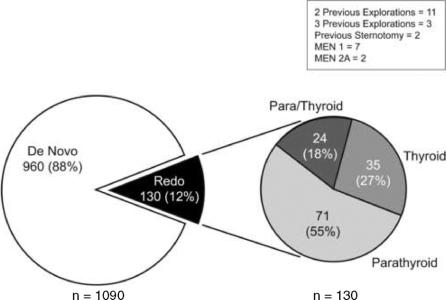
RESULTS
A total of 130 remedial explorations were performed in 128 patients and form the basis for this study. As shown in Table 1, previous parathyroid surgery, often combined with thyroid resections had been performed in 95 cases. Many of these patients had undergone 2 (n = 11) or 3 (n = 2) previous parathyroid explorations. This group included 7 patients with MEN1. A total of 35 patients had undergone previous thyroid resection either for thyroid cancer (n = 4) or benign (n = 31) conditions. The thyroid procedures included 12 total thyroidectomies and 23 lobectomies. Two of these patients had papillary carcinoma and 2 had medullary carcinoma of the thyroid in the setting of MEN2A. The groups are combined and simplified for pictorial representation in Figure 1. Patients who underwent remedial parathyroid surgery, but had other cervical procedures including tracheostomy (n = 3), brachial cleft cyst resection (n = 1), thyroglossal duct cyst resection (n = 1), or anterior cervical fusion (n = 1) were excluded from this analysis, although all were cured during their second cervical exploration. The symptoms and signs in these patients are demonstrated in Table 2. Many had more than one symptom or sign; however, a large percentage had demonstrable evidence of bone disease (57%) or nephrolithiasis (32%). Five patients presented in hypercalcemic crisis. In addition neurocognitive, gastrointestinal, and cardiovascular disease was present in a large proportion of the patients.
TABLE 1. Previous Procedures
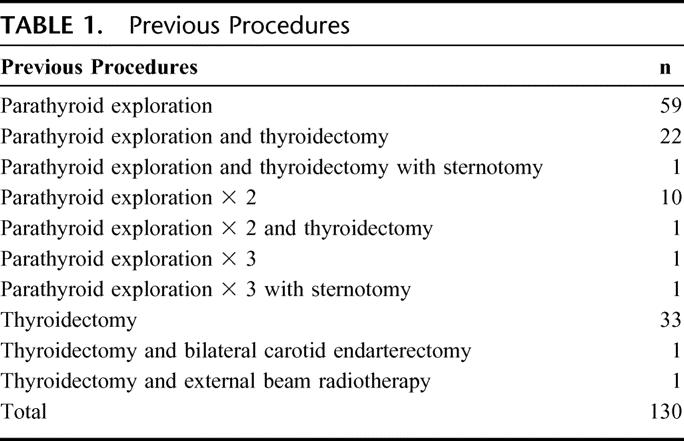
FIGURE 1. Categories of patients who underwent remedial (Redo) cervical exploration for primary hyperparathyroidism. “De novo” represents previously unexplored patients.
TABLE 2. Symptoms and Signs

Preoperative localization was performed in all patients in this remedial series (Fig. 2). The majority underwent sestamibi scanning, using single photon emission computed tomography. A smaller number underwent ultrasound, MRI, CT, venous localization, or ultrasound combined with fine needle aspiration (FNA) of suspected enlarged parathyroid glands. Many patients had more than one localization study. Sestamibi scanning was the most commonly performed imaging procedure and yielded a positive finding in 79% (66 of 90) of patients.11 Ultrasound also had a high yield of 74% (21 of 32), although it was performed in a smaller number of patients. MRI (16 of 34) and CT (10 of 20) scans had similar sensitivities of 47% and 50%, respectively. The most accurate localization study was angiography/venous localization; this was informative in 93% (13 of 14) of patients.12 Its use was limited due to its cost and invasive nature. Ultrasound guided FNA of the suspected parathyroid gland was also an effective technique and was positive in 78% (7 of 9) patients. We have recently used on site PTH analysis for both venous localization and ultrasound guided parathyroid FNA as this adjunct offers immediate feedback to the imaging team and appears to expedite the study thereby improving the accuracy.12 All of these patients underwent focused operations based upon the preoperative imaging data.
