Abstract
Objective:
To determine the utility of hepatic resection (HR) in the treatment of patients with noncolorectal nonendocrine liver metastases (NCNELM).
Summary Background Data:
The place of HR in the treatment of NCNELM remains controversial, primarily due to the limitations of previously published reports and the heterogeneity of primary tumor sites and histologies.
Methods:
A multivariate risk model was developed by analyzing prognostic factors and long-term outcomes in 1452 patients with NCNELM treated with HR at 41 centers from 1983 to 2004.
Results:
Hepatic metastases were solitary in 56% and unilateral in 71% (mean diameter, 50.5 mm). Extrahepatic metastases were present in 22%. The most common primary sites were breast (32%), gastrointestinal (16%), and urologic (14%). The most common histologies were adenocarcinoma (60%), GIST/sarcoma (13.5%), and melanoma (13%). R0 resection was achieved in 83% of patients with a 60-day mortality rate of 2.3% and a major complication rate of 21.5%. Tumor recurred in 67% of patients (liver, 24%; extrahepatic, 18%; both, 25%). Overall and disease-free survivals at 5 years were 36% and 21% and at 10 years were 23% and 15%, respectively. In multivariate analysis, factors associated with poor prognosis were patient age >60 years, nonbreast origin, melanoma or squamous histology, disease-free interval <12 months, extrahepatic metastases, R2 resection, and major hepatectomy (all P ≤ 0.02). A prognostic model based on these factors effectively stratified patients into low-risk (0–3 points, 46% 5-year survival), mid-risk (4–6 points, 33% 5-year survival), and high-risk (>6 points, <10% 5-year survival) groups (P = 0.0001).
Discussion:
HR for NCNELM is safe and effective, with outcomes mainly dependent on primary tumor site and histology. For individual patients, a statistical model based on key prognostic factors could validate the indication for hepatic resection by predicting long-term survivals.
To determine the utility of hepatic surgery for noncolorectal nonendocrine metastases, long-term outcomes for 1452 patients were assessed. Five-year and 10-year overall survivals following hepatectomy were 36% and 23%, respectively. Ten independent prognostic factors were identified, allowing for the development of a risk model that may be helpful in counseling patients regarding the additive benefits of surgical therapy.
Although the liver is a frequent site for tumor metastases, the mechanisms for the development of liver metastases differ based on the location of the primary tumor site. In patients with primary tumors of the gastrointestinal tract (colorectal adenocarcinoma and gut-associated endocrine tumors), the most likely mode of spread to the liver is through portal venous drainage or via direct intraabdominal lymphatic channels. The rationale for liver resection in these cases is that the majority of the patient's tumor burden may be confined to the abdomen. Therefore, adequate treatment of the primary tumor combined with liver resection may provide a chance for cure. This rationale has proven to be correct for colorectal liver metastases, where 5-year survivals are routinely reported to be 40% and 10-year survivals as high as 25% have been documented.1–5
In contrast, most other liver metastases originate from tumors outside of the intraabdominal cavity. Most commonly, metastases from these tumors reach the liver via the systemic circulation, implying that extrahepatic sites may have an equal probability of being involved. Based on this rationale, hepatic resection of noncolorectal liver metastases has been approached with caution.
