Abstract
Objective:
To determine whether detection of hepatocellular carcinoma (HCC) cells by real-time quantitative RT-PCR targeting of alpha-fetoprotein mRNA (AFP mRNA) before or after curative hepatectomy predicts HCC recurrence and patient survival.
Summary Background Data:
The presence of cancer cells in peripheral blood and/or bone marrow in patients with malignant disease has been reported to correlate with outcome.
Methods:
Between July 2000 and June 2005, 136 consecutive HCC patients underwent primary curative hepatectomy. Bone marrow aspirated preoperatively, and peripheral blood samples collected before and after operation were subjected to real-time quantitative RT-PCR analysis using AFP mRNA as a target molecule. Median follow-up was 23 months (range, 6–54 months). Patient survival (PS), disease-free survival (DFS), and clinicopathologic features were compared between patients with positive and negative AFP mRNA.
Results:
Twenty-four patients died (22 from HCC). HCC recurred in 66 patients (hepatic in 37 [56.1%]; hepatic and remote in 17 [25.8%], and remote alone in 12 [18.2%]). Bone marrow was positive for AFP mRNA in 38 patients (27.9%) and negative in 98 (72.1%). One- and 3-year PS was 96.6% and 91.4%, respectively, with negative AFP mRNA versus 86.2% and 55.5%, respectively, with positive AFP mRNA (P < 0.0001). One- and 3-year DFS were 73.2% and 44.8%, respectively, with negative AFP mRNA versus 54.5% and 25.8%, respectively, with positive AFP mRNA (P = 0.0399). Portal vascular invasion, tumor size, multiple tumors, and tumor differentiation correlated with inferior PS and DFS on univariate analysis. On multivariate analysis, positive AFP mRNA was the most important risk factor for PS (P = 0.001) and DFS (P = 0.0165). In addition, positive AFP mRNA in peripheral blood after operation tended to predict reduced DFS.
Conclusion:
AFP mRNA in the bone marrow and systemic circulation during the perioperative period predicts patient survival and recurrence after curative hepatic resection for HCC.
Hepatocellular carcinoma cells were perioperatively detected by real-time quantitative RT-PCR targeting AFP mRNA in bone marrow and peripheral blood of HCC patients who underwent curative hepatectomy. Positive AFP mRNA correlated significantly with portal vein invasion. AFP mRNA in bone marrow predicts patient survival and recurrence after curative hepatectomy for HCC.
Hepatocellular carcinoma (HCC) is the fifth most common cause of mortality from malignant diseases, leading to 250,000 deaths annually worldwide. Total removal of HCC by hepatic resection or transplantation provides the only chance for cure, but a large proportion of patients develop intrahepatic and extrahepatic recurrence after surgery. Various factors are thought to contribute to HCC recurrence and resulting death, including multicentric carcinogenesis in the remnant liver due to underlying hepatitis B virus (HBV) or hepatitis C virus (HCV) induced liver cirrhosis,1 hematogenic spread, or micrometastasis of HCC cells before surgery or during hepatectomy by manipulation of the liver.2
Recently, by using various molecular biologic markers, researchers have been able to detect malignant cells in the systemic circulation and bone marrow; the presence of these cells has been found to correlate with outcome.3–8 With regard to HCC, the reliability of these methods for predicting outcome after resection has remained controversial because of differences in modality, target molecules, primers, patient population, and timing and site of sample collection.9–20 In the present study, we tried to determine whether the presence of HCC cells perioperatively in bone marrow, peripheral blood, and cancerous tissues, detected by means of the most sensitive real-time quantitative RT-PCR, which targets alpha-fetoprotein mRNA (AFP mRNA), could predict recurrence and mortality in 136 consecutive patients after curative hepatic resection.
PATIENTS AND METHODS
Patients
From July 2000 to June 2005, 136 consecutive patients underwent primary curative hepatectomy at the First Department of Surgery, Hokkaido University Hospital. Mean age was 61.0 ± 8.8 years; 112 were male. Thirty-eight had received various treatments before operation (transarterial chemoembolization [TAE], n = 28; percutaneous ethanol injection [PEIT], n = 6; or radio frequency ablation [RFA], n = 4). Child-Pugh staging was A in 132 patients and B in 4. Absence of extrahepatic lesions was confirmed by imaging studies, using helical-computed tomography (helical-CT) and magnetic resonance imaging (MRI) within 1 month before operation. Absence of intrahepatic lesions in the presumed remaining liver was assessed by ultrasonography (US), helical CT, MRI, and, if suspected, by CT during angiography. Extensive hepatic resection (more than lobectomy) was performed in 52 patients, while anatomic minor resection was done in 84 patients. No patient developed a major complication. Patients were discharged from the hospital at an average of 19.8 ± 11.5 days after surgery. They were followed at 3-month intervals by US, thoracoabdominal CT, MRI, and laboratory tests for AFP, AFP-L3, and protein induced by vitamin K absence or antagonists-II (PIVKA-II). Bone scintigraphy was performed when indicated by clinical symptoms. In patients with intrahepatic and/or extrahepatic recurrences, surgical removal, TAE, PEIT, or RFA was repeatedly applied. The median follow-up period was 23 months (range, 6–54 months).
Bone marrow AFP mRNA was also measured in 34 control subjects: 23 living liver donors, 4 patients with cholelithiasis, 2 with echinococcosis, and 5 with other benign diseases. Mean age of these controls was 34.0 ± 11.9 years; 11 were male. Control liver tissues were obtained from 24 living liver donors (mean age, 49.8 ± 7.03 years; 13 males) and control peripheral blood samples from 12 living liver donors and 15 breast cancer patients (mean age, 56.3 ± 11.7 years; 9 males).
Sample Collections
The study was approved by the Institutional Review Board of the Hokkaido University, School of Advanced Medicine. Informed consent was obtained from each of the patients in accordance the Ethics Committees Guidelines for our institution.
Immediately before starting laparotomy, 5 mL of bone morrow was aspirated from the sternum and put into citrate-coated test tubes. For measurements of the peripheral blood, 8 mL was collected into a citrate-coated tube through venipuncture of the cubital vein before starting surgery, at the end of hepatectomy, and 1, 3, and 5 days after operation. The initial 2 mL of bone marrow aspirate and peripheral venous blood were discarded. After removal of the liver, HCC tissues were excised, diced into small pieces, and put into liquid nitrogen. Normal liver tissues were obtained by wedge biopsies and stored similarly.