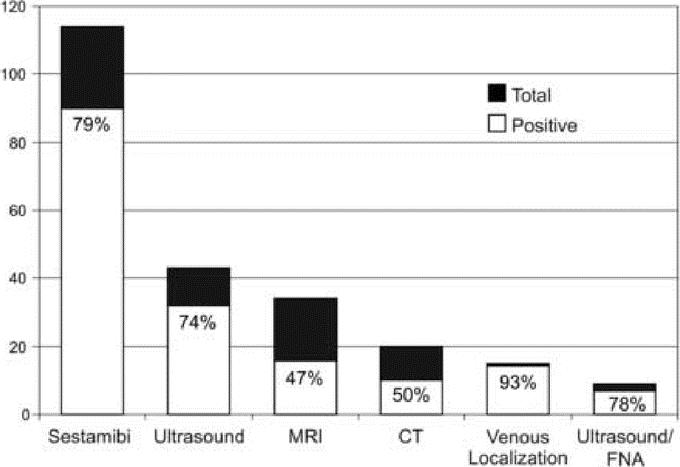
FIGURE 2. Results of preoperative localization studies in patients who underwent remedial cervical exploration for primary hyperparathyroidism.
The majority of operations (n = 107) employed conventional exploration under general endotracheal anesthesia. However, a subset (n = 23, 18%) underwent MIP. Patients were selected for MIP based upon their imaging studies as well as the individual patient's motivation to avoid general anesthesia.
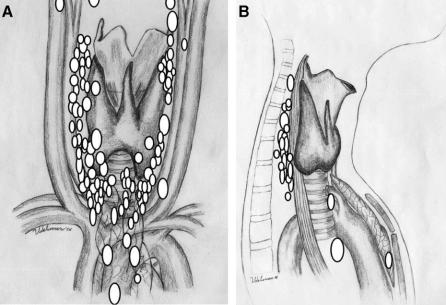
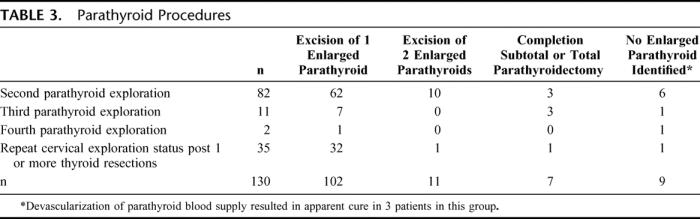
The locations of the enlarged parathyroid glands found at remedial exploration are demonstrated on either an anterior-posterior (Fig. 3A) or lateral (Fig. 3B) projection depending on which image was most relevant. The majority of glands were found in eutopic positions along the lateral surfaces of the thyroid gland, the tracheoesophageal grooves or in the thyro-thymic ligament. Ectopic glands were found in the retroesophageal space (n = 23), mediastinal thymus (n = 9), thyroid (n = 11), carotid sheath (n = 4), undescended in the submandibular space (n = 1), or in the aortopulmonary window (n = 1). The operative procedures are demonstrated in Table 3. The majority of patients [102 (78%)] had a single enlarged parathyroid gland resected. Eleven patients had 2 enlarged parathyroid glands resected and in 7 cases, in the setting of multigland hyperplasia, a subtotal parathyroidectomy (n = 4) or total cervical parathyroidectomy with immediate heterotopic parathyroid autotransplantation (n = 3) was performed. In 9 patients enlarged parathyroids were not identified in spite of extensive exploration. In these cases, ligation of the blood supply to the presumed missing parathyroid gland was performed and in 3 cases there was a dramatic reduction in the intraoperative PTH levels. Long-term follow-up in these patients demonstrated durable cures with serum calcium levels of 10.1 mg/dL (19 months), 9.2 mg/dL (39 months), and 9.6 mg/dL (46 months).
FIGURE 3. Location of enlarged parathyroid glands resected during remedial cervical exploration. Because of overlapping glands, the parathyroid glands are not drawn to scale. Some patients had more than one enlarged gland. Individual glands are depicted on one illustration only. A, Anteroposterior projection. B, Lateral projection.
