Abstract
Objective:
Advantages of thoracoscopic lobectomy for early stage non-small cell lung cancer (NSCLC), as compared with lobectomy by conventional thoracotomy, include less postoperative pain and shorter length of hospitalization. The outcomes after thoracoscopic lobectomy in patients with more complex pulmonary conditions are analyzed to determine safety, efficacy, and versatility.
Methods:
A prospective database of 500 consecutive patients who underwent thoracoscopic lobectomy between June 1999 and January 2006 was queried. Demographic, histopathologic, perioperative, and outcome variables were assessed using standard descriptive statistics and Kaplan-Meier survival analyses.
Results:
Thoracoscopic lobectomy was successfully performed in 492 patients (conversion rate, 1.6%). Pathologic analysis included primary NSCLC in 416 patients (83.2%), centrally located secondary pulmonary malignancy in 37 patients (7.4%), and a variety of benign conditions in 45 patients (9%). Among the 416 patients with NSCLC, pathologic analysis demonstrated stage I in 330 patients (55.3%), stage II in 40 patients (9.6%), and stage III or greater NSCLC in 44 patients (10.6%). The operative and perioperative (30-day) mortality was 0% and 1%, respectively. The overall 2-year survival rate for the entire cohort was 80%, and the 2-year overall survival rates for stage I NSCLC, stage II or greater NSCLC, secondary pulmonary malignancy, and granulomatous disease patients were 85%, 77%, 73%, and 89%, respectively.
Conclusions:
Thoracoscopic lobectomy is applicable to a spectrum of malignant and benign pulmonary disease and is associated with a low perioperative morbidity and mortality rate. Survival rates are comparable to those for lobectomy with thoracotomy.
A review of 500 consecutive patients who underwent thoracoscopic lobectomy at a single institution was performed. Outcome variables included complications and survival. Thoracoscopic lobectomy is a safe, effective, and versatile procedure.
Thoracoscopic, also termed video-assisted thoracoscopic, lobectomy has become accepted as a safe and effective procedure to treat early-stage non-small cell lung cancer (NSCLC).1–10 We have previously reported a pilot series of thoracoscopic lobectomy in 110 early stage lung cancer patients, demonstrating low complication rate and effective short-term oncologic results.1 With increasing experience, the indications for thoracoscopic lobectomy have been expanded. The purpose of this study was to examine the longer-term results of thoracoscopic lobectomy across the spectrum of indications to determine safety, efficacy, and versatility.
METHODS
After obtaining approval from the Institutional Review Board, a prospective database of 500 consecutive patients who underwent thoracoscopic lobectomy between June 1999 and January 2006 was queried. Demographic, histopathologic, perioperative, and outcome variables including overall survival were analyzed. Standard descriptive statistics and Kaplan-Meier survival analyses were used. Because of the possibility that the surgical procedure itself may affect outcome and because many of the patients had benign diagnoses, overall survival was used as an endpoint.
Surgical Technique
Thoracoscopic lobectomy, previously described,11 is performed under general anesthesia with single-lung ventilation, which may be accomplished with either dual-lumen endotracheal tubes or with single-lumen tubes and bronchial blockers. The patient is positioned in full lateral decubitus position with slight flexion of the table at the level of the mid-chest, which allows slight splaying of the ribs to improve exposure in the absence of rib spreading.
Two incisions were used in the majority of patients in this series. The thoracoscope is placed in the 7th or 8th intercostal space in the midaxillary line, and an anterior utility incision is placed in the 5th intercostal space anteriorly (4–5 cm), providing access for hilar dissection. The intercostal spaces are wider in this location, allowing for both better exposure and easier retrieval of specimens. Importantly, rib spreading is not used.