RNA Isolation and Reverse Transcription
Bone marrow and blood samples were prepared for measurement of total RNA using a Blood RNA extraction kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol with minor modifications. Briefly, 5 mL of bone marrow cells and peripheral blood cells were mixed with 25 mL of reagent buffer EL (erythrocyte lysis). They were cooled on ice for 15 minutes and centrifuged, and the cell pellets were collected. They were suspended with 1.35 mL buffer and applied into the reagent columns. They were washed twice with reagent buffer containing ethanol and eluted total RNA with RNase-free water. Extraction of total RNA from liver tissues was performed as described elsewhere.21 In brief, diced samples were homogenized in Trizol (TRI-Reagent, T9424, Sigma Co., St. Louis, MO) on ice, deproteinized with chloroform, washed twice with 70% ethanol, and stored in RNA storage solution (Ambion, RNA Co., Austin, TX). Both tissue and blood RNA extracts were stored at −80°C until analysis. cDNA was generated from 1 μg of total RNA using Moloney murine leukemia virus-reverse transcription (SuperSciptII, Life Technologies, Inc.), plus 20 pmol/L of each dNTP and 10 pmol/L oligo dT primers, in a 20-μL final reaction volume, at 42°C for 60 minutes, followed by heating at 99°C for 5 minutes.
Real-Time Quantitative RT PCR
The LightCycler PCR and detection system (Roche Diagnostics, Mannheim, Germany) was used for amplification. Online quantification real-time RT-PCR was performed in glass capillaries according to the protocol. The cDNA was amplified in the 20-μL PCR reaction mixture containing each dNTP (with dUTP instead of dTTP), 1 × PCR buffer, the specific primers, and magnesium chloride. For detection of AFP, 2 adjacent oligonucleotide probes were applied: the LightCycler Red 640 fluorophore, hAFP-LCR; (5′-CTTGCACACAAAAGCCCACTCCA-3′) and the fluorophore labeled at the 3′ end with fluorescein, hAFP-FITC; (5′-TCGATCCCACTTTTCCAAGTT-3′) (Nihon Gene Research Laboratories, Sendai, Japan). The sense and antisense primers (kindly supplied by Dr. Hiroaki Nagano at Osaka University) for amplifications of AFP were as follows: 5′-TGCAGCCAAAGTGAAGAGGGAAGA-3′) (hAFP-s) and 5′-CATAGCGAGCAGCCCAAAGAAGAA-3′ (hAFP-As). RT-PCR amplification was carried out for one cycle of 95°C for 10 minutes, followed by 5 cycles of 95°C for 10 seconds, 62°C for 15 seconds, and 72°C for 15 seconds. After the final cycle, we used a 10 minutes extension period at 40°C. For detection of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control, 2 adjacent oligonucleotide probes were hGAPDH-LCR; 5′-TTCCGTGTCCCCACTGCCAA-3′ and hGAPDH-FITC; 5-GGGAAGCTCACTGGCATGGC-3′. The sense and antisense primers for amplifications of GAPDH were as follows: 5′-GCCTCCTGCACCACCAACTG-3′ (hGAPDH-S) and 5′-CGACGCCTGCTTCACCACCTTCT-3′ (hGAPDH-As). RT-PCR amplification was carried out for one cycle of 95°C for 10 minutes, followed by 35 cycles of 95°C for 10 seconds, 58°C for 10 seconds, and 72°C for 16 seconds. After the final cycle, we used a 10-minute extension period at 40°C.
Quantification Analysis
Quantification data were analyzed using the LighCycler analysis software (Roche Diagnostics, Mannheim, Germany) as described by the manufacturer's description. In this analysis, the background fluorescence was removed by setting noise band. The crossing point for the calculation of amplified PCR product was set the intersection of the best-fit line through the log-linear lesion and the noise band. The standard curve was a plot of the “crossing point” versus the copy number of DNA fragments constructed into the cloning vector.
Statistical Analysis
Cumulative survival and disease-free survival rates were computed according to the Kaplan-Meier method and compared between groups by the log-rank test. The Cox proportional hazards model was used for multivariate analysis. Statistical analyses using standard tests (χ2, t test) were performed where appropriate. Significance was defined as a P value <0.05. Statistical analyses were performed using StatView 5.0 Windows (SAS Institute Inc., Cary, NY).
RESULTS
Patient Outcome
Mortality
Twenty-four patients died; 22 from HCC, 1 from liver failure, and 1 from another malignant disease. Actuarial 1-, 2-, and 3-year patient survival rates were 93.5%, 85.8%, and 77.5%, respectively.
HCC Recurrence
HCC recurred in 66 patients (48.5%); only in the remnant liver in 37 (56.1%), remnant liver and remote in 17 (25.8%), and remote alone in 12 (18.2%). Actuarial 1-, 2-, and 3-year disease-free survival rates were 66.9%, 50.5%, and 39.1%, respectively.
Real-Time Quantitative RT-PCR
Sensitivity of the System
To determine the sensitivity of the assay, HuH7 HCC cells, serially diluted from 2 to 2 × 107 cells, were mixed into 2-mL peripheral blood samples donated from healthy volunteers. The real-time amplification plots showed a strong linear relationship between AFP mRNA quantity and the HCC cell count (Fig. 1). The amplified AFP mRNA could be detected in samples containing a minimum of 2 HCC cells. Hence, the sensitivity was determined theoretically at 1 AFP mRNA-positive cell in approximately to 1 × 107 of mononuclear blood cells.
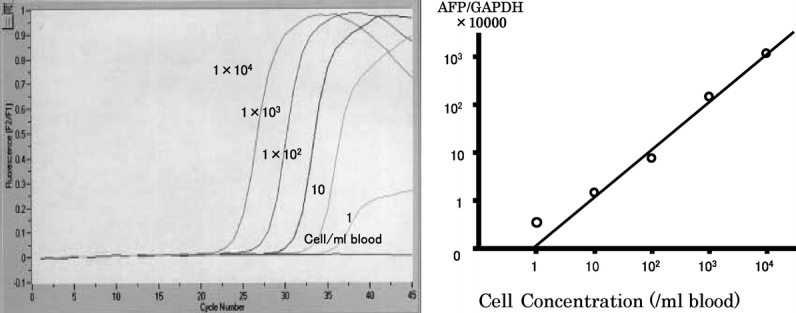
FIGURE 1. The real-time amplification plots showed strong linear relationship between AFP mRNA quantity and the count of containing HCC cells. The sensitivity was determined theoretically at 1 AFP mRNA-positive cell in approximately to 107 white blood cells from healthy donor.