TABLE 3. Parathyroid Procedures
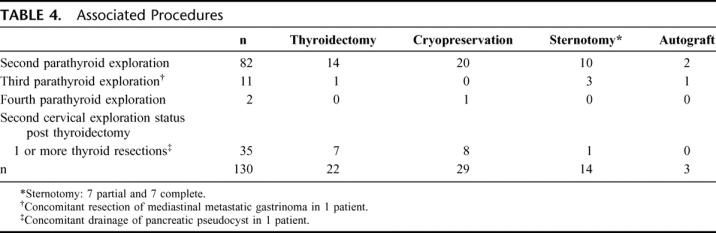
In addition, to parathyroid resection, these patients frequently underwent associated procedures as shown in Table 4. These included thyroid lobectomy (n = 22), parathyroid cryopreservation (n = 29), partial sternotomy (n = 7), complete sternotomy (n = 7), and immediate heterotopic parathyroid autografting to the brachioradialis muscle (n = 3).
TABLE 4. Associated Procedures
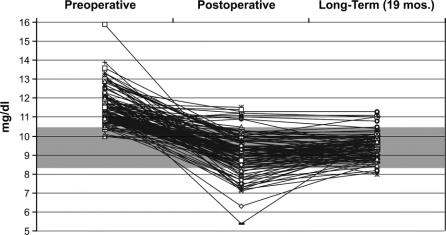
The levels of serum preoperative (n = 130), postoperative (n = 130), and long-term calcium (n = 102) levels are depicted in Figure 4. Most patients presented with elevated preoperative serum calcium levels, however, a subset (n = 9) had serum calcium levels that were at or within the upper limits of the reference range (8.4–10.5 mg/dL). All of these patients had significant symptoms and signs of primary hyperparathyroidism and had serum PTH levels that were inappropriately elevated relative to their serum calcium levels. In the early postoperative period (3–7 days), there were 9 patients who had postoperative hypercalcemia. In 2 of these cases, the serum calcium normalized once the patients stopped taking exogenous calcium supplementation. Many patients had hypocalcemia in the early postoperative period (3–7 days). In all but 4 cases, these levels returned to the normal range in long-term follow-up. The mean long-term follow-up for the entire group was 19 months.
FIGURE 4. Preoperative (n = 130), postoperative (n = 130), and long-term (n = 102) serum calcium levels in patients who underwent remedial cervical exploration for primary hyperparathyroidism. The shaded area represents the reference range for serum calcium (8.4–10.5 mg/dL). Preoperative levels were obtained 1 week prior to surgery. Postoperative levels were obtained 3 to 7 days postoperatively. The mean long-term follow-up for the entire group was 19 months.
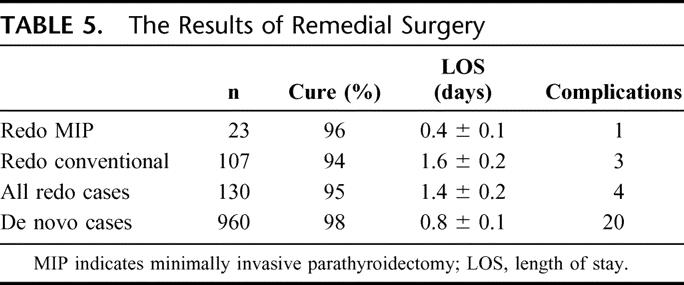
The results of remedial surgery are demonstrated in Table 5 and are compared with a concurrent group of 960 patients that underwent de novo exploration (no prior history of cervical exploration). The cure rates of 96% and 94% for redo MIP and redo conventional exploration, respectively, compare favorably with the 98% cure rate achieved in the concurrent group of previously unexplored patients. Similarly, the mean length of stay (LOS) of 0.4 ± 0.1 day (redo MIP) and 1.6 ± 0.2 days (redo conventional) were also attractive compared with the unexplored (de novo) group of 0.8 ± 0.1 day.
TABLE 5. The Results of Remedial Surgery
There were 7 failed remedial explorations in 6 patients. Four of these patients had multigland hyperplasia, 1 of which was in the setting of MEN2A following total thyroidectomy for medullary carcinoma of the thyroid. One patient had 4 enlarged cervical parathyroid glands removed but has persistent disease from an occult supranummary gland.
