Abstract
Objective:
To determine the effects of parenteral nutrition (PN) on LTβR in gut-associated lymphoid tissue (GALT), particularly the intestine and Peyer's patches (PP).
Summary Background Data:
Lack of enteral stimulation with PN impairs mucosal immunity and reduces IgA levels through depression of GALT cytokines (IL-4 and IL-10) and GALT specific adhesion molecules. We have shown that each is critical to intact mucosal immunity through effects on lymphocyte homing, IgA production, and resistance to antibacterial and antiviral immunity. IgA is the principal specific immunologic mucosal defense. LTβR stimulation controls production of IL-4, the adhesion molecule MAdCAM-1, and other key components of GALT, all of which are important in increasing IgA levels and maintaining mucosal defenses.
Methods:
Experiment 1: LTβR expression in intestine and PP was analyzed by Western blot after 5 days of chow, a complex enteral diet (CED), or PN. Diets were isocaloric and isonitrogenous except for chow. Experiment 2: After completing pilot experiments to determine the appropriate dose of the LTβR agonistic antibody, mice received chow, PN + 5 μg of anti-LTβR mAb (2 times/d, i.v.) or PN + isotype control antibody. PP lymphocytes and intestinal IgA levels were measured after 2 days.
Results:
Lack of enteral stimulation with PN significantly decreased LTβR expression in intestine and PP compared with chow and CED. LTβR stimulation with an agonistic anti-LTβR mAb significantly increased PP lymphocyte counts and intestinal IgA in PN fed-mice.
Conclusions:
LTβR expression is critical for GALT control mechanisms and intact mucosal immunity. PN reduces LTβR expression, PP lymphocytes, and intestinal IgA production. Exogenous LTβR stimulation reverses PN-induced depression of gut mucosal immunity.
Expression of Peyer's patch lymphotoxin β receptor (LTβR), a critical control molecule in mucosal immunity, decreases significantly with lack of enteral feeding in association with reduced Peyer's patch lymphocytes and intestinal IgA levels. Agonistic antibody stimulation of LTβR reverses PN-induced depression of gut mucosal immunity.
Enteral feeding significantly reduces the incidence of infectious complications in critically ill and critically injured patients. This has been observed in multiple clinical trials comparing enteral feeding with either parenteral feeding or starvation1–6 and confirmed by meta-analysis of the published literature.7 The primary benefit of enteral stimulation is a reduction in pneumonia with a less frequent, but still significant reduction in intra-abdominal abscess formation. Our laboratory has demonstrated a significant gap in immune defenses that occurs when adequate nutrition is provided parenterally rather than enterally.8–14 This immunologic gap in mucosal-associated lymphoid tissue (MALT), and its function increases susceptibility of the respiratory tract to invasive pathogens experimentally. Indeed, administration of parenteral feeding to mice with established antiviral or antibacterial defenses destroys these defenses9,12 and impairs the ability of animals to respond to new infectious respiratory challenges.15
The MALT contains 50% of total body immunity producing 70% of the body's immunoglobulin as sIgA.16 Naive T and B cells expressing α4β7 and l-selectin enter the MALT through the Peyer's patches (PP), which express mucosal addresin adhesion molecule-1 (MAdCAM-1).17 Intercellular adhesion molecule-1 and specific chemokines interact with α4β7 and l-selectin on the T and B cells to draw them into the PP for sensitization and stimulation.18 The stimulated T and B cells migrate via mesenteric lymph nodes, the thoracic duct, and into the vascular tree for distribution to a gut associated lymphoid tissue (GALT) and extraintestinal sites of mucosal function. Within these sites the Th-2 type IgA stimulating cytokines IL-4, IL-5, IL-6, and IL-10 stimulate maturation of the B cells into plasma cells and the production of IgA for mucosal defenses.19 IL-4 also plays a significant role in the PP in the stimulation and maintenance of MAdCAM-1 expression.20
Lack of enteral feeding alters both the cytokine and cellular milieu of the MALT. Within hours, one can detect reductions in mRNA MAdCAM-1 and decreased expression of MAdCAM-1 in PP within 24 to 48 hours.21 Over the first 3 days, there are progressive decreases in T and B cells within the PP and lamina propria with simultaneous decreases in intestinal and respiratory IgA levels.11 IL-4 levels plummet within the PP, and both IL-4 and IL-10 levels decrease in gut homogenates and IL-4 and IL-10 mRNA decreases in isolated GALT lamina propria cells.19,22 IFNγ, a Th-1 IgA-inhibiting cytokine, is not affected. A unifying concept tying enteral feeding with regulation of MAdCAM-1 expression and the Th-2 cytokine IL-4 is the lymphotoxin beta receptor (LTβR). LTβR is found within the stroma of PP and the lamina propria.23 Within these sites, after its stimulation by the heterotrimer of lymphotoxin α and β (LTα1β2) present on T and B lymphocytes, LTβR provides the signal for IL-4 and MAdCAM-1 through the activation of NFκB.24 Within the lamina propria, LTβR signaling is also important for IgA production.25 In this work. we hypothesized that lack of enteral feeding affects critical GALT and MALT defense mechanisms through reduction in LTβR expression and activation resulting in impaired mucosal defenses, which are reversible by LTβR stimulation. This hypothesis was tested by quantization of LTβR levels in intestine samples from animals receiving varying degrees of enteral stimulation and through the administration of an agonistic LTβR mAb.