Bone Marrow Samples
AFP mRNA was undetectable in 31 of 34 control subjects (91.1%), while the median number of GAPDH mRNA copies was 5,136,681.8 (range, 22,700–11,560,000). Three control subjects were positive for AFP mRNA; AFP mRNA of 0.8, 0.51, and 0.7 copies; GAPDH mRNA of 4,171,000, 3,363,000, and 622,000 copies; and ratios of 1.92 × 10−7, 1.52 × 10−7, and 1.13 × 10−7, respectively. Thus, 1.92 × 10−7 was designated as a cutoff value for AFP mRNA/GAPDH mRNA. Of the 136 patients with HCC, 38 (27.9%) were positive for AFP mRNA and 98 (72.1%) were negative. Microvascular and macrovascular invasion was found to be a single significant risk factor for positive bone marrow AFP mRNA (P < 0.0152).
Peripheral Blood Samples
Because of an electricity problem during storage, preoperative and postoperative blood samples were available for analysis from only 37 patients (27.2%). In 76 patients (55.8%), only preoperative blood samples could be analyzed, and in another 55 (40.4%), postoperative samples could be evaluated. The remaining blood samples and those collected postoperatively from nonmalignant control patients were denaturated during storage.
No AFP mRNA was detected in control preoperative samples while GAPDH mRNA levels were a median of 177,500 copies (range, 19,720–17,620,000). Assuming zero as a cutoff value for AFP mRNA/GAPDH mRNA in blood samples, 7 patients (9.2%) were positive and 69 (90.8%) were negative preoperatively. Of 55 postoperative blood samples, 10 (18.2%) were positive and 45 (81.8%) were negative.
Normal Liver and HCC Tissues
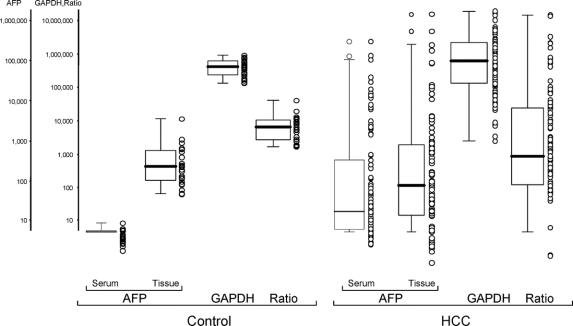
AFP mRNA was detected in all 24 tissue samples from living liver donors, with a median of 248.5 copies (range, 51.9–950.7 copies). In 35 of the 136 HCC tissues, the quality of RNA extracts was inappropriate for measurements. Of the remaining 101 HCC tissue samples, 93 (92%) were positive for AFP mRNA (median, 912.4; range, 0.1–15,698,500). The remaining 8 tissue samples (8%) showed no detectable AFP mRNA. GAPDH mRNA was almost identical in both normal and HCC tissues. The ratio in normal liver tissues was higher, compared with HCC tissues whose ratio showed wide distribution from zero to more than 15,000,000 (Fig 2).
FIGURE 2. The ratio of normal liver tissues was higher than that of HCC tissues. The range of the ratio of normal liver tissue was close but that of HCC tissues was widely, from zero to more than 15,000,000.
Clinical Significance of AFP mRNA Measurements
Patient Survival
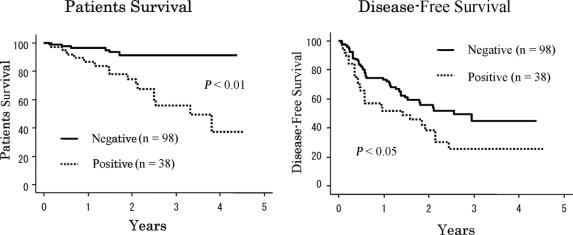
Sixteen (72.7%) of the 22 patients who died of HCC had positive AFP mRNA in their bone marrow; the remaining 6 (27.3%) were negative. One- and 3-year survival rates were 86.5% and 55.5%, respectively, with positive AFP mRNA, compared with 96.6% and 91.4%, respectively, with negative AFP mRNA (Fig. 3). The difference was highly significant (P < 0.001). On univariate analysis, preoperative serum albumin and AFP levels, tumor number, tumor size, vascular invasion, intrahepatic metastasis, preoperative management, Milan criteria, and positive AFP mRNA in bone marrow were found to be important risk factors (Table 1). On multivariate analysis, positive AFP mRNA, vascular invasion, and preoperative albumin level were independent risk factors for patient survival (Table 2). There was no difference in patient survival between groups with positive and negative AFP mRNA in preoperative and postoperative peripheral blood samples, nor was any survival difference found to be associated with AFP mRNA tissue levels.
FIGURE 3. Survival rates and disease-free survival rates of negative AFP mRNA in bone marrow were higher than with positive AFP mRNA in bone marrow (P < 0.0001, P = 0.0399).
TABLE 1. Univariate Analysis of the Factors Affecting Survival
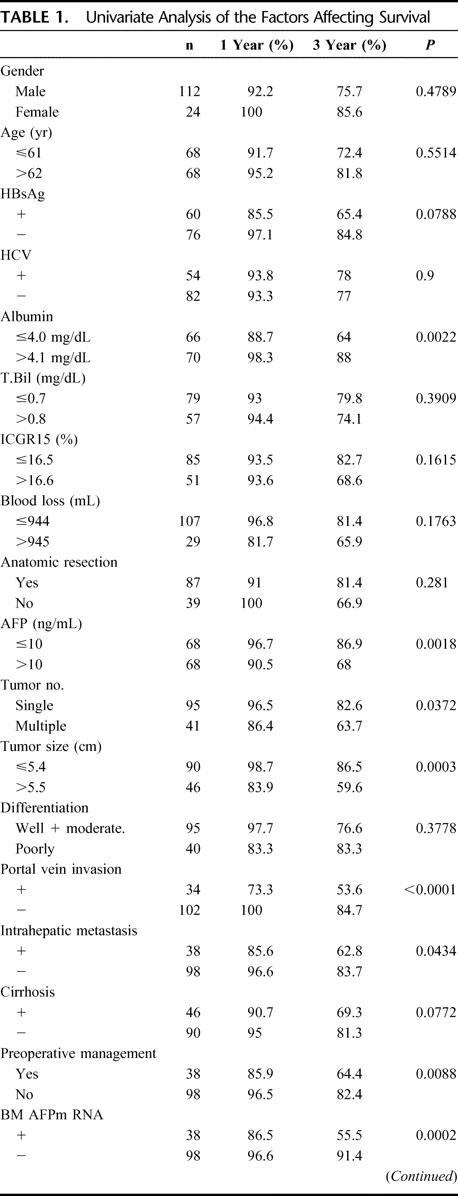
TABLE 1. (Continued)
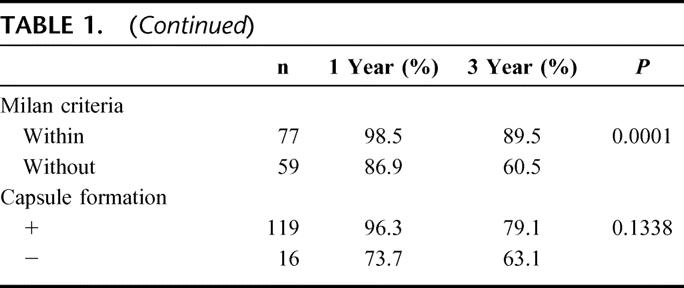
TABLE 2. Multivariate Analysis of the Factors Affecting Survival and Disease-Free Survival
HCC Recurrence
As shown in Figure 3, 1- and 3-year disease-free survival rates were 73.2% and 44.8%, respectively, with negative bone marrow AFP mRNA, versus 54.5% and 25.8%, respectively, with positive bone marrow AFP mRNA (P < 0.0399). On univariate analysis, age, HBV infection, indocyanine green (ICG), blood loss, AFP level, tumor number, tumor size, histopathologic differentiation, vascular invasion, intrahepatic metastasis, preoperative management, positive bone marrow AFP mRNA, and Milan criteria were important risk factors for HCC recurrence (Table 3). On multivariate analysis, ICG and positive bone marrow AFP mRNA were independent risk factors for recurrence (Table 2).