Many of the first reports to examine outcomes for patients with noncolorectal liver metastases treated with hepatic resection included patients with both endocrine and nonendocrine metastases6–15 (Table 1). These analyses demonstrated that patients with endocrine metastases were a unique group with a better prognosis than patients with noncolorectal nonendocrine metastases. Several subsequent studies on this topic have accounted for these survival differences and have excluded patients with endocrine metastases.16–23
TABLE 1. Review of Reports Describing Patients With Noncolorectal Liver Metastases Treated With Hepatic Resection
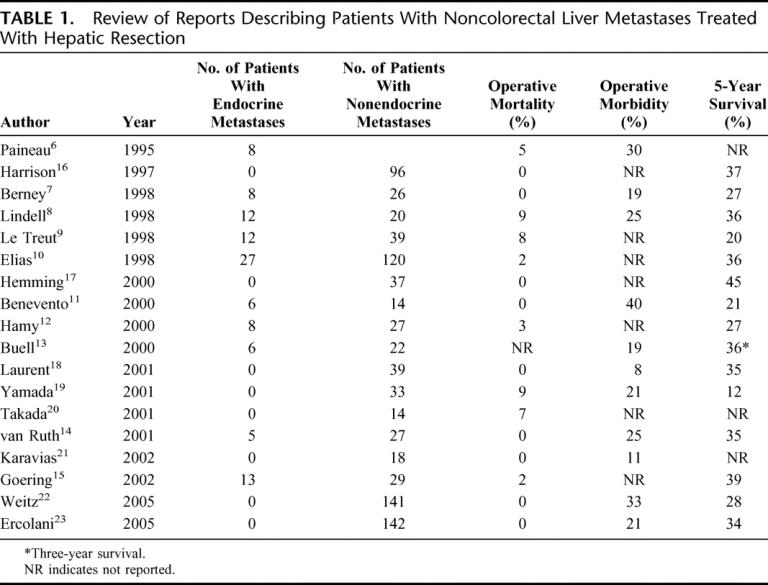
These studies have suggested that hepatic resection is safe and approximately as effective as hepatic resection for colorectal liver metastases, with reported 5-year survivals between 30% and 40% (Table 1). Although these data have contributed to our understanding of the natural history of these diseases and their responses to surgical therapy, the efficacy of liver surgery for patients with noncolorectal nonendocrine metastases has remained unclear because of the heterogeneity of primary tumor types, the frequent inclusion of patients with endocrine tumor metastases, and the limited numbers of patients reported.
To minimize the limitations of previous studies, our study was designed to analyze the outcomes for a large number of patients with noncolorectal nonendocrine metastases treated with hepatic resection at multiple centers. Overall survivals in this cohort were determined and analysis of prognostic factors was robust enough to create a risk-model for prognosis that may be helpful in selecting patients for resection.
METHODS
Patients
Study data were collected from a noncolorectal nonendocrine liver metastases specific questionnaire designed to capture patient, primary tumor, liver metastases, hepatectomy, and outcome variables. Discrepancies identified in reported data were corrected through further communication between the statistician/data manager and the specific center involved.
In total, 41 centers contributed historical and long-term outcomes data for 1452 patients with noncolorectal nonendocrine liver metastases consecutively treated with hepatic resection from 1983 to 2004.24
Statistical Considerations
Recurrence patterns and cause of death were assessed to determine overall, recurrence-free, and disease-free survivals for the entire cohort. For univariate analysis of prognostic factors, survivals were determined with Kaplan-Meier survival curves and compared using the log-rank test. Factors with univariate significance at a level of P ≤ 0.15 were entered into multivariate Cox proportional hazards models. In multivariate models, a P value ≤0.05 was considered evidence of independent statistical significance. Prognostic factors identified in multivariate analysis were selected for inclusion in a risk model based on the relative risk ratio of the prognostic factor weighted by the number of patients demonstrating the factor. The power of the model to differentiate long-term outcomes based on risk factors was determined by analyzing risk groups with the log-rank test and Kaplan-Meier curves. All statistical calculations were performed with SAS software (SAS Institute, Inc., Cary, NC).
RESULTS
Evolution of Surgical Treatment of Noncolorectal Nonendocrine Liver Metastases
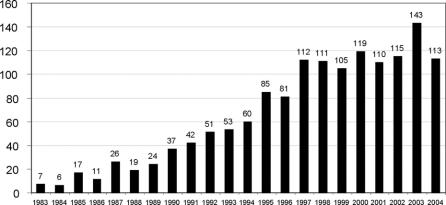
Trends in hepatectomy utilization were determined by assessing the number of hepatectomies performed per annum during the study period. This analysis identified an increase in the use of hepatic resection over time for patients with noncolorectal nonendocrine liver metastases (Fig. 1). During the 1980s, the median number of hepatectomies for noncolorectal nonendocrine metastases per annum did not exceed 17. During the 1990s, this number rose to 70 and, during the most recent decade of the 2000s, the median number of hepatectomies per year was 115 with a peak of 143 cases in 2003. Four centers registered more than 100 patients (range, 113–231 patients), representing 50% of all patients contributed to the study. For the remainder of the patients, the distribution of center volume was as follows: 16 centers entered less than 10 patients, 19 centers entered from 10 to 50 patients, and 2 centers entered from 50 to 100 patients.
FIGURE 1. Annual number of hepatic resections for patients with noncolorectal nonendocrine liver metastases at 41 centers from 1983 to 2004.