METHODS
Animals
Male Institute of Cancer Research mice were purchased from Harlan (Indianapolis, IN) and housed in the Animal Research Facility of the William S. Middleton Memorial Veterans Hospital, an American Association for Accreditation of Laboratory Animal Care accredited conventional facility. Mice were left to acclimatize for 1 week with free access to chow diet (PMI Nutrition International, St. Louis, MO) and water, under controlled conditions of temperature and humidity with a 12:12 hour light:dark cycle.
Experiment 1: Effects of Route of Nutrition on Gut LTβR Expression
Mice received catheters for IV infusion after intraperitoneal injection of a ketamine (100 mg/kg body weight) and acepromazine maleate (5 mg/kg body weight) mixture. A silicone rubber catheter (0.012″ I.D. by 0.025″ O.D.; Helix Medical, Inc., Carpinteria, CA) was inserted into the vena cava through the right jugular vein. The distal end of the catheter was tunneled subcutaneously and exited the midpoint of the tail. For intragastric feeding, mice received a gastrostomy catheter (0.020″ I.D. and 0.037″ O.D., Helix Medical, Inc.) fixed with a “purse string” suture and tunneled as the IV catheter. Mice were partially immobilized by tail restraint to protect the catheter during infusion. This technique of infusion in the mouse has proven to be an acceptable method of nutrition support and does not produce physical or biochemical evidence of stress.26
Thirty-three mice were randomized to receive chow (n = 11), parenteral nutrition (PN, n = 12), or the complex enteral diet (CED) (n = 10). Mice received 0.9% saline at a rate of 4 mL/d with free access to water and chow for 2 days after surgery. Parenteral fed (PN) mice initially received 4 mL/day of PN and advanced to a goal rate of 10 mL/day by the third day. The PN solution contains 6.0% amino acids, 34.9% dextrose (6002 kJ/L), electrolytes, and multivitamins, with a nonprotein calorie/nitrogen ratio of 535.8 kJ/g N. CED mice received 4 mL/day of Nutren (Nestle, Chicago, IL) via gastrostomy with the rate increased to a goal of 14 mL/day. Nutren contains 12.7% carbohydrate, 3.8% fat, and 4% protein (4186 kJ/L) in addition to electrolyte and vitamins. The nonprotein calorie/nitrogen ratio of the CED is 549.4 kJ/g N; therefore, PN and CED diets were almost isocaloric and isonitrogenous. These feedings meet the calculated nutrient requirements of mice weighing 25 to 30 g.
After 5 days of dietary treatments, animals were anesthetized and exsanguinated by cardiac puncture. The small intestine was removed, and the mesenteric fat and external vasculature were dissected away. After washing with 20 mL cold calcium and magnesium-free Hanks balanced salt solution (HBSS, BioWhittaker, Walkersville, MD), the PP were removed from the serosal side of the intestine. The intestine and PP were stored at −80°C until the levels of LTβR expression were measured by Western blot.
Western Blot for LTβR Expression
Tissues were homogenized in RIPA lysis buffer (Upstate, Lake Placid, NY) containing 1% of a protease inhibitor cocktail (Sigma-Aldrich). The homogenates were incubated on ice for 30 minutes, centrifuged at 16,000g for 10 minutes at 4°C, and the supernatants stored at −20°C until assayed. Protein concentration of each preparation was determined by the Coomassie dye-binding method using bovine serum albumin as standard. Solubilized protein was denatured at 95°C for 10 minutes with sodium dodecylsulfate and β-mercaptoethanol, and 20 μg of protein was separated in a denaturing 10% polyacrylamide gel. The proteins were transferred to a polyvinylidene fluoride membrane using a Tris-glycine buffer plus 20% methanol at 80 V for 50 minutes at 4°C. The membrane was blocked with 5% nonfat dry milk prepared in Tris-buffered saline with 0.5% Tween-20 (TBS-Tween) for 1 hour at room temperature with constant agitation, followed by incubation with a goat anti-LTβR antibody (R&D Systems, Minneapolis, MN) diluted 1:100 overnight at 4°C with agitation. The membrane was washed and incubated with rabbit anti-goat IgG-HRP conjugate (Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted 1:40,000 for 1 hour at room temperature. After the last wash, the membrane was incubated for 5 minutes with the substrate for HRP (ECL reagent, Pierce Biotechnology, Rockford, IL) and the bands detected using photographic film. The density of the protein bands was analyzed and quantified with the TotalLab imaging software (Nonlinear Dynamics, Durham, NC).
Experiment 2: Effects of LTβR Stimulation on Gut Mucosal Immunity
Previous work demonstrated that intestinal IgA levels and PP lymphocyte counts dropped after 2 days of PN.11 We first determined the optimal dose and time for the administration of the agonistic LTβR mAb in PN-fed mice. A single intravenous dose of 0.05, 0.5, or 5 μg of LTβR (n = 2–3 mice/dose) for 2 days had no effect on PP cell number nor on intestinal IgA levels (data not shown). Increasing the dose of the agonistic anti-LTβR mAb to 2 times/day resulted in increases in both PP cell number (at 0.5 and 5.0 μg) and in IgA levels (at 5.0 and 50 μg). Administration 3 times/day did not improve these responses. Therefore, a dose of 5 μg, i.v. of the agonistic LTβR mAb administered twice a day was selected for these experiments.