Hilar dissection is carried out through the anterior incision. Dissection of the pulmonary vessels and bronchi is performed in the same manner as in open surgery. Endoscopic linear staplers are used for individual vessel and bronchial ligation. Once the lobe is completely resected, it is placed in a specimen bag for retrieval; this technique avoids implantation of tumor cells into the incision. Mediastinal lymph node dissection is also performed, similar to conventional techniques.9
RESULTS
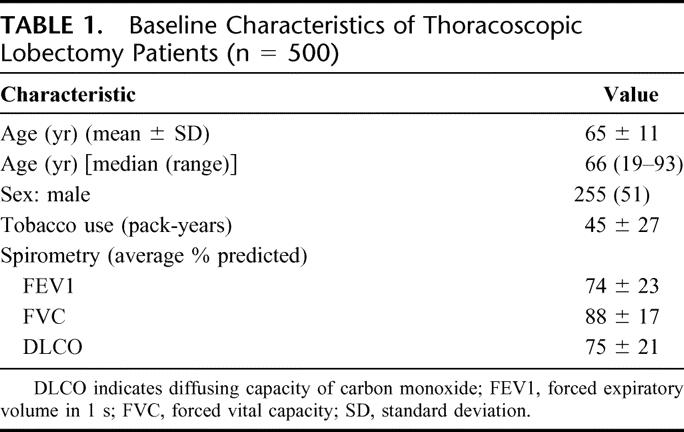
Demographic information is presented in Table 1. Mean age of the 255 men and 245 women was 65 ± 11 years. Median follow-up was 24 months. The mean preoperative FEV1 was 74% ± 22% of predicted, with a range of 21% to 138%. The preoperative indication for surgery was known or suspected NSCLC in 451 patients (90%), secondary malignancy or other etiology in 42 patients (8%), and infection in 7 patients (2%).
TABLE 1. Baseline Characteristics of Thoracoscopic Lobectomy Patients (n = 500)
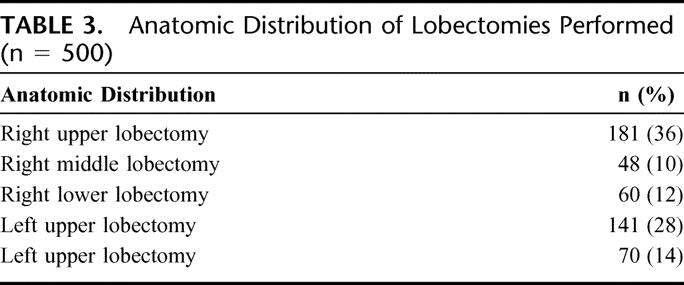
Thoracoscopic lobectomy was successfully performed in 492 of the 500 patients (conversion rate, 1.6%). Of the 8 conversions, 1 was for hemorrhage (controlled thoracoscopically during conversion), 4 for dense hilar adenopathy (benign), 1 for oncologic concerns, and 2 for bronchial complications (Table 2). All conversions were performed with hemodynamic stability and no further sequelae after conversion. Anatomic resections included all lobes; thoracoscopic pneumonectomy was not performed (Table 3).
TABLE 2. Conversions
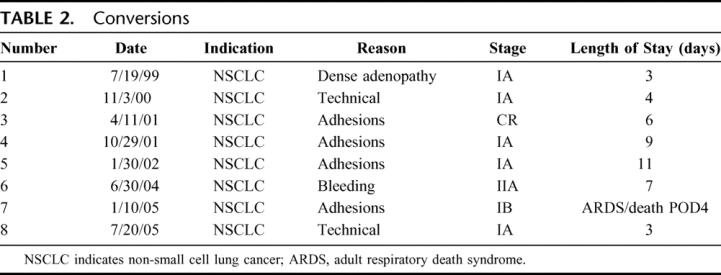
TABLE 3. Anatomic Distribution of Lobectomies Performed (n = 500)
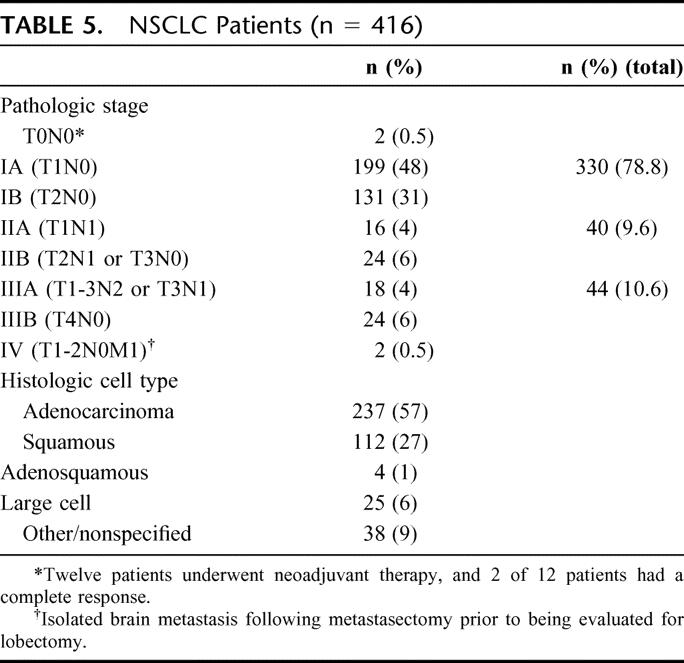
All resections were considered R0: negative surgical margins and all pathologic lymph nodes resected. Pathologic analysis included primary NSCLC in 416 patients (83.2%), centrally located secondary pulmonary malignancy in 37 patients (7.4%), and a variety of benign conditions in 45 patients (9%; Table 4). For the 416 patients with NSCLC, pathologic analysis demonstrated stage I in 330 patients (55.3%), stage II in 40 patients (9.6%), and stage III or greater NSCLC in 44 patients (10.6%; Table 5). The patients with stage IV disease had previously undergone metastasectomy for isolated cerebral metastasis and mediastinoscopy to exclude N2 disease (T1-T2N0M1). The histopathologic distribution is also demonstrated in Table 5.