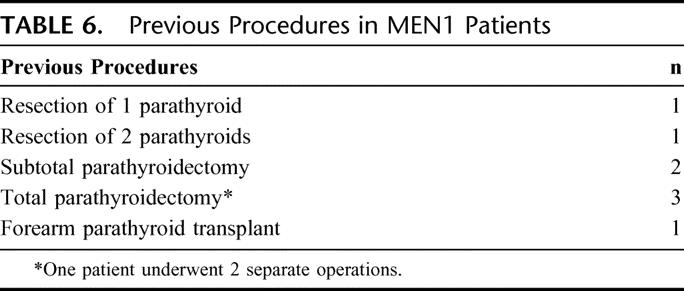
There were 7 patients with MEN1 who underwent remedial exploration for persistent (n = 3) or recurrent (n = 4) primary hyperparathyroidism. The previous operations are demonstrated in Table 6. At reexploration, 2 patients underwent resection of parathyroid glands in eutopic positions. Five patients underwent resection of ectopic parathyroid glands in the carotid sheath (n = 1), thyroid (n = 1), or mediastinum (n = 3). The mediastinal glands required sternotomies (1 redo sternotomy) for resection of a low intrathymic parathyroid gland, aorta-pulmonary parathyroid gland with pericardial involvement, and a thymic parathyroid gland that was attached to a 7-cm metastatic mediastinal gastrinoma. Cryopreservation was used in all of these cases, and all had normalization of their postoperative serum calcium levels.
TABLE 6. Previous Procedures in MEN1 Patients
Long-term follow-up calcium levels were obtained in 102 patients with a mean follow-up for the entire series of 19 months. Long-term recurrences occurred in 2 patients at 13 and 17 months, respectively, after an initially successful remedial exploration. Both of these patients had multigland hyperplasia and are being managed nonoperatively with mild hypercalcemia.
There were 2 patients with MEN2A who had undergone a previous total or partial thyroidectomy. At reexploration, one patient underwent a completion thyroidectomy, bilateral modified radical neck dissections, and curative redo parathyroidectomy with cryopreservation. The other MEN2A patient failed remedial exploration that included an extensive cervical exploration with median sternotomy with resection of a 60-mg parathyroid gland.
Minimally invasive parathyroidectomy was performed in 23 patients who required remedial cervical exploration for primary hyperparathyroidism. There were 13 females and 10 males with a mean group age of 63 years. Their previous procedures included parathyroid exploration (n = 10), staged thyroid resection and parathyroid exploration (n = 5), thyroid lobectomy (n = 6), total thyroidectomy (n = 1), and 1 patient who had undergone 3 separate thyroid resections. The mean preoperative calcium of 11.34 ± 0.15 mg/dL normalized to 8.99 ± 0.16 mg/dL following successful resection of a single parathyroid gland in 22 of these patients. One patient failed reexploration despite extending the exploration bilaterally. Twelve of these procedures were performed on an outpatient basis, and none has recurred with a mean follow-up of 12 months. One recurrent nerve injury occurred in this group.
There were 4 complications in 130 consecutive remedial procedures for an overall complication rate of 3.1%. These complications included 3 permanent recurrent nerve injuries and one mild stroke.
DISCUSSION
This series of 130 remedial cervical explorations for primary hyperparathyroidism confirms important well-established principles and demonstrates several new trends in parathyroid surgery. Recurrent or persistent primary hyperparathyroidism is not uncommon in the setting of multigland parathyroid hyperplasia,13 and remedial parathyroid exploration is more difficult and more expensive than de novo cases.14
All patients who are evaluated for remedial cervical exploration for primary hyperparathyroidism benefit from a carefully performed history and physical examination with detailed review of the previous operative and pathologic findings. The diagnosis should be unequivocally confirmed and the indications for surgery carefully reevaluated. Imaging procedures are routinely performed in these patients. Most patients with persistent or recurrent primary hyperparathyroidism who are reexplored ultimately prove to have their disease in their neck; or if it is present in their mediastinum, it is usually accessible by a cervical approach.8,9
Newer trends have also emerged. It has been stated that one should not operate upon these patients unless at least 2 preoperative imaging studies are both positive and concordant.8 We and others15 have not adhered to this principle. The NIH group suggested that ultrasound and sestamibi scans may be adequate preoperative localization in a subset of patients who require reoperation for parathyroid adenomas. We have accepted a positive sestamibi scan alone as adequate imaging when it is convincing and rational based upon previous operative and pathologic findings.