Two days after cannulation, 35 mice were randomized to chow (n = 11), PN plus 5 μg of agonistic LTβR mAb (n = 11), or PN plus isotype control antibody (IsoAb; n = 13). Chow and PN diets were identical to experiment 1. Chow mice received 200 μL of vehicle (PBS). PN + agonistic LTβR mAb, and PN + IsoAb groups received the same volume of hamster agonistic LTβR mAb or hamster IgG1κ, respectively.
Mice received IV catheters as described in experiment 1 with a 2 day recovery. After 2 days of treatment, mice were killed by exsanguination under anesthesia. The small intestine was excised from the duodenal bulb to ileocecal valve and the lumen flushed with 20 mL of HBSS. The intestinal contents were collected in plastic tubes and stored at −80°C for IgA analysis.
IgA Antibody Quantification
Total IgA in the intestinal wash was measured using a sandwich ELISA method. Briefly, 96-well ELISA plates (BD Biosciences, Bedford, MA) were coated with 50 μL of a 10 μg/mL goat anti-mouse IgA, α-chain specific (Sigma-Aldrich) in 0.1 mol/L coating buffer (carbonate-bicarbonate, pH 9.6), and incubated overnight at 4°C. The plate was washed and blocked with 100 μL of a 5% nonfat dry milk solution in TBS-Tween for 1 hour at room temperature. One hundred microliters of intestinal wash (diluted 1:100) or the IgA standards (seven 2-fold dilutions, from 1000 to 7.8 ng/mL, Sigma-Aldrich) were added in triplicate, and the plate incubated for 1 hour at room temperature. After 3 washes, 100 μL of a 1:500 dilution of the secondary antibody, goat anti-mouse IgA, α-chain specific-HRP conjugate (Sigma-Aldrich) were added and incubated for 1 hour at room temperature. The plate was washed, and 100 μL of the substrate solution (H2O2 and o-phenylenediamine) were added and incubated for 12 minutes at room temperature. The reaction was stopped by the addition of 50 μL of 2 mol/L H2SO4 and the absorbance read at 490 nm in a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). The concentration of IgA in the samples was obtained by the intrapolation of their absorbance values into the IgA standard curve, which was calculated using a 4-parameter logistic fit provided by the SOFTmax PRO software (Molecular Devices, Sunnyvale, CA).
Cell Isolation
Lymphocyte isolations from PP were performed following previously described protocol with minor modifications.8 Briefly, PP were excised from the serosal side of the intestine and teased apart. The fragments were incubated with RPMI-1640 (Mediatech Inc, Herndon, VA) containing 40 U/mL collagenase Type I (Sigma), 5% fetal bovine serum, 100 U/mL penicillin-streptomycin, and 2 mmol/L l-glutamine for 60 minutes at 37°C with constant rocking. The slurry was passed through a 100 μm nylon cell strainer (BD Falcon, Bedford, MA) and centrifuged at 1500 rpm for 5 minutes. The PP lymphocyte numbers counted in a hemacytometer after Trypan blue (Cellgro, Mediatech, Inc.) staining.
Statistical Analyses
All values were expressed as mean ± SD. Statistical analysis was performed by analysis of variance, followed by the Fisher's protected least significant difference post hoc test.
RESULTS
Experiment 1: Effects of Route of Nutrition on Gut LTβR Expression
Body Weight Change
There were no significant differences between groups in pre-experiment body weight (Table 1). At the end of feeding, chow mice weighed significantly more than CED or PN mice with no significant differences between CED and PN animals. Chow mice have approximately 1.5 g of residual feces, while the GI tracts of PN mice are empty. CED animals hold approximately 0.5 g of feces. Therefore, body weight differences are exaggerated in the chow group.
TABLE 1. Mice Body Weight
LTβR Expression in the Small Intestine and the PP
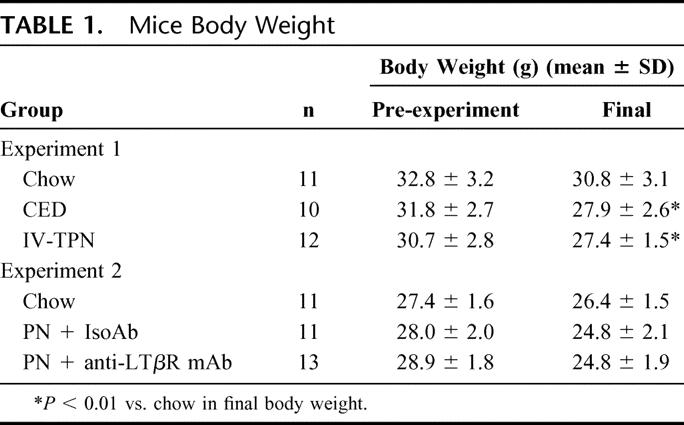
Lack of enteral stimulation with PN affected intestinal (Fig. 1) and PP (Fig. 2) LTβR expression. PN significantly reduced LTβR expression in the intestine (PN: 113 ± 42 vs. Chow: 167 ± 14 vs. CED: 167 ± 23 units, P < 0.05 for each) and in PP (PN: 104 ± 47 vs. Chow: 146 ± 38 vs. CED: 136 ± 26 units, P < 0.05 for each). There were no differences between chow and CED animals.