TABLE 3. Univariate Analysis of the Factors Affecting Disease-Free Survival
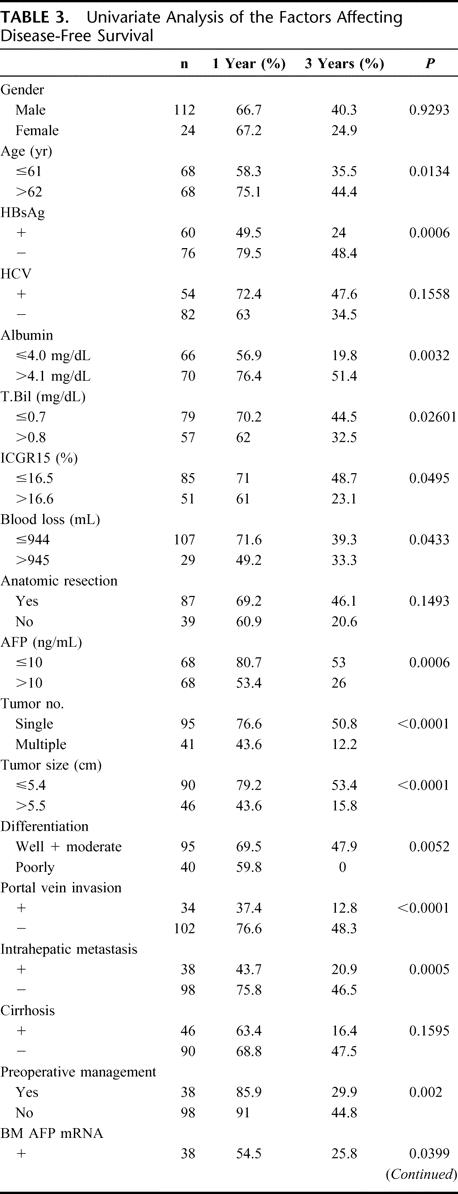
TABLE 3. (Continued)
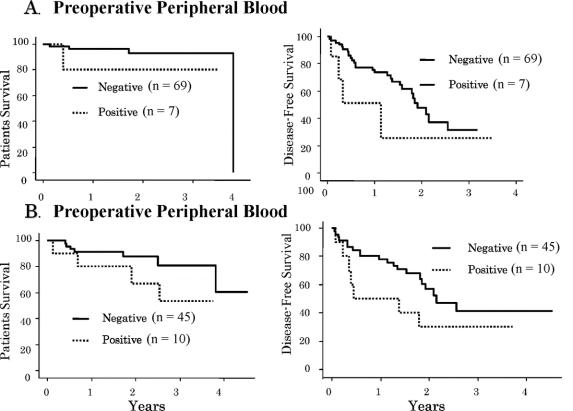
Recurrence developed in 4 of 7 patients (57%) with positive AFP mRNA in preoperative peripheral blood samples, compared with 28 of 69 (40.6%) negative patients. Positive patients had a significantly higher incidence of remote recurrence (3 of 4 vs. 6 of 28, P < 0.03). The incidence of remote recurrence was also higher in patients with positive postoperative blood samples. Of the 10 who were positive, 7 developed recurrence, with remote recurrence in 5. In 45 patients with negative postoperative samples, 20 had recurrence, with remote recurrence in 6 (5 of 7 vs. 6 of 20, P < 0.07).
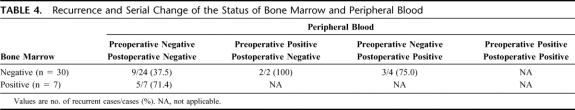
Although the difference was not statistically significant, patients with negative AFP mRNA in preoperative and postoperative blood had a higher disease-free survival rate (Fig. 4). More importantly, of the 37 patients from whom both preoperative and postoperative blood samples were available, those who were positive for AFP mRNA before or after surgery showed a higher rate of HCC recurrence, even though bone marrow AFP mRNA was negative (Table 4).
FIGURE 4. A, Survival rates and disease-free survival rates of negative AFP mRNA in preoperative peripheral blood tended to be higher than with positive AFP mRNA in bone marrow (P = 0.1205, P = 0.1035). B, Survival rates and disease-free survival rates of negative AFP mRNA in postoperative peripheral blood tended to be higher than with positive AFP mRNA in bone marrow (P = 0.1710, P = 0.5784).
TABLE 4. Recurrence and Serial Change of the Status of Bone Marrow and Peripheral Blood
Ratios of AFP mRNA/GAPDH mRNA in HCC tissues showed no correlation with recurrence, with preoperative serum AFP levels, nor with AFP mRNA in bone marrow and peripheral blood (Fig. 2).