Ultrasound-guided parathyroid FNA has been reported as a localization technique for remedial parathyroid surgery.8,16 The addition of on-site PTH analysis is extremely helpful as it yields real-time feedback to the ultrasonographer, thereby confirming whether a suspected lesion is in fact a parathyroid gland. Ultrasound-based procedures are particularly attractive due to their low cost and morbidity.
Minimally invasive techniques are appropriate in a subset of patients who require remedial cervical exploration. The concept of a directed image-guided surgical approach is an outgrowth of the strategy used during remedial exploration. Operative adjuncts are routinely used to improve the adequacy of resection, thereby offering intraoperative evidence of cure. We and others have relied on the intraoperative rapid PTH assay.4,5,7 This technique appears to result in improved success rates in some but not all series.17,18 It has been suggested that more stringent criteria should be considered in the reoperative setting.19 Although some groups have used sestamibi-based intraoperative gamma probes to help localize parathyroid glands in the reoperative setting, we have not used this technique.20
There is a subset of patients in whom parathyroid glands are not identified during remedial exploration. In this setting, extensive, bilateral explorations are performed to search for parathyroid glands in eutopic and ectopic sites, including the retroesophageal space, thymus gland, carotid sheaths, and submandibular region for undescended glands. If the occult gland is still not identified, additional intraoperative adjuncts are used, including ultrasound, and bilateral internal jugular vein sampling to determine if an ipsilateral PTH gradient is present.21 This technique has guided us to explore upstream and locate occult undescended or partially descended glands. Partial or complete thyroid lobectomy is performed depending on the suspected location of the missing gland. If an aggressive cervical approach to the anterior and posterior mediastinum fails to reveal the culprit parathyroid gland, a partial or complete median sternotomy is considered.22
Despite all of these maneuvers, there still remains a subset of patients in whom the elusive parathyroid is not identified. In this setting, ligation of the blood supply to the missing parathyroid gland is performed. This usually involves ligation of the ipsilateral inferior thyroid artery but may also involve devascularization of other arterial branches. The concept of this technique is based upon the fact that the parathyroid gland has an end-organ blood supply that usually emanates from a single arterial branch as initially suggested by Sandström23 and subsequently proven by Halsted and Evans.24 In this series, this technique resulted in curative salvage of 3 patients who would have otherwise failed remedial exploration.
Surgeons who perform reoperative parathyroid surgery must consider the possibility that the resected parathyroid gland may represent the only residual functioning parathyroid tissue. Under these circumstances, immediate heterotopic parathyroid autotransplantation and cryopreservation may be appropriate. These patients are at risk for postoperative hypocalcemia that, at times, can be life-threatening. Although we have successfully performed outpatient remedial parathyroid surgery, it is only offered to select patients where we are confident that adequate parathyroid tissue resides in situ.
The most advantageous time to cure primary hyperparathyroidism is during the first surgical exploration.25 It is the obligation of the initial surgeon to perform a meticulous exploration evaluating both eutopic and ectopic sites. It is imperative that, if the surgeon is unable to locate an enlarged parathyroid gland, normal glands should not be removed and their blood supply should not be compromised. When normal glands are resected during unsuccessful parathyroid exploration, the patient is placed at risk for postoperative hypoparathyroidism during subsequent explorations. Although this lesson was first learned with Captain Martell, it continues to plague parathyroid surgeons and their patients.
ACKNOWLEDGMENTS
The nurses, endocrinologists, operative assistants, and house officers contributed to the care of every one of these patients. The authors thank Dotty Franco for her expert assistance in the completion of this manuscript.
Discussions
Dr. Quan-Yang Duh (San Francisco, California): I congratulate Dr. Udelsman for this outstanding paper. It sets the standard of treatment for this challenging group of parents who require reoperative parathyroid surgery. These 130 cases come from the extensive experience of a single endocrine surgeon of more than a thousand. The high success rate of 95% and low complication rate is extraordinary, and they are indistinguishable from the results of first-time parathyroid exploration.
This paper also showed even for reoperation, minimally invasive parathyroidectomy was possible in 23 of these patients. Although the term “minimally invasive parathyroidectomy” has been used to describe several types of parathyroid operations, in this paper it means an image-directed, focused parathyroidectomy, performed under local-regional anesthesia. This is a significant achievement, since prior to this paper it is usually assumed such approach can only be used for first-time parathyroidectomy. I have 5 questions for Dr. Udelsman.