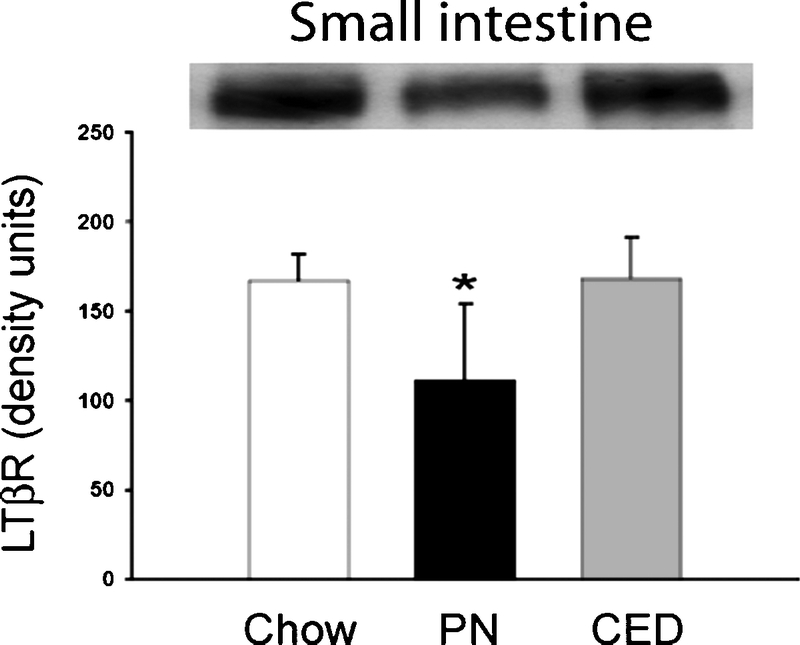
FIGURE 1. Western blot for LTβR in small intestine samples from chow, PN, and CED fed mice. PN decreased LTβR expression in the small intestine. *P < 0.05 for PN mice versus chow and CED mice.
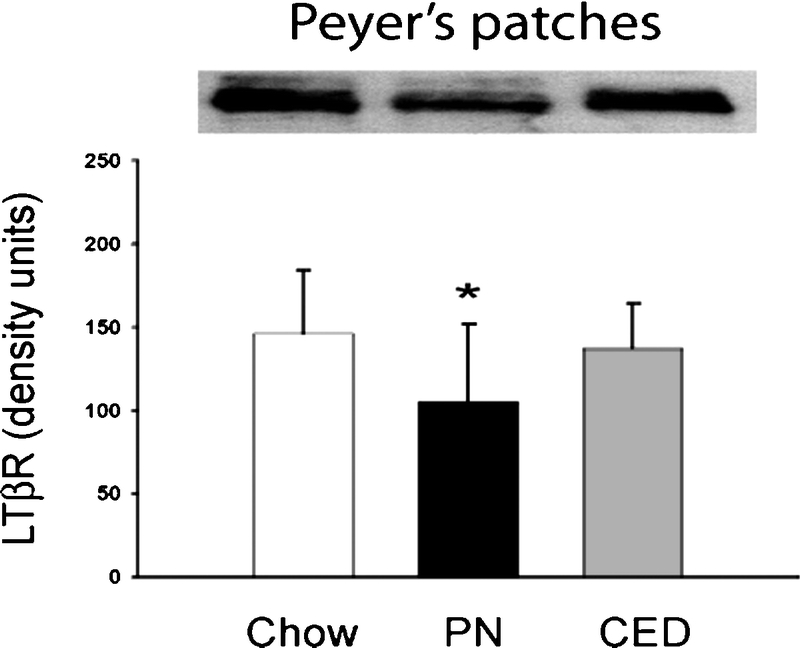
FIGURE 2. Western blot for LTβR in Peyer's patches from chow, PN, and CED fed mice. PN decreased LTβR expression in the Peyer's patches. *P < 0.05 for PN mice versus chow and CED mice.
Experiment 2: Effects of LTβR Stimulation on Gut Mucosal Immunity
Body Weight Change
The precannulation body weights of all groups were similar (Table 1). PN + IsoAb and PN + agonistic LTβR mAb mice weighed less than chow mice after the completion of the experiment, but these differences were not statistically significant.
Lymphocytes Numbers in PP
PP lymphocytes counts were significantly lower in the PN + IsoAb mice (cells) than chow mice (18.4 ± 5.6 × 106 vs. 29.6 ± 8.1 × 106 cells, P < 0.05) (Fig. 3). Administration of the agonistic LTβR mAb significantly increased cell counts (24.5 ± 7.3 × 106 vs. 18.4 ± 5.6 × 106 cells, P < 0.05) compared with PN + IsoAb group. There was with no significant difference between the chow and PN + agonistic LTβR mAb group.