DISCUSSION
The present prospective study, in 136 consecutive patients who underwent curative hepatic resection for HCC, has demonstrated that detection of AFP mRNA perioperatively in bone marrow and in peripheral blood by real-time quantitative RT-PCR is closely correlated with patient mortality and HCC recurrence. Patients with detectable AFP mRNA in bone marrow aspirates collected before skin incision had higher incidences of vascular invasion and HCC recurrence as well as reduced survival, indicating that HCC cells have already migrated into the blood stream and bone marrow before surgery. In addition, although our findings are inconclusive due to the limited number of available samples, it appears that positive AFP mRNA in peripheral venous blood after surgery relates to clinical outcome, even when preoperative measurement of AFP mRNA in bone marrow and peripheral blood is negative. Shedding of HCC cells into hepatic tributaries by surgical manipulation may occur during the procedure.
Circulating malignant cells in bone marrow and/or peripheral blood have been correlated with remote metastasis and reduced survival in patients with breast cancer, colorectal cancer, gastric cancer, non-small cell lung cancer, and pancreatic cancer.1–6 Most of these studies used immunohistochemistry with various cytokeratin antibodies for cancer cell detection. In HCC patients, similar studies have been conducted, for the most part using a RT-PCR technique, with different molecular targets, such as albumin and AFP. The results are contradictory, however (Table 5). Kar and Carr22 and Hillarie et al23 first correlated the presence of circulating HCC cells detected by albumin mRNA with advanced cancer stage and outcome, but the clinical significance of their finding was soon denied by others on the basis of to “illegitimate” transcription of the albumin gene in mononuclear cells.24 Later, Komeda et al10 proposed that AFP mRNA be used for detection because it is expressed prominently in HCC but only weakly in normal hepatocytes. By using sequential dilutions of Hep G2 cells as a control, they showed that 36% of HCC patients, but no control subjects, were positive for AFP mRNA in peripheral blood. In addition, positivity was correlated with TNM stage, serum AFP level, intrahepatic and extrahepatic metastasis, and portal vein thrombus. In contrast, however, Limoine et al11 could not confirm any importance of AFP mRNA in peripheral blood. Their intraoperative assessments showed positive values in 40% of HCC patients, 63% of patients with other malignancies, and 52% in subjects without malignancy. Furthermore, HCC recurrence was not correlated with positive AFP mRNA before surgery, during surgery, or after surgery. Although their choice of AFP primers was different, the conflict between their results and others’ may indicate the limitation of the qualitative, or semi-quantitative, analyses that were applied in these studies.
TABLE 5. Reports of AFPmRNA for Hepatocellular Carcinoma
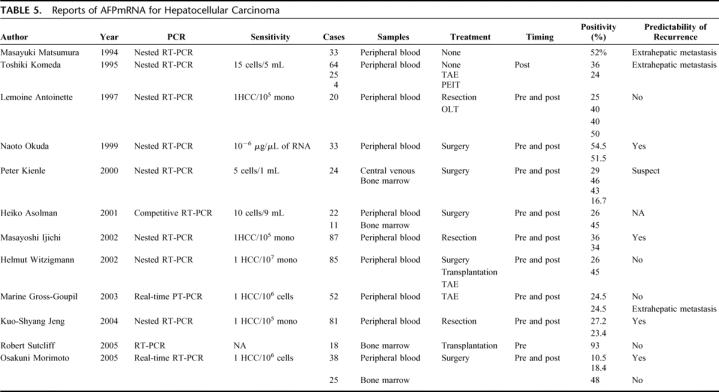
In the present study, we used a real-time RT-PCR method to determine quantitative differences of AFP mRNA in bone marrow, peripheral blood, HCC, and normal liver tissues. Concomitantly, we measured GAPDH mRNA, which is expressed as a “housekeeping gene” in all mammalian cells. Simultaneous measurement of both genes enables establishment of cutoff values, as well as comparison of AFP mRNA concentrations between HCC patients and normal controls, and even among HCC patients. With bone marrow samples, the ratio of 1.92 × 10−7 was set as a cutoff from 3 positive control subjects, and 27.9% of HCC patients were positive. The figure with HCC was similar to those reported by others: 16.7%13 to 48%.20 Presence of AFP in mononuclear cells in normal subjects has been found previously.25 More importantly, while AFP mRNA, GAPDH mRNA, and their ratios in liver tissues were generally constant among normal control subjects, they were markedly different among HCC patients, indicating highly variable AFP synthesis activity among individual HCC cells. Using the same real-time quantitative RT-PCR technique, Gross-Goupil et al17 demonstrated a higher incidence of extrahepatic metastases in patients who had positive AFP mRNA in blood before or after TAE.
The importance of bone marrow micrometastasis for prediction of recurrence and patient survival has been shown mostly with extrahepatic solid-organ malignancies.3–7 However, the relevance of these findings for patients with HCC has not been well evaluated and demonstrated. Aselmann et al,14 using real-time quantitative RT-PCR with AFP mRNA and GAPDH mRNA, found that 5 of 11 (45%) HCC patients had AFP mRNA in bone marrow, but there were no control data or postoperative follow-up. By using the same analytical method, Morimoto et al20 reported positive bone marrow AFP mRNA in 48% of 25 HCC patients. They found no correlation of the positivity with disease-free survival during 4 years of follow-up. Using immunohistochemistry of bone marrow aspirates with Hep Par-1 (Dako, United Kingdom), Sutcliffe et al19 described positivity of 6% by staining compared with 57% by qualitative albumin mRNA RT-PCR. In 18 patients undergoing liver transplantation, the positive predictive values of staining and albumin mRNA RT-PCR for posttransplant tumor recurrence were 42% and 0%, respectively. Thus, they pointed to a role for immunohistochemical analysis, not of RT-PCR, for prediction.
In contrast, in the present study, using real-time quantitative RT-PCR, 27.9% of HCC patients were found to have AFP mRNA in their bone marrow before operation. These patients had higher hepatic and extrahepatic recurrence than negative patients. Although management strategies and their applications were not significantly different between the 2 groups (unpublished data), positive patients showed markedly reduced survival.
Interestingly, 41 (41.2%) of the 98 bone marrow-negative patients developed HCC recurrence after operation. The explanation for these recurrences is unclear, but their etiologies may involve 1) multicentric carcinogenesis, 2) systemic circulation of HCC cells without residing in bone marrow, 3) spreading of HCC cells by surgical manipulation during surgery, and 4) absent or minimum AFP mRNA in HCC cells.