Patient and Tumor Characteristics
The mean age of patients was 53 years (range, 10–87 years). The male-to-female ratio was 1:1.7. The most common primary tumor sites represented were breast cancer (460, 32%), gastrointestinal cancer (230, 16%), urologic cancer (206, 14%), and melanoma (148, 10%). For the 81% of patients with known primary tumor histology, the most common histologies were adenocarcinoma (60%), sarcoma and other stromal tumors (14%), and melanoma (13%).
Primary tumors had been treated with surgical resection in 90% of patients. Preoperative and postoperative chemotherapy was used to treat primary tumors in 13% and 42% of patients, respectively. The liver metastases were synchronous (diagnosed within 3 months of primary tumor treatment) in 24% of patients and metastases were metachronous in 76% of patients. Overall, the mean time from treatment of the primary tumor to diagnosis of the metastases was 38 months (range, 0–448 months).
Forty-two percent of patients received adjuvant chemotherapy prior to hepatic resection. For 8% of patients, liver metastases were initially considered unresectable and resection was only attempted following significant response to this therapy. Liver metastases were solitary in 56% of patients and numbered less than 4 in 83% of patients. In 71% of patients, metastases were unilateral. The mean size of the largest metastasis was 51 mm (range, 7–270 mm). At least one extrahepatic metastatic disease site was present either prior to or at the time of liver resection in 22% of patients.
Hepatectomy Characteristics
The mean time from diagnosis of liver metastases to hepatic resection was 7 months. Anatomic resections based on the segments defined by Couinaud25 were performed in 77% of patients. Major hepatectomy, defined as resection of more than 2 anatomic segments, was required in the majority of patients (55%). Negative resection margins (R0) were achieved in 83% of hepatectomies. Microscopic (R1) and gross positive (R2) margins were present following 8% and 9% of hepatectomies, respectively.
During the 2-month period following hepatectomy, the perioperative mortality was 2.3%. Morbidity was classified as “local” if it occurred within the field of liver resection and “general” if distant from the operative field. Local complications were reported in 14% of patients and general complications were reported in 15% of patients. The mean postoperative inpatient hospitalization was 14 days (range, 1–106 days).
Cohort Survivals
Overall Survivals
After a mean follow-up interval of 31 months (range, 0–258 months), the overall 5-year and 10-year survivals for the entire cohort were 36% and 23%, respectively, with a median overall survival of 35 months (Fig. 2A). A total of 209 patients (14%) were actual survivors 5 years following first hepatectomy, of whom 46 patients (4%) were alive after 10 years.
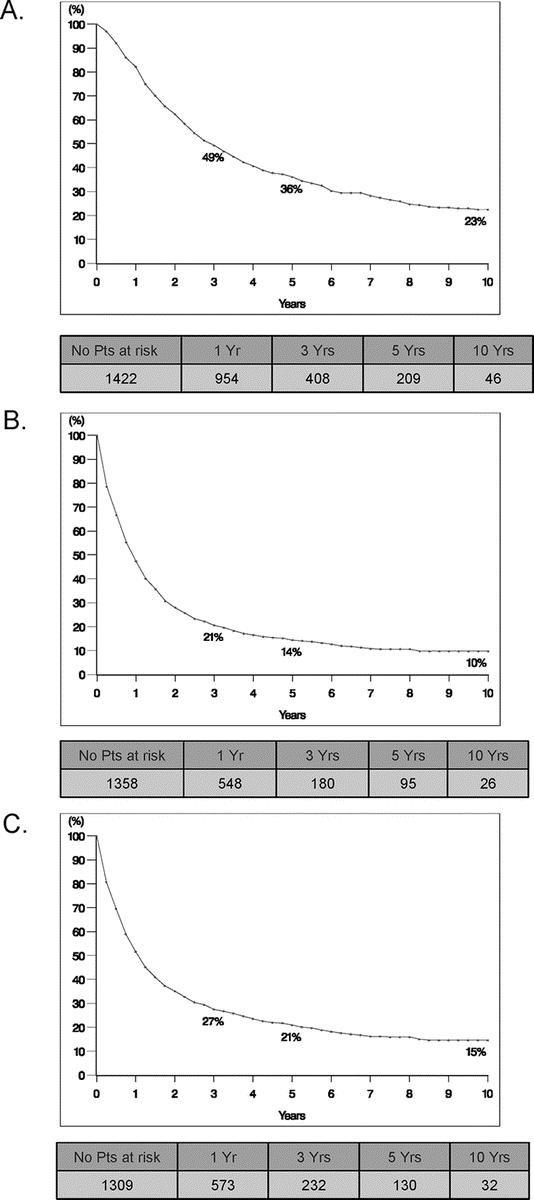
FIGURE 2. Survivals for study patients with noncolorectal nonendocrine liver metastases following hepatic resection. A, Overall survival. B, Recurrence-free survival. C, Disease-free survival.