FIGURE 3. Effects of agonistic anti-LTβR antibody on Peyer's patch lymphocyte cell counts. PN-induced decreased cell counts were reversed by injection of the agonistic anti-LTβR mAb. *P < 0.05 for PN + IsoAb mice versus chow or PN + agonistic anti-LTβR mAb mice.
Intestinal IgA Levels
Intestinal IgA levels were significantly lower in PN + IsoAb mice compared with chow mice (121 ± 55 μg vs. 205 ± 57 μg, P < 0.05) (Fig. 4). Administration of the agonistic LTβR mAb significantly increased intestinal IgA levels above the PN + IsoAb group (177 ± 78 μg vs. 121 ± 55 μg, P < 0.05). There was no significant difference in intestinal IgA levels between chow and PN + agonistic LTβR mAb groups.
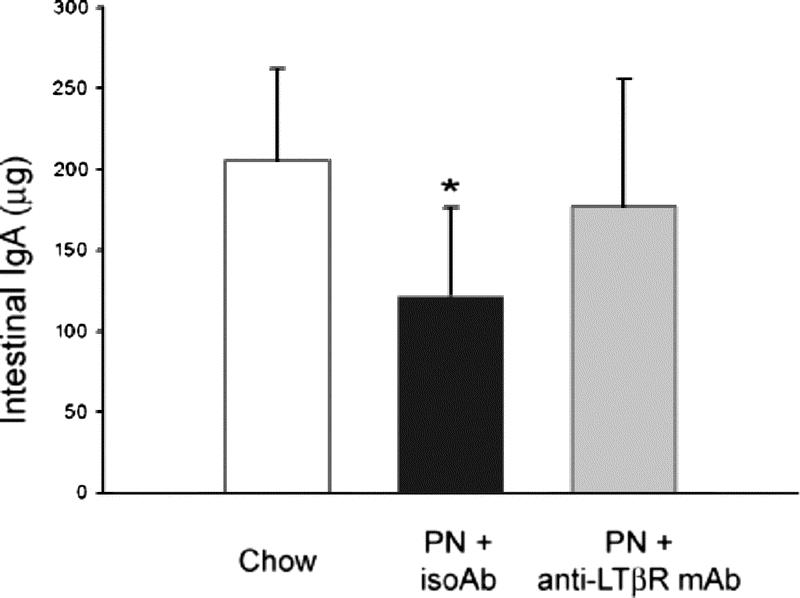
FIGURE 4. Effects of agonistic anti-LTβR antibody on intestinal IgA levels. Administration of agonistic LTβR antibody restored PN-induced depression of intestinal IgA levels. *P < 0.05 for PN + IsoAb mice versus chow or PN + agonistic anti-LTβR mAb mice.
DISCUSSION
Lack of enteral feeding creates a defect in established respiratory antiviral and antibacterial defenses of mice. After prior intranasal viral immunization, mice sterilize their respiratory tracts within hours of viral rechallenge via viral-specific IgA, which binds the virus and stops viral proliferation and shedding. Similarly, intratracheal administration of a lethal dose of Pseudomonas can be rendered nonlethal by prior immunization of mice with Pseudomonas antigen in liposomes. “Starving” the gut (but administering parenteral feeding to avoid lethal malnutrition) severely impairs the antiviral and antibacterial defenses, but the defect is reversible in 3 to 5 days with enteral stimulation. These results are consistent with the significant increase in pneumonia occurring in critically injured patients randomized to parenteral feeding compared with those patients fed enterally. Experimentally, it is clear that enteral feeding does more than just stimulate processes of digestion and absorption. Enteral feeding stimulates and maintains normal immunologic defenses necessary for an intact immunologic barrier against the external environment. Lack of enteral feeding creates an immunologic “gap” in this defense.
The MALT contains 50% of total body immunity and produces 70% of the body's total immunoglobulin in the form of secretory IgA (sIgA). The cells responsible for sIgA in the lamina propria of the aerodigestive tract are continually repopulated through education of naive T and B cells within the PP. The naive T and B cells express α4β7 and l-selectin on their surfaces which interact with MAdCAM-1 and Intercellular adhesion molecule-1 expressed on the high endothelial venules of the PP. Once the lymphocytes have migrated into the PP, they are sensitized and stimulated through interaction with dendritic cells and migrate to mesenteric lymph nodes where they mature and/or proliferate. After release into the thoracic duct, they are distributed by the vascular system to intestinal and extraintestinal sites. Approximately 80% of circulating murine lymphocytes are α4β7, supporting the role of the vascular tree in this immunologic system. Within a few hours of stopping oral intake, decreases in MAdCAM-1 mRNA are detectable with RT-PCR and after 24 hours, MAdCAM-1 protein expression drop in PP. Within 2 to 3 days of discontinuing enteral stimulation, dramatic decreases (by up to 50%) in T and B cells occur in the PP, lamina propria, and intraepithelial spaces. These changes occur simultaneously with decreases in the lamina propria T cell CD4/CD8 ratio and in the Th-2 type IgA-stimulating cytokines. These cytokines (IL-4 and IL-10) drop in proportion to respiratory and intestinal IgA levels. IL-4 levels also drop in the PP where IL-4 normally serves as an important stimulus for MAdCAM-1 expression. These cellular changes (but not the cytokine changes) in the PP and lamina propria of chow fed mice are reproduced if MAdCAM-1 is blocked by a specific blocking anti-MAdCAM-1 monoclonal antibody, MECA-367.