The annual rate of HCC development in HCV-induced liver cirrhosis is reportedly more than 6%.26 Kumada et al1 reported that overall recurrence rates after initial treatment with PEIT or hepatic resection in 112 HCC patients were 23.7% at 1 year, 64.5% at 3 years, and 76.1% at 5 years; these findings are similar to others’ and to ours. Each half of these recurrences were found either from intrahepatic metastasis or from multicentric occurrence. Therefore, in nearly half of our 41 recurred patients with negative bone marrow, recurrence is thought to be due to multicentric carcinogenesis. In patients who had negative bone marrow and negative peripheral blood, recurrence developed in 37.5%, versus 71.4% (5 of 7) in patients with positive bone marrow but negative blood (Table 4). It is important to note that both of 2 patients who had negative bone marrow but positive peripheral blood before operation developed HCC recurrence postoperatively (Table 4). Thus, it may be necessary to analyze both bone marrow and peripheral blood simultaneously to satisfactory predict the outcome before operation. Even more importantly, 3 (75%) of the 4 patients who became positive in peripheral blood after hepatectomy developed HCC recurrence, although both bone marrow and blood were negative preoperatively. In addition, patients who were negative in bone marrow but developed recurrence were found to have multiple larger tumors with higher incidence of vascular invasion than those without recurrence (unpublished data). Thus, hematogenic spreading of HCC cells by liver manipulation during operation might have a greater role as a cause of HCC recurrence, even after curative resection of HCC. Indeed, using a similar method, Gross-Goupil et al17 reported that 33% to 50% of patients with positive AFP mRNA in peripheral blood before and/or after TAE developed remote metastases, compared with 8% of negative patients, similar to those reports by Ijichi et al15 and Morimot et al.20 Finally, even with an advanced real-time quantitative RT-PCR using AFP mRNA as a target molecule, it is not possible to detect all of the HCC cells in the bone marrow and systemic circulation. Of the 101 HCC tissues, 8 specimens showed no AFP mRNA signal despite intact GAPDH mRNA. A novel genetic marker that is specific for HCC or combination of multiple markers may improve the detectability and prediction.
CONCLUSION
Along with the degree of impaired liver function and microscopic and macroscopic vascular invasion, perioperative detection of AFP mRNA in bone marrow and peripheral venous blood by real-time quantitative RT-PCR significantly correlated with outcome of patients undergoing curative hepatectomy for HCC. Migration of HCC cells in the systemic circulation before operation and hematologic spreading of HCC cells by surgical manipulation during operation were suspected as possible sources of HCC recurrence. AFP mRNA/GAPDH mRNA ratios in bone marrow and HCC tissues had no relationship with recurrence rate. In addition, 8% of HCC tissues were devoid of AFP mRNA. Thus, more sophisticated techniques, HCC-specific markers, and preventive maneuvers to suppress HCC cell spreading during operation are needed to improve predictability and to reduce HCC recurrence after curative resection.
ACKNOWLEDGMENTS
The authors thank Miss Miyuki Kasegai, Miss Mizuho Kasai, and Miss Kozue Mori for their technical assistant, Mr. Minoru Ohta for statistical analysis, Miss Kazuko Asari for typing the manuscript, and Mrs. Nancy Ehlich Lapid for critical review.
Discussions
Dr. Ronald W. Busuttil (Los Angeles, California): As you know, for several decades we have struggled to identify an accurate, reproducible reoperative marker for HCC that is likely to recur. Currently, even the best imaging techniques, whether done preoperatively or not, really overestimate or underestimate the stage of HCC by as much as 30%. So to address this issue, Dr. Todo and his group have proposed an interesting preoperative method to molecularly identify systemic disease in a cohort of patients undergoing curative hepatectomy, and I am sure this also applies to liver transplantation. Using highly sensitive RT-PCR, alpha-fetoprotein was documented in both the bone marrow and serum of these patients and may provide a mechanism to predict tumor recurrence and subsequent mortality.
I think this is very important because, as you know, in the setting of worldwide epidemic of viral hepatitis, which is the most important cause of development of HCC, we have to get a better handle on how to predict how these patients will do. So I have several questions for you.
First, in the discussion of the manuscript, you said that there were 38 patients who were found to be positive for AFP in the bone marrow and these had a poor 1- and 3-year disease-free survival of about 55% and 26%, respectively. However, those that were found to be negative for AFP also had a poor survival of 44%. Thus, over 50% of the negative patients still suffered a disease recurrence. Did the authors find a significant difference in tumor size that might have been causative for this discrepancy? And did a higher percentage of those negative patients have a higher incidence of risk factors for biologic aggressiveness such as viral hepatitis or poor differentiation?
The second question is that you clearly demonstrate that normal liver tissue from living donors produces AFP with all 24 controls testing positive. Conversely, 18% of the HCC specimens were negative. How do you rectify this discrepancy?
Third, it is interesting that the multivariate analysis only identified bone marrow AFP and Indocyanine green 15 a minute as significant. Do you think that indices such as tumor size, tumor number, and the Milan criteria, which have previously been found to be predictive, were not found to be predictive and why?
And lastly, while these are some important observations, what is the next step? Does additional technology such as gene array provide an adjunct to this strategy? What other molecular markers should we be considering in our search for an accurate, reproducible, and clinically practical surrogate for systemic disease?
Dr. Satoru Todo (Sapporo, Japan): Thank you, Dr. Busuttil for your comments and questions. Answering to the first question, we found that the patients who had negative bone marrow but developed HCC recurrence after hepatic resection had larger and multiple tumors with more vascular invasion than those who were negative and had no recurrence, although the data were not shown in the presentation.
With regard to the second question, it was a surprise also to us that nearly 10% of HCC tissues did not show any AFP mRNA message. The similar finding was also reported by others (Miyamoto et al. Dig Dis Sci, 2000;45:1376). According to them, 12% of the 24 HCC tissues were without AFP mRNA. Besides, several human HCC cell lines are known not to produce AFP by a western blot analysis (Yokoo et al. Hepatology, 2004;40:609).
With regard to the third question, we included all of the pathological risk factors, such as tumor size, tumor number, and the Milan criteria, at statistical analysis. These factors were found significant by univariate analysis, but only positive bone marrow and ICG values were determined as independent risk factor by multivariate analysis. If we analyze many variables with a small number of samples, unexpected risk factor(s) are often found significant. But I believe our results are not the case. Identification of positive bone marrow as a risk factor might reflect sequential events of HCC development, invasion, and metastasis. Poorly differentiated cancer cells tend to grow rapidly into larger tumor, invade more into surrounding tissues and vasculatures, often migrate into systemic circulation, and localize finally at remote sites and bone marrow.