Disease-Free Survivals
During posthepatectomy follow-up, recurrent liver metastases were identified in 49% of patients. These metastases were solely intrahepatic in 24% of patients and were associated with extrahepatic metastases in 25% of patients. From the group of 331 patients with only intrahepatic recurrences, 105 patients (32%) underwent a second hepatectomy. Subsequent intrahepatic metastases were treated with third hepatectomy in 14 patients, fourth hepatectomy in 2 patients, and fifth hepatectomy in 2 patients. Initial extrahepatic metastases were surgically treated in 329 patients (23%), and subsequent recurrences were surgically treated in 121 of these 329 patients (37%).
Following first hepatectomy, the 5-year and 10-year recurrence-free survivals were 14% and 10%, respectively, with a median recurrence-free survival of 11 months (Fig. 2B). When accounting for patients who were free of disease at latest follow-up (following resection of recurrent intrahepatic and/or extrahepatic metastases), the 5-year and 10-year disease-free survivals were 21% and 15%, respectively, with a median disease-free survival of 13 months (Fig. 2C).
Analysis of Outcomes Based on Primary Tumor Site
Breast Primary Tumors
Patients with liver metastases from breast primary tumors represented the largest subset in the series (n = 460) (Table 2). Following hepatic resection, these patients experienced 5-year and 10-year survivals of 41% and 22%, respectively, with a median survival of 45 months.
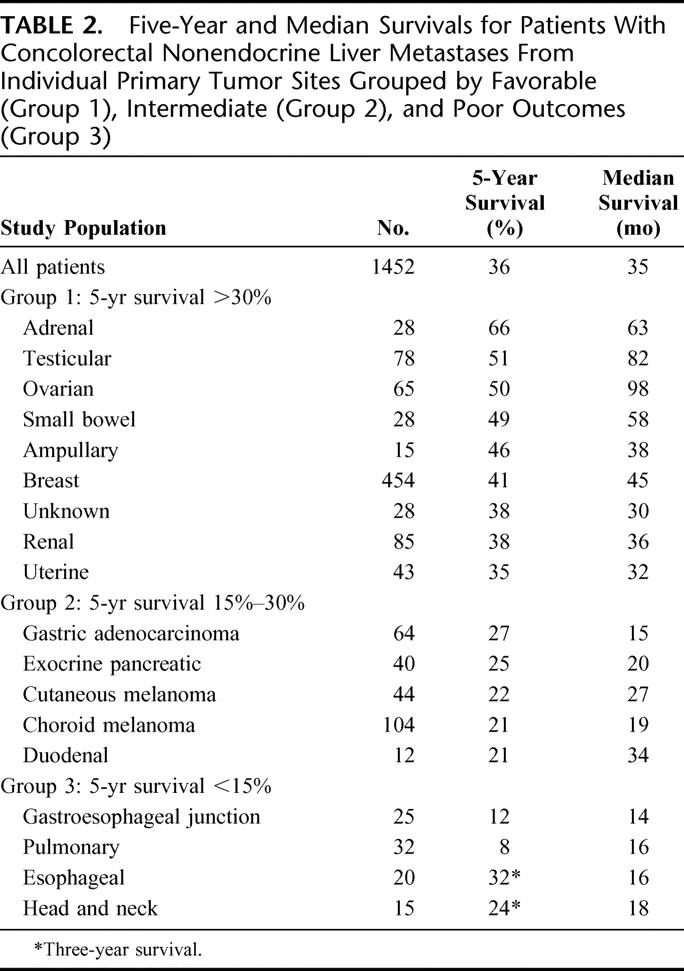
TABLE 2. Five-Year and Median Survivals for Patients With Concolorectal Nonendocrine Liver Metastases From Individual Primary Tumor Sites Grouped by Favorable (Group 1), Intermediate (Group 2), and Poor Outcomes (Group 3)
Gastrointestinal Primary Tumors
The second largest subset of patients in the series had liver metastases that originated from gastrointestinal primary tumors (n = 230). In general, these patients experienced favorable to intermediate survivals following resection, including an overall 5-year survival of 31% and a median survival of 26 months. However, within the gastrointestinal site category, some groups experienced relatively better survivals (ie, small bowel 5-year survival, 49%), whereas other sites experienced poor outcomes (ie, gastroesophageal junction 5-year survival, 12%).