Functional LTβR is critical to an intact mucosal immune system. LTβR is found with the stroma of the PP and lamina propria. In PP, LTβR is required for differentiation of dendritic cells and for antigen presentation. In this site, it also stimulates the production of MAdCAM-1 and IL-4 through the activation of NFκB.24 Blockade of LTβR signaling reduces cells in lymph nodes27 and PP28 and reduces chemokine and MAdCAM-1 mRNA.27 In lamina propria, LTβR signal is important for IgA production. LTβR−/− mice fail to develop PP or lymph nodes and have decreased MAdCAM-1, IL-4, IL-10, and IgA levels. Stimulation of LTβR signaling is initiated via the TNF family of cytokines and in particular by members of the TNF superfamily lymphotoxin alpha (LTα) and LT beta (LTβ) present on activated T and B lymphocytes. The heterotrimer LTα1β2 expressed on these cells binds LTβR with high affinity.29
Lack of enteral feeding (with preservation of nutritional status using parenteral feeding) interferes with this LTβR-driven control system. To our knowledge, this is the first report demonstrating that enteral feeding regulates LTβR expression and that lack of enteral stimulation with PN impairs its expression. Our experiments convincingly showed that lack of enteral feeding reduced LTβR expression in the PP and intestine, PP lymphocyte counts, and intestinal IgA production. Exogenous administration of an LTβR agonist antibody restored these PN-induced decreased cell counts and intestinal IgA levels proving that down-regulation of LTβR expression is one mechanism of impaired mucosal immune system induced by lack of enteral feeding. In this experiment, there was no significant correlation between PP lymphocytes numbers and intestinal IgA levels (r = 0.29; P = 0.095, Pearson's coefficient). This is consistent with the finding that PP is not essential for intestinal IgA production.30 However, reduction of LTβR expression explains the reduction in IL-4 within the PP and lamina propria, the reduction in IL-10 within the lamina propria, and significant depression in MAdCAM-1 in the PP. Each of these has been shown to play a key role in maintaining an intact mucosal defense.
Since NFκB signaling from LTβR is responsible for production of IL-4, IL-10, and MAdCAM-1, we hypothesize that decreased NFκB activation via inhibited LTβR drive are related to these results. These effects in toto result in reduced intestinal IgA levels starting with impaired entry of naive T and B cells into the MALT and concluding with reductions in the Th-2 type IgA stimulating cytokines. Our current investigations are focused on these pathways consistent with our overall goal to identify new nontoxic strategies to decrease the infectious complications in the patients dependent on PN as their only source of nutrition support.
Discussions
Dr. Frederick A. Moore (Houston, Texas): Over 15 years ago, our group in Denver and Dr. Kudsk's group in Memphis independently performed prospective, randomized controlled trials that showed that early enteral nutrition compared to total parenteral nutrition reduces infections after major torso trauma. In the early 1990s, I gave numerous presentations describing these studies and was surprised at the resistance of some audiences in accepting what I perceived to be irrefutable evidence. In retrospect, I now recognize several reasons for this resistance.
First, TPN was invented by surgeons, and some people felt it was sacrilegious to propose that TPN could be bad. Second, while it is easy to talk somebody into the benefits of early enteral nutrition, in fact, when you go into the clinical arena and you try to administer early enteral nutrition, it is difficult. So in day-to-day practice, it is much easier to be a disbeliever and only start TPN in those patients who develop complications. Third, and probably the most important reason for the resistance, is that surgeons are trained to be skeptics. And back in the early 1990s, we really didn't have a good mechanistic explanation. Early on, we overemphasized the role of bacterial translocation. However, as we began to study the clinical relevance of bacterial translocation in our patients, it became painfully obvious that this could not be the unifying mechanism.
Dr. Kudsk has been working for over a decade on elucidating an alternative hypothesis. His studies demonstrate that the gut is a very important immunologic organ and if you starve it your patients will become immunocompromised. He has developed an excellent mouse model in which he has extensively characterized the affector and effector pathways of gut associated lymphoid tissue (GALT). He has repeatedly shown that lack of enteral stimulation rapidly downregulates the GALT. This causes systemic immunosuppression and renders the experimental animals susceptible to infectious challenges.
The data presented today are just a small piece of a big puzzle that he has been solving over the past decade. Given his extensive experience in this model, I see no need to challenge the methodology or the results. My comments will focus on relevance.
In this work presented today, you have been focused on a specific receptor that most of us have never heard of. You state in the paper that it is a receptor found in the stroma of Peyer's patches and in the lamina propria. Is it found elsewhere? Now, the immune response has redundant regulators. And I am always humbled in my own work, just when I think I know what is happening, somebody comes up with a new blocker or a new mediator. So do you believe that this is the key regulator? You have shown that bombesin protects against adverse effects of TPN in your GALT model. Is bombesin working through this receptor?