TABLE 4. Pathologic Analysis: Thoracoscopic Lobectomies (n = 500)
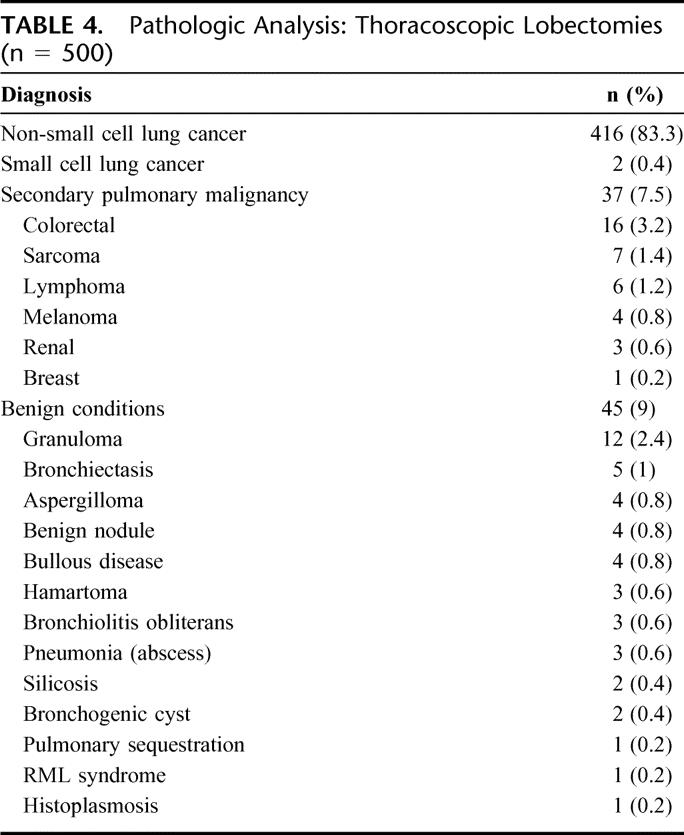
TABLE 5. NSCLC Patients (n = 416)
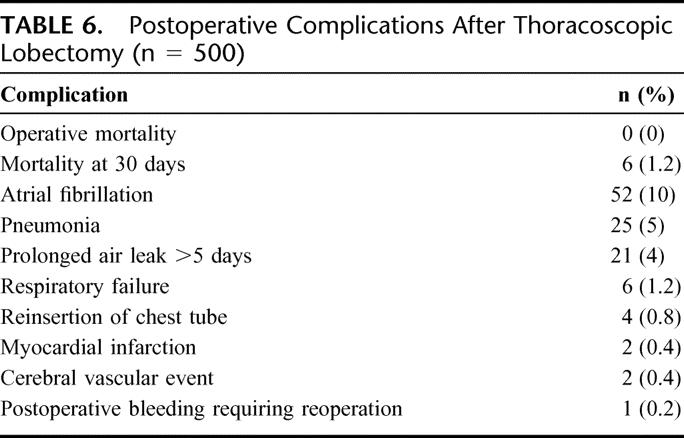
The median chest tube duration was 3 days, and the median length of hospitalization was also 3 days (range, 2–50 days). The operative and perioperative (30-day) mortality were 0% and 1.2% (6 patients), respectively. Postoperative complications (Table 6) included atrial fibrillation (10%) pneumonia (5%), prolonged air leak (4%), and respiratory failure (1.2%). This series included 12 patients who underwent induction chemotherapy for NSCLC, with or without radiation therapy. There was no operative or 30-day mortality in this subset, and the median length of hospitalization was 3 days. In addition, there were no significant differences in postoperative complications between patients with benign or malignant disease.