Question 1: What are the selection criteria or exclusion criteria for minimally invasive reoperative parathyroidectomy? How do they differ from those of first-time parathyroidectomy? Is it important that the area of anticipated reexploration has not previously been explored? Did you only do it in those who have previous limited parathyroid exploration or thyroid lobectomy in the contralateral side of the neck?
Question 2: How useful was intraoperative PTH monitoring? In the subgroup of 23 patients who had minimally invasive parathyroidectomy, 22 had successful resection of a single tumor. PTH monitoring would not have changed their outcome. The 1 remaining patient had persistent disease despite extensive exploration. So intraoperative PTH monitoring did not change the outcome in any of the 23 patients who underwent minimally invasive parathyroidectomy. In the conventional surgery group, however, 22% had multiple tumors removed. How accurate was intraoperative PTH monitoring in predicting these multiple tumors?
Question 3: How many of the parathyroid tumors removed were upper glands and how many were lower glands? I noticed in your beautiful illustrations that most of the tumors were posterior and thus were probably upper glands. Were there more upper gland tumors in this series? If there were, why? As you know, there is a well-defined trap that can cause persistent disease. The radiologist misinterprets a low-lying upper parathyroid tumor as a lower gland. The surgeon then performs a focused exploration, removing the lower gland, and misses the true upper gland tumor. How often did you see such cases in your series?
Question 4: Where and how do you ligate the inferior thyroid artery when you cannot find the tumor? You had 3 patients who were cured after you ligated the inferior thyroid artery without finding the tumor. Since the inferior thyroid artery arborizes and have collateral vessel communicating with the superior thyroid artery, do you need to tie off the main inferior thyroid artery and the various branches?
Lastly, do you do your own neck ultrasound now? Only a few patients had ultrasonography in your series compared with Sestamibi scan. Many endocrine surgeons now perform neck ultrasound in the office and in the operating room. Do you think this will change your strategy on what localization studies to use in these patients?
Dr. Robert Udelsman (New Haven, Connecticut): Dr. Duh, thank you for your insightful questions. The first question was to define the selection criteria for minimally invasive parathyroidectomy in the remedial setting. There are 2 criteria. The first is imageable disease. And the second is a motivated patient. Those are the only criteria.
The fact is that, in the group of 23 patients who underwent minimally invasive parathyroidectomy in the remedial setting, 13 had undergone extensive previous bilateral explorations, 4 of which also included simultaneous thyroidectomies. Two of the patients had undergone previous total thyroidectomies. A subset, had undergone limited parathyroid explorations, and 6 had undergone thyroid lobectomies. Therefore, more than half the patients had undergone previous extensive surgery. It is not the extent of previous surgery that determines eligibility, it is whether we have adequate localization and if the patient is motivated.
The second question you asked was about intraoperative PTH monitoring. As you know, we use it routinely. You are absolutely correct, in the minimally invasive remedial group, the 22 patients who were cured would have been cured with or without the assay. We found the lesion, we took it out and the intraoperative PTH fell. What it did do was inform us that the patient was cured, thereby allowing to us to terminate the exploration.
More importantly is the group with occult multigland disease. That is where the assay is most useful. The surgeon believes the patient is cured following removal of the Sestamibi identified lesion, but the fact is that occult disease is still present. This occurred in 8 patients where the assay suggested additional disease. In some cases, we had already closed the patient; however, based on the assay, we immediately reexplored and found additional disease. Therefore, 8 patients benefitted from the assay.
The next question you asked was the location of upper versus lower parathyroid glands. There were 72 upper glands and 69 lower glands, for a total of 141 glands in 130 patients. You are correct; there were 23 retroesophageal glands confirming the fact that this location is often overlooked during initial exploration. As you know, that is an easy place to explore using the lateral approach.
You addressed the issue of devascularization when one cannot locate the suspected adenoma. This concept was first developed by Sandström, who defined the parathyroid blood supply. However, it was Halsted and Evans who injected the inferior thyroid artery and showed that each parathyroid gland had a single end artery blood supply. So after an extensive exploration when we can't find a single gland, we ligate the ipsilateral main trunk of the inferior thyroid artery.
There are arborizations to the superior thyroid artery, but by that point we have already devascularized most if not all of the branches.
