Abstract
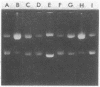
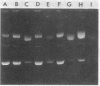
Dnacin B1 preferentially inhibited the incorporation of [3H]thymidine into acid-insoluble fractions in Escherichia coli. At a sublethal concentration, dnacin B1 caused filamentous growth in E. coli and induced prophage lambda. The antibiotic also showed potent bactericidal activity against repair-deficient E. coli strains, such as recA, recB, and polA strains, In in vitro studies, dnacin Ba raised the melting temperatures of various double-stranded DNAs. In addition, the antibiotic showed DNA-cleaving activity against PM2 DNA in the presence of reducing agents, and the activity was suppressed by scavengers for oxygen free radicals and an iron-specific chelator, desferrioxamine E. The stimulation of the generation of superoxide radical by dnacin B1 was confirmed by measuring the reduction of neotetrazolium. Therefore, it can be presumed that the primary cellular target of dnacin B1 is DNA in susceptible cells, and the autooxidation of DNA-bound dnacin B1 causes the generation of oxygen-free radicals that result in the damage of DNA and the inhibition of its synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- Canellakis E. S., Fico R. M., Sarris A. H., Shaw Y. H. Diacridines - double intercalators as chemotherapeutic agents. Biochem Pharmacol. 1976 Jan 15;25(2):231–236. doi: 10.1016/0006-2952(76)90304-x. [DOI] [PubMed] [Google Scholar]
- Cone R., Hasan S. K., Lown J. W., Morgan A. R. The mechanism of the degradation of DNA by streptonigrin. Can J Biochem. 1976 Mar;54(3):219–223. doi: 10.1139/o76-034. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L. H., Petrusek R. Proposed structure of the anthramycin-DNA adduct. Nature. 1979 Nov 29;282(5738):529–531. doi: 10.1038/282529a0. [DOI] [PubMed] [Google Scholar]
- Hurley L. H. Pyrrolo(1,4)benzodiazepine antitumor antibiotics. Comparative aspects of anthramycin, tomaymycin and sibiromycin. J Antibiot (Tokyo) 1977 May;30(5):349–370. doi: 10.7164/antibiotics.30.349. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U. Disulfiram inhibits DNA breakage by hydroxyradical-producing agents. Biochim Biophys Acta. 1980 Aug 26;609(1):75–83. doi: 10.1016/0005-2787(80)90202-6. [DOI] [PubMed] [Google Scholar]
- Lesko S. A., Lorentzen R. J., Ts'o P. O. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980 Jun 24;19(13):3023–3028. doi: 10.1021/bi00554a029. [DOI] [PubMed] [Google Scholar]
- Lloyd R. S., Haidle C. W., Robberson D. L. Bleomycin-specific fragmentation of double-stranded DNA. Biochemistry. 1978 May 16;17(10):1890–1896. doi: 10.1021/bi00603a014. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Joshua A. V. Molecular mechanism of binding of pyrrolo(1,4)benzodiazepine antitumour agents to deoxyribonucleic acid--anthramycin and tomaymycin. Biochem Pharmacol. 1979 Jul 1;28(13):2017–2026. doi: 10.1016/0006-2952(79)90218-1. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Sim S. K., Majumdar K. C., Chang R. Y. Strand scission of DNA by bound adriamycin and daunorubicin in the presence of reducing agents. Biochem Biophys Res Commun. 1977 Jun 6;76(3):705–710. doi: 10.1016/0006-291x(77)91557-1. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Weir G. Studies related to antitumor antibiotics. Part XIV. Reactions of mitomycin B with DNA. Can J Biochem. 1978 May;56(5):269–304. [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Mong S., Strong J. E., Bush J. A., Crooke S. T. Use of covalently closed circular deoxyribonucleic acid for prescreening for antitumor compounds. Antimicrob Agents Chemother. 1979 Sep;16(3):398–405. doi: 10.1128/aac.16.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi M., Tanida S., Asai M., Kishi T. Dnacins, new antibiotics II. Isolation and characterization. J Antibiot (Tokyo) 1980 Dec;33(12):1449–1456. doi: 10.7164/antibiotics.33.1449. [DOI] [PubMed] [Google Scholar]
- Petrusek R. L., Anderson G. L., Garner T. F., Fannin Q. L., Kaplan D. J., Zimmer S. G., Hurley L. H. Pyrrol[1,4]benzodiazepine antibiotics. Proposed structures and characteristics of the in vitro deoxyribonucleic acid adducts of anthramycin, tomaymycin, sibiromycin, and neothramycins A and B. Biochemistry. 1981 Mar 3;20(5):1111–1119. doi: 10.1021/bi00508a011. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., DiMarco A., Zunino F. The interaction of daunomycin with polydeoxynucleotides. Eur J Biochem. 1978 Apr 17;85(2):487–492. doi: 10.1111/j.1432-1033.1978.tb12264.x. [DOI] [PubMed] [Google Scholar]
- Someya A., Tanaka N. DNA strand scission induced by adriamycin and aclacinomycin A. J Antibiot (Tokyo) 1979 Aug;32(8):839–845. doi: 10.7164/antibiotics.32.839. [DOI] [PubMed] [Google Scholar]
- Tanida S., Hasegawa T., Muroi M., Higashide E. Dnacins, new antibiotics. I. Producing organism, fermentation, and antimicrobial activities. J Antibiot (Tokyo) 1980 Dec;33(12):1443–1448. doi: 10.7164/antibiotics.33.1443. [DOI] [PubMed] [Google Scholar]
- Tanida S., Hasegawa T., Yamano T., Yoneda M. Inhibition of coliphage multiplication and R plasmid transfer by desdanine. J Antibiot (Tokyo) 1976 Jul;29(7):754–758. doi: 10.7164/antibiotics.29.754. [DOI] [PubMed] [Google Scholar]
- Ueda K., Morita J., Yamashita K., Komano T. Inactivation of bacteriophage phi X174 by mitomycin C in the presence of sodium hydrosulfite and cupric ions. Chem Biol Interact. 1980 Feb;29(2):145–158. doi: 10.1016/0009-2797(80)90029-0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Thrum H., Pitra C. Binding of analogues of the antibiotics distamycin A and netropsin to native DNA. Effect of chomophore systems and basic residues of the oligopeptides on thermal stability, conformation and template activity of the DNA complexes. Eur J Biochem. 1972 Mar 15;26(1):81–89. doi: 10.1111/j.1432-1033.1972.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Zunino F., Gambetta R., Di Marco A., Zaccara A. Interaction of daunomycin and its derivatives with DNA. Biochim Biophys Acta. 1972 Sep 14;277(3):489–498. doi: 10.1016/0005-2787(72)90092-5. [DOI] [PubMed] [Google Scholar]