TABLE 6. Postoperative Complications After Thoracoscopic Lobectomy (n = 500)
The overall 2-year survival rate for the entire cohort was 80%, and the 2-year overall survival rates for stage I NSCLC, stage II or greater NSCLC, secondary pulmonary malignancy, and granulomatous disease or other etiology were 85%, 77%, 73%, and 89%, respectively (Fig. 1).
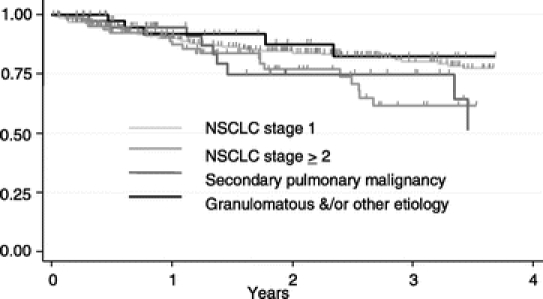
FIGURE 1. Kaplan-Meier analysis of overall survival after thoracoscopic lobectomy by etiology (log-rank P value = 0.099).
DISCUSSION
Advantages of a thoracoscopic approach to anatomic lung resection include decreased blood loss,11,12 decreased pain,12–14 shorter length of stay,10–13 more rapid return to preoperative activity,11–13 improved shoulder function,14 preserved postoperative pulmonary function,15,16 and decreased inflammatory response (which may confer superior immunologic function).17 These benefits are achieved with equivalent oncologic effectiveness.
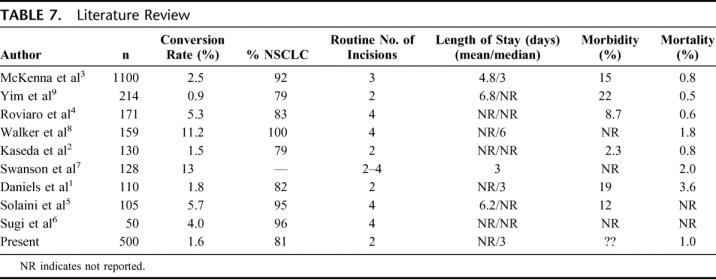
Safety of thoracoscopic lobectomy has been addressed in many studies (Table 7).1–9 In the present series, no patient died intraoperatively. In a large international survey, including 1578 thoracoscopic lobectomies, only 1 patient died intraoperatively, secondary to a myocardial infarction.18 Conversion to open lobectomy occurred in 8 patients (1.6%) in the present series, which is consistent with the conversion rate of 1% to 11% reported in most large series in the literature (Table 7). Perioperative mortality in these series was 1.2%, which is consistent with other thoracoscopic series (Table 7), as well as with published series of lobectomy with thoracotomy.19,20 These results indicate that thoracoscopic lobectomy is safe and efficacious, with results comparable to thoracotomy.
TABLE 7. Literature Review
The oncologic efficacy of thoracoscopic lobectomy has been questioned. Using the procedure as described, utilizing individual vessel dissection and ligation performed through the small utility incision, the same operation is performed thoracoscopically as is performed conventionally via an open approach. This issue was addressed prospectively in Japan by Sugi et al, who randomized 100 patients to either thoracoscopic lobectomy or open lobectomy and found no survival difference.21 Numerous reports from single institutions have reported survival rates for stage IA NSCLC, which are similar to historical figures for open lobectomy.3,4,9
The appropriate surgical management of patients with NSCLC includes accurate staging of the mediastinum.19,22–24 Recent reports documenting disappointing results for noninvasive mediastinal lymph node staging23,24 emphasize the need for adequate mediastinal assessment during definitive resection. Complete mediastinal lymph node dissection may be performed as effectively thoracoscopically as with thoracotomy. While our database did not assess number of lymph nodes examined pathologically, the low rate of mediastinum as the first site of failure (2%) validates the approach.