Your last question was about ultrasound. I do not routinely perform the ultrasound myself. It is a good technique and I encourage surgeons to do it. We do it in the operating room and our ultrasonographers perform preoperative ultrasounds when requested.
Dr. Robert M. Beazley (Boston, Massachusetts): Dr. Udelsman, I congratulate you on a splendid series and your remarkable 95% cure rate. No doubt, this series reflects your long experience with this disease and your meticulous technique and intuitive surgical judgment, which are both part of the success equation. I feel it is quite fitting that Paper Committee included your paper in this Boston meeting because the first reoperative parathyroidectomy was performed in this city. I note that both Dr. Churchill and Dr. Cope were members of this organization, and that when Dr. Churchill began to do parathyroid surgery he sent Dr. Cope to the anatomy lab and the autopsy room to study the location of the parathyroids. We might miss fewer glands if younger surgeons were to adopt Dr. Cope's approach.
I was impressed with your Sestamibi localization results, but would like to know what you do when all tests are nonlocalizing or when there is discordance between results? Also, which patients are not candidates for the minimal procedure? Lastly, your manuscript prompted me to review the 1975 NCI report by Beazley, Costa, who I understand is now Vice Chief of Pathology at Yale, and Alf Ketcham. I was pleased to see that you still use the “lateral approach” and that from time to time employ Dr John Doppman's arteriography and selective venous assay for localization. However, I was not so pleased with our results compared with yours. I am proud that endocrine surgeons, and you in particular, working diligently, have made very significant improvements over the past 30 years in the management of persistent hyperparathyroidism.
Lastly, in light of yesterday's discussion concerning “competence and the surgeon's age,” in redo surgery, I feel surgical volume is important regardless whether or not the operator can still manage to see 5-0 Prolene suture!
Dr. Robert Udelsman (New Haven, Connecticut): Thank you, Dr. Beazley, for those fine comments. And I appreciate your earlier work from NCI.
What do we do in the remedial setting when we have noninformative noninvasive preoperative imaging? We move on to invasive studies, including arteriography, venous sampling, and ultrasound guided samples. Hopefully, we find something along the way. But what do we do at the end of the day when all studies are negative? It is a judgment call. I do not like exploring patients in the remedial setting if I have no idea what the lesion is. The only time I will do that is when the previous operation was grossly inadequate. But if an experienced surgeon could not find disease I have to ask what am I going to do to be successful? If the symptoms and signs are significant, we may be forced to operate.
Otherwise, I might elect to follow the patients on medical therapy. The next question is: who is not a candidate for minimally invasive parathyroidectomy? First of all, all of our unexplored patients with primary hyperparathyroidism who do not appear to have multigland disease are candidates for minimally invasive parathyroidectomy. That applies to 85% of our patients. Contraindications include patients who cannot cooperate during an operation. If they won't lie still on the operating table, I will not put their recurrent nerves at risk. There are also patients who refuse to have a local procedure. However, the sicker they are, the more logical it is to use minimally invasive techniques.
The lateral approach described by yourself and Ketcham is a wonderful technique because you can avoid scared tissue planes.
As you indicated, these are difficult operations. It is not just technical skills; it is sound judgment in the operating room that will result in optimal outcomes.
Dr. Gerard M. Doherty (Ann Arbor, Michigan): My 1 question is about intraoperative nerve monitoring. Do you ever use that in the reoperative patients and does that enter into your judgment to do them under local? The need for nerve monitoring enter into your judgment to perform the operation under local anesthesia?
Dr. Robert Udelsman (New Haven, Connecticut): Our patients are awake, and yes, we occasionally use nerve monitoring when we use general anesthesia. I don't find it very useful.
Footnotes
Reprints: Robert Udelsman, MD, MBA, Yale University School of Medicine, P.O. Box 208062, New Haven, CT 06520-8062. E-mail: Robert.udelsman@yale.edu.