I would next like to focus on the clinical relevance of this model. In your clinical studies compared to our clinical studies, you enrolled on the most severely injured patients and emphasize that this is the group that benefits most by early enteral nutrition. Unfortunately, this is a group who will not tolerate full dose enteral nutrition early after trauma. Do you see the same positive effects on the GALT with less than full dose enteral nutrition? Is there a dose-response effect? Second, your animals are not severely stressed. Do you see similar effects in stressed animals?
Finally, in 1996 you published a noteworthy clinical trial in which you showed that feeding with an immune-enhancing enteral diet compared to a standard enteral diet further reduces nosocomial infections. This would indicate that the type of nutrients is important in modulating the immune response. Are these immune-enhancing nutrients working at the GALT level? I am particularly interested in glutamine. There are now 3 clinical trials in which high dose enteral glutamine has been shown to reduce infections. Do you think glutamine modulates GALT?
In conclusion, I congratulate Dr. Kudsk on his continued intriguing research. I look forward to the next piece of the puzzle and for the interventional trial he is proposing.
Dr. Kenneth A. Kudsk (Madison, Wisconsin): I agree with you. Enteral feeding is difficult and a challenge to administer it safely. There are certainly people who require parenteral nutrition, and we are trying to define whether parenteral feeding produces an immunologic gap which is important.
LT beta receptor is found throughout the immune system, particularly in lymph nodes, within the lamina propria, and within Peyer's patches. It is not found, for example, in nonimmune sites such as liver tissue, muscle tissue, and others. In fact, if animals are born without this particular molecule, they have no lymph nodes and no Peyer's patches, and they have an inadequate mucosal immune system.
Is it the key? Not entirely. We know from our work with bombesin and with glutamine supplementation of parenteral nutrition that we can improve mucosal immunity. But they do not work through MAdCAM, so I doubt that lymphotoxin B receptor is being affected by glutamine or bombesin. It is one thing to bring cells into and distribute them through the system, but it is another thing to keep these cells viable and functional. When you think about it, glutamine is upregulated during times of stress when we are not eating. I think it is a backup system under these conditions. When it becomes a conditional essential amino acid, it supports the system, which isn't getting normal types of enteral stimulation. Does bombesin work on this molecule? No, it doesn't because bombesin does not affect MAdCAM either. So these are probably peripheral effects on the mucosal immune system rather than entry and distribution effects.
You asked about the various models and dose response. I haven't varied doses, although it appears from other work, for example, Wes Alexander's work with the burn guinea pig model, that you need approximately 50% of the enteral feeding in order to see a beneficial effect. And I think that has been confirmed in another model.
Stress is different. Two things we've found in this system. First, if you give parenterally fed animals an infectious challenge they do not respond to make new immune GALT cells the way a chow animal does. So when our patients come in the intensive care unit, if you can draw the analogy, if they are met with a new sea of bacteria requiring an immunologic response, but TPN does not support that generation of a new response.
Second, while I haven't published any of my clinical data yet, I do know there is a very interesting thing that happens. When trauma patients are studied with quantitative bronchoalveolar lavage for IgA, there is a spike within 24 to 36 hours and then levels plummet. We took that into the animal model, gave them a simple stress, and at 6 hours there is an IgA spike associated with IL-6. By 24 hours, it is gone. If you pretreat animals with intravenous nutrition, you take away that spike.
Dr. John A. Mannick (Boston, Massachusetts): Dr. Kudsk, congratulations on the latest piece in this very nice body of work. If my memory serves me correct, lymphotoxin beta is really essential for germinal center formation anywhere in the lymphoid system. And I can imagine that it probably doesn't have anything much at all to do with migration. What does the architecture look like? Have we lost all germinal center formation and the mesenteric lymph nodes in the Peyer's patches when this phenomenon occurs? Is that what is going on?
Dr. Kenneth A. Kudsk (Madison, Wisconsin): Dendritic cells are dependent upon it. I have not done any immunohistochemistry on the Peyer's patches. All I know is that there is a marked reduction in cells. We tried to develop a dendritic cell separation experiment, but we really haven't gotten very far with it. I can't tell you much about the architecture changes within germinal centers.
Dr. John A. Mannick (Boston, Massachusetts): Finally, I probably missed it, but did you give these animals any lymphotoxin beta in any studies? Granted, its name means it is a toxic compound, so you have to be careful with the dose, but have you tried administering it and did I just miss that in your presentation?
Dr. Kenneth A. Kudsk (Madison, Wisconsin): No, we haven't given any lymphotoxin beta. It is a particular kind of lymphotoxin that causes these effects. Lymphotoxin used to be thought of as just a backup for TNA alpha. But there are different families within the TNA family and there only one, lymphotoxin alpha 1 beta 2, which works on LT beta receptor. Other lymphotoxins don't work there. We have not injected any.
Dr. Bruce M. Wolfe (Portland, Oregon): Your title, as well as Dr. Moore in his comments, suggests that the TPN itself is somehow deleterious. Are these effects due to withholding enteral feeding or may there be some negative impact of the macro nutrients in the TPN itself? In the clinical model, for example, we have learned that the fatty acid profile in the currently available IV fat emulsion is suboptimal, and we have also relatively recently learned of the critical importance of controlling hyperglycemia when TPN is administered.