Increasing experience with thoracoscopic lobectomy has allowed expansion of the indications for lobectomy. Approximately 16% of lobectomies were performed for indications other than NSCLC, representing a larger fraction than some other large series.2,3,25,26 Central metastases and extensive lobar infections make wedge resection difficult, and thoracoscopic lobectomy has become the preferred approach in many of these patients. Despite the inflammation and adhesions involved in resection of granulomatous lung disease, which has caused conversion in up to 15% of these patients,26 none of the conversions in the present series occurred in this subset.
Thoracoscopic lobectomy has also been found to be safe and effective after induction chemotherapy, with or without radiation therapy.27 The present series included 12 patients who underwent induction therapy, with no operative or 30-day mortality. In this series, the thoracoscopic approach was associated with significantly shorter chest tube duration and length of hospitalization, with no difference in survival.
CONCLUSION
Thoracoscopic approach to lobectomy is a safe and effective procedure. While it has historically been applied only to early stage NSCLC, this report demonstrates good results in patients with a spectrum of indications for lobectomy, including patients stage II and III NSCLC, patients treated with induction therapy, patients with central secondary pulmonary malignancy, and patients with granulomatous lung disease. Oncologic outcomes for patients with lung cancer are as equivalent to those published for open lobectomy. Thoracoscopic lobectomy is a safe, oncologically effective, and versatile procedure.
Discussions
Dr. James D. Luketich (Pittsburgh, Pennsylvania): I would like to thank the authors and the Association for the opportunity to review and discuss this important contribution to the literature. And thank you, Dr. Onaitis, for the manuscript prior to this meeting.
The obvious strengths of this review include the excellent surgical results with the low conversion rate of 1.8% and a very low complication rate. Importantly, the authors have demonstrated good oncologic outcomes compared to historical open controls at a median follow-up of 24 months. I have several questions.
Can you comment on how many patients had a mediastinoscopy before VATS lobectomy? And although you mentioned complete mediastinal node dissection, more specifically what was the total mediastinal lymph node count? In your manuscript, you mention this may not be a part of your current database, and I wonder why not, and have you corrected this and are you individually pulling the path reports to assess this, as I think this would enhance your final manuscript. Also, recording oncologic issues, did you note any port site recurrences?
Your conversion rate is commendable and obviously dependent upon 2 issues: 1) surgical experience and 2) surgical selection. Regarding the issue of surgical experience, I note 6 MDs on the author list. Can you elaborate on who actually did the lobectomies thoracoscopically and the percent done by Dr. D'Amico? And were there credentialing criteria to actually perform VATS lobectomy in your institution?
Were there VATS lobectomies performed by other surgeons in your center that not included in this study? And do all the thoracic surgeons in your center now perform VATS lobectomies? Also, during this 6-year period, how many open lobectomies were performed at your institution, and what is the current overall of lobes now performed by VATS versus open?
Can you make any comments to the audience at large on the advisability of a thoracic surgeon considering adding VATS lobectomy to his practice? How might they get started in terms of safety, proctoring courses, and credentialing? My point is that before the average open thoracic surgeon can extrapolate these excellent results, I believe a note of caution is warranted as there is a learning curve and not all surgeons have the same skills necessary to achieve these excellent results in a basic fashion. Of note, Dr. McKenna, in a 2006 paper on VATS lobectomy, stated that only 5% of lobectomies in the United States are performed by VATS, underlining my comments there.
Regarding selections, how do you select cases and were there any absolute contraindications to VATS lobectomy? It seems like a very low number of patients had received neoadjuvant therapy prior to lobectomy, and what is your stance on this at the present time? I note in your reference list you have a separate publication on a series that received neoadjuvant therapy, and are they included here?
Dr. Mark W. Onaitis (Durham, North Carolina): Thank you, Dr. Luketich, and of course we recognize your seminal work in minimally invasive thoracic surgery, particularly esophageal surgery. I will try to answer these questions in order.
In our practice, we perform mediastinoscopy and bronchoscopy on all stage I lung cancer patients. So nearly every patient in this series underwent mediastinoscopy.
Regarding our lymph node dissection, you are right, and we mention in the manuscript that we did not count the lymph nodes in the specimens. That is something that we hope to do in the future. We did go back and pull all the pathology reports, but those didn't make it into the database before we published our paper.