REFERENCES
- 1.Mandl F. Therapeutisher versuch bein falls von ostitis fibrosa generalisata mittles. Extirpation eines epithelkörperchen tumors. Wien Klin Wochenschr Zentral. 1926;143:245–284. [Google Scholar]
- 2.Carney JA. The glandulae parathyroideae of Ivar Sandström. Am J Surg Pathol. 1996;20:1123–1144. [DOI] [PubMed] [Google Scholar]
- 3.Bauer W, Albright F, Aub JC. A case of osteitis fibrosa cystica (osteomalacia?) with evidence of hyperactivity of the parathyroid bodies: a metabolic study. J Clin Invest. 1930;8:228–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irvin GL III, Sfakianakis G, Yeung L, et al. Ambulatory parathyroidectomy for primary hyperparathyroidism. Arch Surg. 1996;131:1074–1078. [DOI] [PubMed] [Google Scholar]
- 6.LoGerfo P. Bilateral neck exploration for parathyroidectomy under local anesthesia: a viable technique for patients with coexisting thyroid disease with or without sestamibi scanning. Surgery. 1999;126:1011–1015. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Mack E, Starling JR. A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: which is most reliable? Ann Surg. 2005;242:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaskowiak N, Norton JA, Alexander HR, et al. A prospective trial evaluating a standard approach to reoperation for missed parathyroid adenoma. Ann Surg. 1996;224:308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beazley RM, Costa J, Ketcham AS. Reoperative parathyroid surgery. Am J Surg. 1975;130:427–429. [DOI] [PubMed] [Google Scholar]
- 10.Carling T, Udelsman R. Parathyroid surgery in familial hyperparathyroid disorders. J Intern Med. 2005;257:27–37. [DOI] [PubMed] [Google Scholar]
- 11.Civelek A, Ozalp E, Donovan P, et al. Prospective evaluation of delayed Tc-99m sestamibi SPECT scintigraphy for preoperative localization of primary hyperthyroidism. Surgery. 2002;131:149–157. [DOI] [PubMed] [Google Scholar]
- 12.Udelsman R, Aruny JE, Donovan PI, et al. Rapid parathyroid hormone analysis during venous localization. Ann Surg. 2003;237:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivlen MH, Bartlett DL, Libutti SK, et al. Reoperation for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery. 2001;130:991–998. [DOI] [PubMed] [Google Scholar]
- 14.Doherty GM, Weber B, Norton JA. Cost of unsuccessful surgery for primary hyperparathyroidism. Surgery. 1994;116:954–957. [PubMed] [Google Scholar]
- 15.Feingold DL, Alexander HR, Chen CC, et al. Ultrasound and sestamibi scan as the only preoperative imaging tests in reoperation for parathyroid adenomas. Surgery. 2000;128:1103–1110. [DOI] [PubMed] [Google Scholar]
- 16.MacFarlane MP, Fraker DL, Shawker TH, et al. Use of preoperative fine-needle aspiration in patients undergoing reoperation for primary hyperparathyroidism. Surgery. 1994;116:959–964. [PubMed] [Google Scholar]
- 17.Irvin GL III, Molinari AS, Figueroa C, et al. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999;229:874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebag F, Shen W, Brunaud L, et al. Intraoperative parathyroid hormone assay and parathyroid reoperations. Surgery. 2003;134:1049–1055. [DOI] [PubMed] [Google Scholar]
- 19.Thompson GB, Grant CS, Perrier ND, et al. Reoperative parathyroid surgery in the era of sestamibi scanning and intraoperative parathyroid hormone monitoring. Arch Surg. 1999;134:699–705. [DOI] [PubMed] [Google Scholar]
- 20.Norman J, Denham D. Minimally invasive radioguided parathyroidectomy in the reoperative neck. Surgery. 1998;124:1088–1093. [DOI] [PubMed] [Google Scholar]
- 21.Norton JA, Shawker TH, Jones BL, et al. Intraoperative ultrasound and reoperative parathyroid surgery: an initial evaluation. World J Surg. 1986;10:631–639. [DOI] [PubMed] [Google Scholar]
- 22.Gold JS, Donovan PI, Udelsman R. Partial median sternotomy: an attractive approach to mediastinal parathyroid disease. World J Surg. In press. [DOI] [PubMed]
- 23.Sandström. Om en ny körtel hos menniskan och atskilliga däggdjur. Ups Läk Forh. 1880;15:441–471. [Google Scholar]
- 24.Halsted WS, Evans HM. The parathyroid glandules: their blood supply, and their preservation in operation upon the thyroid gland. Ann Surg. 1907;46:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caron NR, Sturgeon C, Clark OH. Persistent and recurrent hyperparathyroidism. Curr Treat Opt Oncol. 2004;5:335–345. [DOI] [PubMed] [Google Scholar]