The last question is very important. The next step should include cDNA microarray, CGH array, proteomics, and other molecular analyzing techniques. However, these methods require too much effort and are not without risks to the patients because HCC tissues need to be obtained prior to operation. Using the same HCC tissues as analyzed in this study, cDNA array revealed a combination of 30 to 40 genes could predict intrahepatic metastasis and patient outcome. CGH array showed importance of amplification for prediction of the long arm of chromosome 8, where an oncogene, cMyc, lacotes. Similarly, Pittsburgh group (Finkelstein et al. Hepatology, 2003;37:871) reported reliability of genomic analysis of multiple biopsy samples from one patient for prediction of HCC recurrence after liver transplantation. Using the same real-time quantitative RTCR method, we analyzed 40 patients undergoing living donor liver transplantation. Our current strategy is to exclude positive bone marrow patients from waiting list, but 1 positive patient underwent the procedure for social reasons and developed recurrence at 6 months and died at 11 months. Another 2 patients who had negative bone marrow developed HCC recurrence after 1 year. One died at 2 years, and the other is still alive without recurrence for more than 3 years after partial resection of a small metastatic lung nodule. Since both patients had lower serum AFP levels, less than 50 μg/mL, null or suppressed production of AFP by HCC cells was estimated with these cases. Thus, the method is not 100% accurate for recurrence prediction.
Dr. Charles M. Miller (Cleveland, Ohio): It seems to me that the 2 really important things in stratifying patients for recurrence are degree of differentiation of the tumor and vascular invasion. And our problem really is the best we do with vascular invasion preoperatively is macrovascular invasion and that the finding of microvascular invasion is left to analysis of the specimens after surgery. The use of this new technique looking at AFP mRNA may become a very important tool in helping us understand preoperatively whether or not there is microscopic vascular invasion. So this is really an important issue. I have 3 rather brief questions.
There have been some reports that preoperative minimally invasive therapy such as chemoembolization can cause tumor dissemination. Did you analyze your groups that had the different forms of pre-resection treatment as to whether they had a higher incidence of bone marrow AFP or not?
When there is systemic dissemination of the AFP, especially in the bone marrow, why do most of the recurrences still occur in the liver and not peripherally?
Finally, I thought that the use of the sternum for the site of bone marrow was interesting. I suspect it was easy. Do you think that you would have the same findings in bone marrow from the iliac crest or other places?
Dr. Satoru Todo (Sapporo, Japan): Thank you, Charlie, for your questions. Although TAE was shown to induce HCC cell spread into systemic circulation by French group, they and we did not evaluate bone marrow status. Hematologic spread of HCC cells by TAE is, I believe, dependent on the technique by radiologists. About 10 years ago, you reported importance of pretransplant TAE to reduce HCC recurrence after liver transplantation in patients who exceeded the Milan criteria. Recently, Yao and his group reported usefulness of TAE and locoregional therapy for downstaging of HCC in patients while waiting liver transplantation.
Why does most of HCC recurrence happen in the liver? One reason is de novo carcinogenesis within cirrhotic liver, and the other is “seeds and soil” phenomenon as described by Paget more than 100 years ago. HCC cells,“the seeds,” in bone marrow migrate out, and reach and survive in the liver, “the soil.” Molecular mechanisms of the phenomenon are now under intense investigation worldwide.
Answering to the third question, we use the sternum because it is easy to access. Of course, there are several studies reporting higher detectability of cancer cell in bone marrow by accessing multiple sites, such as the sternum, rib and iliac crest, or multiple samplings from one site.
Dr. Henri Bismuth (Villejuif, France): I had the privilege to read the paper, and I like it. I have two brief questions.
One, you say that the detection of AFP mRNA in the bone marrow collates well with well-known factors of size and number of tumors and portal invasion. So why do you perform such analyses to achieve the same results?
My second question is how do you explain that Indocyanine green clearance, that factor of disease-free survival, as this is only a liver function test.
My third question is: today the main question for the surgical treatment of HCC is resection or transplantation. Have the results helped for the choice between these 2 surgeries?
Dr. Satoru Todo (Sapporo, Japan): Most of the pathological parameters used for the estimation of clinical outcome have been examined with removed or explanted livers. Besides, preoperative imaging studies are not always accurate. Our aim was to obtain reliable risk factor prior to operation.
With regard to the second question, ICG clearance test has been shown to correlate with the degree of liver cirrhosis, and degree of liver cirrhosis, or fibrosis, correlates with the incidence of HCC development. If liver cirrhosis is mild, F1, annual rate of HCC occurrence is about 2%, and when liver cirrhosis advances to F4, the rate increases to 6% to 7%.
The ultimate object of our study is to establish a novel method to identify the patients who would, or would not, get benefit from liver transplantation for the treatment of HCC. Of course, I know, a choice of resection or transplantation for HCC treatment is very important issue in surgery, but it is impossible to perform liver transplantation in all HCC patients whenever indicated. Particularly in Japan, we have more than 30,000 HCC patient deaths every year, and had only 40 cadaveric organ donors for the last 8 years. According to our experience at Hokkaido University, 8 of the 40 HCC patients who underwent living liver transplantation had received liver resection before. Technical difficulty was moderately higher in these patients, but posttransplant morbidity and outcome were not different from the other patients. Thus, I believe that hepatic resection, if resectable, should be considered first as a bridge to liver transplantation.
Dr. Jean C. Emond (New York, New York): I just wanted to ask you about 1 point. I have always been surprised that we can cure any of these patients with transplantation since we take away their immune system. I was wondering whether you think that patients with a good prognosis have a favorable relationship between the tumor and the host, or do you think people with Milan criteria tumors have zero circulating tumor cells? My point is: do you think the people we cure have zero circulating tumors cells or do you just think they are patients with a favorable immune response to the tumor?
Dr. Satoru Todo (Sapporo, Japan): Dr. Edmond, we cannot cure HCC patients by hepatic resection alone because, as I mentioned, HCC develops in multicentric fashion in cirrhotic livers even if there are no circulating HCC cells. On the other hand, in the experimental study, only 0.01% of malignant cells are known to survive, and most of them die in the circulation or are eliminated by immune system. Liver transplantation is an only hope of cure if patients have zero circulating cancer cells.
Dr. Patricia K. Donahoe (Boston, Massachusetts): Probably 1 of the biggest thrusts of the NCI, the National Cancer Institute of our National Institutes of Health, is biomarkers. And the difficulty is the positive and negative controls and the choice of biomarkers, particularly with RNA. For instance, if you have a negative in the bone marrow, do you use another probe to check whether it can be detected by another probe?