The conversion rate is low. If you look at the literature across large series, it is about 2% to 5%. That probably reflects a learning curve. We think that the conversion rate will stay that low with the expansion of surgeons performing the procedure. We do tend to explore our patients with thoracoscopy often, even if thorascopic lobectomy is unlikely. And we don't consider those necessarily conversions, but we do perform a large amount of thoracoscopic exploration as well.
In terms of surgeons performing the procedure, approximately 80% of the cases were performed by Dr. D'Amico. But all 4 of our attending thoracic surgeons are performing the procedure now. So 80% of the cases approximately at Duke at the present time are thoracoscopic lobectomies, leaving 20% open lobectomies. Three of those surgeons have learned thoracoscopic lobectomy at Duke. One of them was a resident at Duke. All of these cases are performed by the thoracic residents at Duke, and by the time people finish, they are very able to do this procedure on their own.
In addition, Duke, and I know Pittsburgh also, put on several courses a year in order to teach thorascopic lobectomy to surgeons. We feel that these courses are an excellent way to approach people who have not performed thoracoscopic lobectomy in the future to learn thoracoscopic surgeries.
Assessment of the learning curve is difficult. If you look at our database at in our first 110, which I mentioned at the beginning and our last group of patients, there has not been a decrease in the number of conversions. But our convergent rate was low at the beginning. So it is difficult to point out the learning curve in this series, although I think it is steep.
In terms of patient selection, the only absolute contraindications to thoracoscopic lobectomy in our hands have been the need for chest wall resection or calcified lymph nodes that would be difficult to remove from around the hilum.
We have performed segmentectomy, lobectomy, bilobectomy. We also don't perform sleeve or pneumonectomy because we don't feel that these patients benefit from the small incisions. We feel like the outcome is more based on the disease process. We resect tumors up to 6 cm in size, although we have gone up to 9 cm in size. The larger tumor is difficult to get out without rib spreading, which is how we try to achieve all these cases.
In terms of induction therapy, I did mention 12 patients who underwent induction therapy. Those 12 patients were in the series. Dr. Petersen presented that work at the Southern Thoracic Surgical Association. All patients had complete resection after VATS restaging with 1 conversion.
Dr. Antoon Lerut (Leuven, Belgium): I would like to congratulate the authors for a very fine presentation. I have a couple of questions.
The first reflects some concerns about the indication for secondary malignancies. It is well known that you need to very carefully palpate the parenchyma regularly revealing the occurrence of very small metastatic lesions not seen even on high-resolution CT scans. So I wonder what your experience is and how you can achieve this goal thoracoscopically, which I think is still the gold standard in this kind of surgery.
My second question relates to your analgesia policy, in particular, the epidural analgesia, which is the classic type of analgesia in open surgeryd1.
Dr. Mark W. Onaitis (Durham, North Carolina): Regarding the first question, you are right that palpation is difficult with a thoracoscopic lobectomy. With our incision, it is possible to place a finger into the thoracic space and feel for other lesions. But we think, with the combination of advanced scanning as well as learning to palpate the lung using the light weight clamp, that we are able to avoid operating on patients who have disseminated disease.
In terms of analgesia, we work closely with our anesthesiologists. Patients who have a high probability of having a thoracoscopic lobectomy generally do not have epidural anesthesia, whereas those that think there may be a higher chance that they are going to be converted do undergo epidural anesthesia. And, of course, that decision is made between the anesthesiologist and the patient.
Dr. Leslie J. Kohman (Syracuse, New York): My question is, do you exclude any patients from this approach due to obesity?
Dr. Mark W. Onaitis (Durham, North Carolina): That is a good question. We have not excluded patients due to obesity in our series. And we have not had any contraindications due to body type at all in our series.
Footnotes
Reprints: Mark W. Onaitis, MD, Duke University Medical Center, Box 31068, Durham, NC 27710. E-mail: mwo@duke.edu.