Dr. Kenneth A. Kudsk (Madison, Wisconsin): That is a good question: is it that TPN is bad or that enteral is good? The fact that we can administer bombesin, which is similar to gastrin releasing peptide since they have the same functional 7 amino acids, and reverse most of these parenteral feeding changes in the mucosal immune anti-viral and anti-bacterial defenses, tells me that it may be the release of these neuropeptides and the hormones in response to feeding which maintains the system rather than a negative effect of the parenteral nutrition.
I don't think it is the hyperglycemia, because it is totally reversible with enteral feeding. I looked at hyperglycemia in my patients in the enteral-parenteral trial. The difference in glucose levels between the enterally and parenterally fed patients was 15 milligrams percent. I don't think I can explain the difference in infectious complication rates based totally on glucose.
Footnotes
Supported by NIH Grant No. R01 GM53439-06A1.
Reprints: Kenneth A. Kudsk, MD, 600 Highland Ave., H4/736 CSC, Madison, WI 53792-7375. E-mail: kudsk@surgery.wisc.edu.
REFERENCES
- 1.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg. 1992;216:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma: reduced septic morbidity. J Trauma. 1989;29:916–923. [DOI] [PubMed] [Google Scholar]
- 3.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets following severe trauma: an immune-enhancing diet (IED) reduces septic complications. Ann Surg. 1996;224:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore FA, Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma: a prospective randomized study. J Trauma. 1986;26:874–881. [DOI] [PubMed] [Google Scholar]
- 6.Moore FA, Moore EE, Kudsk KA, et al. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994;37:607–615. [DOI] [PubMed] [Google Scholar]
- 7.Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature? Nutrition. 2004;20:843–848. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Kudsk KA, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44–52. [DOI] [PubMed] [Google Scholar]
- 9.Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janu P, Li J, Renegar KB, et al. Recovery of gut-associated lymphoid tissue (GALT) and upper respiratory tract (URT) immunity following parenteral nutrition (TPN). Ann Surg. 1997;225:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132:1303–1309. [DOI] [PubMed] [Google Scholar]
- 12.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999;229:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renegar KB, Kudsk KA, DeWitt RC, et al. Impairment of mucosal immunity by parenteral nutrition: depressed nasotracheal influenza-specific secretory IgA levels and transport of parenterally fed mice. Ann Surg. 2001;233:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renegar KB, Johnson C, DeWitt RC, et al. Impairment of mucosal immunity by total parenteral nutrition (TPN): requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001;166:819–825. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CD, Kudsk KA, Fukatsu K, et al. Route of nutrition influences generation of antibody-forming cells (AFCs) and initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003;237:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. [DOI] [PubMed] [Google Scholar]
- 17.Sikorski EE, Hallmann R, Berg EL, et al. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-α and IL-1. J Immunol. 1993;151:5239–5250. [PubMed] [Google Scholar]
- 18.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Kudsk KA, DeWitt RC, et al. Route and type of nutrition influence IgA-mediated intestinal cytokines. Ann Surg. 1999;229:662–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller R, Krahl T, Sarvetnick N. Tissue-specific expression of interleukin-4 induces extracellular matrix accumulation and extravasation of B cells. Lab Invest. 1997;76:117–128. [PubMed] [Google Scholar]
- 21.Zarzaur BL, Fukatsu K, Johnson CJ, et al. A temporal study of diet-induced changes in Peyer patch MAdCAM-1 expression. Surg Forum. 2001;52:194–196. [Google Scholar]
- 22.Fukatsu K, Kudsk KA, Wu Y, et al. TPN decreases IL-4 and IL-10 mRNA expression in lamina propria cells but GLN supplementation preserves the expression. Shock. 2001;15:318–322. [DOI] [PubMed] [Google Scholar]
- 23.Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Immunology. 2003;3:642–655. [DOI] [PubMed] [Google Scholar]
- 24.Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525. [DOI] [PubMed] [Google Scholar]
- 25.Fagarasan S, Honjo T. Regulation of IgA synthesis at mucosal surfaces. Curr Opin Immunol. 2004;16:277–283. [DOI] [PubMed] [Google Scholar]
- 26.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. J Parenter Enteral Nutr. 1983;7:582–586. [DOI] [PubMed] [Google Scholar]
- 27.Dohi T, Rennert PD, Fujihashi K, et al. Elimination of colonic patches with lymphotoxin β receptor-Ig prevents Th2 cell-type colitis. J. Immunol. 2001;167:2781–2790. [DOI] [PubMed] [Google Scholar]
- 28.Browning JL, Allaire N, Ngam-ek A, et al. Lymphtoxin-β receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. [DOI] [PubMed] [Google Scholar]
- 29.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. [DOI] [PubMed] [Google Scholar]
- 30.Chin R, Wang J, Fu YX. Lymphoid microenvironment in the gut for immunoglobulin A and inflammation. Immunol Rev. 2003;195:190–201. [DOI] [PubMed] [Google Scholar]