Secondarily, white cells are ubiquitous in their expression of RNA. So have you used that as a control? In other words, are you recognizing ubiquitous RNA from white cells that is going to be contaminating both the marrow and the tumors?
And is there a good protein? Do you correlate your RNA with protein? Is there a good antibody for AFP to correlate with your findings?
Dr. Satoru Todo (Sapporo, Japan): Thank you, Dr. Donahoe, for your valuable questions. As you pointed out, selection of RNA biomarker is a very important issue because of a high possibility of false-positive or false-negative results. With negative bone marrow, particularly in patients with undetectable AFP mRNA in HCC tissues, we are now tying to determine several another biomarkers by cDNA microarray or CGH array to argument sensitivity and specificity by such analysis. Since AFP mRNA is detected in normal cells, we used GAPDH mRNA as a reference gene to set up a cutoff level. GAPDH is expressed in all mammalian cells ubiquitously. Even with such efforts, our quantitative, not qualitative, method has limitations because we merely detect RNA signals without seeing cancer cells. Recently, French researchers successfully isolated cancer cells in the circulation, one cell in 1 ml of blood, by screening the cell size. The method appears practical and accurate. Unfortunately, we did not study the correlation of AFP mRNA with AFP protein in this study yet.
Footnotes
Reprints: Toshiya Kamiyama, MD, Department of General Surgery, Graduate School of Medicine, Hokkaido University, North 15, West 7, Kita-ku, Sapporo, 060-8638, Japan. E-mail: t-kamiya@med.hokudai.ac.jp.
REFERENCES
- 1.Kumada T, Nakano S, Takeda I, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka N, Okamoto E, Fujihara S, et al. Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer. 1992;70:2263–2267. [DOI] [PubMed] [Google Scholar]
- 3.Diel IJ, Kaufmann M, Goerner R, et al. Detection of tumor cells in bone marrow of patients with primary breast cancer: a prognostic factor for distant metastasis. J Clin Oncol. 1992;10:1534–1539. [DOI] [PubMed] [Google Scholar]
- 4.Lindemann F, Schlimok G, Dirschedl P, et al. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685–689. [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Izbicki J, Passlick B, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347:649–653. [DOI] [PubMed] [Google Scholar]
- 6.Soeth E, Vogel I, Roder C, et al. Comparative analysis of bone marrow and venous blood isolates from gastrointestinal cancer patients for the detection of disseminated tumor cells using reverse transcription PCR. Cancer Res. 1997;57:3106–3110. [PubMed] [Google Scholar]
- 7.Wiedswang G, Borgen E, Karesen R, et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol. 2003;21:3469–3478. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YL, Feng JG, Gou JM, et al. Detection of CK20mRNA in peripheral blood of pancreatic cancer and its clinical significance. World J Gastroenterol. 2005;11:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura M, Niwa Y, Kato N, et al. Detection of alpha-fetoprotein mRNA, an indicator of hematogenous spreading hepatocellular carcinoma, in the circulation: a possible predictor of metastatic hepatocellular carcinoma. Hepatology. 1994;20:1418–1425. [DOI] [PubMed] [Google Scholar]
- 10.Komeda T, Fukuda Y, Sando T, et al. Sensitive detection of circulating hepatocellular carcinoma cells in peripheral venous blood. Cancer. 1995;75:2214–2219. [DOI] [PubMed] [Google Scholar]
- 11.Lemoine A, Bricon TL, Salvucci M, et al. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein mRNA in humans during liver surgery. Ann Surg. 1997;226:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda N, Nakao A, Takeda S, et al. Clinical significance of alpha-fetoprotein mRNA during perioperative period in HCC. Hepatogastroenterology. 1999;46:381–386. [PubMed] [Google Scholar]
- 13.Kienle P, Weitz J, Klaes R, et al. Detection of isolated disseminated tumor cells in bone marrow and blood samples of patients with hepatocellular carcinoma. Arch Surg. 2000;135:213–218. [DOI] [PubMed] [Google Scholar]
- 14.Aselmann H, Wolfes H, Rohde F, et al. Quantification of alpha 1-fetoprotein mRNA in peripheral blood and bone marrow: a tool for perioperative evaluation of patients with hepatocellular carcinoma. Langenbecks Arch Surg. 2001;386:118–123. [DOI] [PubMed] [Google Scholar]
- 15.Ijichi M, Takayama T, Matsumura M, et al. α-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: a prospective study. Hepatology. 2002;35:853–860. [DOI] [PubMed] [Google Scholar]
- 16.Witzigmann H, Geissler F, Benedix F, et al. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein messenger RNA in patients with hepatocellular carcinoma. Surgery. 2002;131:34–43. [DOI] [PubMed] [Google Scholar]
- 17.Gross-Goupil M, Saffroy R, Azoulay D, et al. Real time quantification of AFP mRNA to assess hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Ann Surg. 2003;238:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeng KS, Sheen IS, Tsai YC. Circulating messenger RNA of alpha-fetoprotein: a possible risk factor of recurrence after resection of hepatocellular carcinoma. Arch Surg. 2004;139:1055–1060. [DOI] [PubMed] [Google Scholar]
- 19.Sutcliffe R, Maguire D, Murphy P, et al. Detection and clinical significance of bone marrow micrometastases in patients undergoing liver transplantation for hepatocellular carcinoma. Transplantation. 2005;80:88–94. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto O, Nagano H, Miyamoto A, et al. Association between recurrence of hepatocellular carcinoma and alpha-fetoprotein messenger RNA levels in peripheral blood. Surg Today. 2005;35:1033–1041. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. [DOI] [PubMed] [Google Scholar]
- 22.Kar S, Carr BI. Related articles: detection of liver cells in peripheral blood of patients with advanced-stage hepatocellular carcinoma. Hepatology. 1995;21:403–407. [PubMed] [Google Scholar]
- 23.Hillaire S, Barbu V, Boucher E, et al. Albumin messenger RNA as a marker of circulating hepatocytes in hepatocellular carcinoma. Gastroenterology. 1994;106:239–242. [DOI] [PubMed] [Google Scholar]
- 24.Chelly J, Concordet JP, Kaplan JC, et al. Illegitimate transcription: transcription of any gene in any cell typa. Proc Natl Acad Sci USA. 1989;86:2617–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteban C, Trojan J, Macho A, et al. Activation of an alpha-fetoprotein/receptor pathway in human normal and malignant peripheral blood mononuclear cells. Leukemia. 1993;7:1807–1816. [PubMed] [Google Scholar]
- 26.Oka H, Kurioka N, Kim K, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12:680–687. [DOI] [PubMed] [Google Scholar]