REFERENCES
- 1.Daniels LJ, Balderson SS, Onaitis MW, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg. 2002;74:860–864. [DOI] [PubMed] [Google Scholar]
- 2.Kaseda S, Aoki T, Hangai N. Video-assisted thoracic surgery (VATS) lobectomy: the Japanese experience. Semin Thorac Cardiovasc Surg. 1998;10:300–304. [DOI] [PubMed] [Google Scholar]
- 3.McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Ann Thorac Surg. 2006;81:421–426. [DOI] [PubMed] [Google Scholar]
- 4.Roviaro G, Varoli F, Vergani C, et al. Video-assisted thoracoscopic surgery (VATS) major pulmonary resections: the Italian experience. Semin Thorac Cardiovasc Surg. 1998;10:313–320. [DOI] [PubMed] [Google Scholar]
- 5.Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery major pulmonary resections: present experience. Eur J Cardiothorac Surg. 2001;20:437–442. [DOI] [PubMed] [Google Scholar]
- 6.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy reduces cytokine production more than conventional open thoracotomy. Jpn J Thorac Cardiovasc Surg. 2000;48:161–165. [DOI] [PubMed] [Google Scholar]
- 7.Swanson SJ, Herndon J, D'Amico TA, et al. Results of CALGB 39802: feasibility of VATS lobectomy for lung cancer. Proc Am Soc Clin Oncol. 2002;21:290. [Google Scholar]
- 8.Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg. 2003;23:397–402. [DOI] [PubMed] [Google Scholar]
- 9.Yim APC, Izzat MB, Liu H, et al. Thoracoscopic major lung resections: an Asian perspective. Semin Thorac Cardiovasc Surg. 1998;10:326–331. [DOI] [PubMed] [Google Scholar]
- 10.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case control study. Ann Thorac Surg. 1999;68:194–200. [DOI] [PubMed] [Google Scholar]
- 11.Burfeind WR, D'Amico TA. Thoracoscopic lobectomy. Oper Tech Thorac Cardiovasc Surg. 2004;9:98–114. [Google Scholar]
- 12.Suguira H, Morikawa T, Kaji M, et al. Long-tern benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc. 1999;9:403–410. [PubMed] [Google Scholar]
- 13.Guidicelli R, Thomas P, Lonjon R, et al. Video-assisted minithoracotomy versus muscle-sparing thoracotomy for performing lobectomy. Ann Thorac Surg. 1994;58:712–718. [DOI] [PubMed] [Google Scholar]
- 14.Li WWL, Lee RLM, Lee TW, et al. The impact of thoracic surgical access on early shoulder function: video-assisted thoracic surgery versus posterolateral thoracotomy. Eur J Cardiothorac Surg. 2003;23:390–396. [DOI] [PubMed] [Google Scholar]
- 15.Nakata M, Sakei H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2000;70:938–941. [DOI] [PubMed] [Google Scholar]
- 16.Nomori H, Ohtsuka T, Horio H, et al. Difference in the impairment of vital capacity and 6-minute walking after a lobectomy after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an antero-axillary thoracotomy, and a posterolateral thoracotomy. Surg Today. 2003;33:7–12. [DOI] [PubMed] [Google Scholar]
- 17.Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–365. [DOI] [PubMed] [Google Scholar]
- 18.Angellilo-Mackinlay TA. VATS lobectomy: an international survey. IVth International Symposium on Thoracoscopy and Video-Assisted Thoracic Surgery. Sao Paulo, Brazil, 1997. [Google Scholar]
- 19.Allen MS, Darling GE, Pechet TV, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–1020. [DOI] [PubMed] [Google Scholar]
- 20.Ginsberg RJ, Hill LD, Eagan RT, et al. Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg. 1983;86:654–658. [PubMed] [Google Scholar]
- 21.Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy reduces cytokine production more than conventional open thoracotomy. Jpn J Thorac Cardiovasc Surg. 2000;48:161–165. [DOI] [PubMed] [Google Scholar]
- 22.Martini N. Mediastinal lymph node dissection for lung cancer: the Memorial experience. Chest Surg Clin North Am. 1995;5:189–203. [PubMed] [Google Scholar]
- 23.Gonzalez-Stawinski GV, Lemaire A, Merchant F, et al. A comparative analysis of positron emission tomography and mediastinoscopy in staging non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1900–1905. [DOI] [PubMed] [Google Scholar]
- 24.Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1943–1951. [DOI] [PubMed] [Google Scholar]
- 25.Yim APC. VATS major pulmonary resection revisited: controversies, techniques, and results. Ann Thorac Surg. 2002;74:615–623. [DOI] [PubMed] [Google Scholar]
- 26.Weber A, Stammberger U, Inci I, et al. Thoracoscopic lobectomy for benign disease: a single centre study on 64 cases. Eur J Cardiothorac Surg. 2001;20:443–448. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RP, Pham DK, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg. 2006;82:214–218. [DOI] [PubMed] [Google Scholar]