Urologic Primary Tumors
Metastases from urologic primary tumors represented the third largest subset in the series (n = 206). These primary tumor sites were associated with a 5-year survival of 48% and a median survival of 51 months. In descending order, adrenal, testicular, and renal metastases were associated with 5-year survivals of 66%, 51%, and 38%, respectively.
Melanoma Primary Tumors
Melanomas represented the fourth highest contribution of a tumor site to the study (n = 148). This included 104 patients with choroid melanoma and 44 patients with cutaneous melanoma. The 5-year survivals for each of these melanoma types were 21% and 22%, respectively. Analysis of each of these subgroups found that choroid melanomas were commonly associated with multiple intrahepatic tumors but were less likely than cutaneous melanomas to be associated with extrahepatic disease.
Gynecologic Primary Tumors
Patients with gynecologic primary tumors accounted for the fifth highest contribution to the study (n = 126). Overall these tumor sites were associated with a 5-year survival of 48%. However, the 5-year survival for patients with ovarian primary tumor sites (50%) exceeded that of patients with uterine primaries (35%).
Pancreaticobiliary Primary Tumors
Patients with primary tumors of pancreatic or biliary origin represented the sixth largest contribution to the study (n = 84). This included 41 patients with an exocrine pancreatic primary, 23 patients with gallbladder primary, 15 patients with ampullary primary, and 5 patients with other biliary primary tumor locations. In general, this subgroup experienced an intermediate 5-year survival of 27%. Only those patients with ampullary primary tumors had a favorable 5-year survival (46%). Patients with liver metastases from pancreatic primary tumors had a 5-year survival of 25%, and the subset with pancreatic adenocarcinoma had a 5-year survival of 20%.
Head, Neck, and Pulmonary Primary Tumors
Patients with head, neck, and pulmonary primary tumors represented the seventh highest contribution to the study (n = 50). In general, these patients had tumors of squamous cell histology and they experienced poor outcomes following hepatic resection with 5-year survivals less than 15%.
Primary Tumors of Unknown Origin
Twenty-nine patients with tumors of unknown origin were included in the study. This indication was associated with a 5-year survival of 38%.
Analysis of Outcomes Based on Primary Tumor Histology
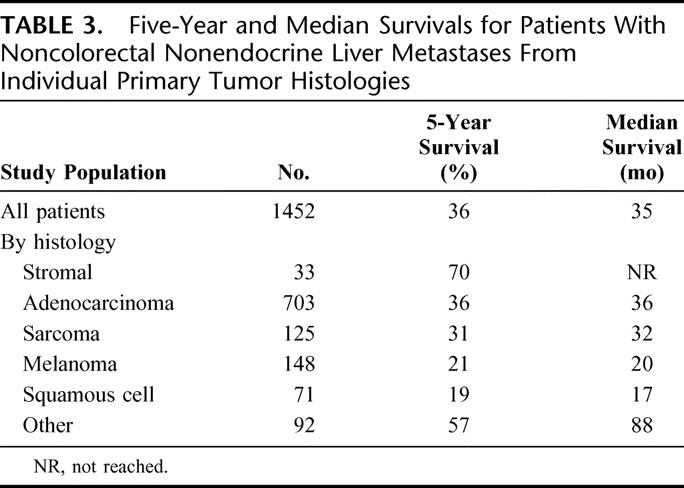
In addition to primary tumor site, patients were categorized based on primary tumor histology (Table 3). After excluding the 280 patients with indeterminate primary tumor histology, the relative distribution included adenocarcinoma in 60% of patients, sarcomas and stromal tumors in 14% of patients, melanoma in 13% of patients, squamous cell tumors in 6%, and other mixed histologies in 7%. When survivals were assessed based on the primary tumor histology, the highest 5-year survivals were observed in patients with stromal (mainly GIST) tumors (70%), adenocarcinomas (36%), and sarcomas (31%), while intermediate 5-year survivals were seen in patients with melanoma (21%) and squamous cell tumors (19%).
TABLE 3. Five-Year and Median Survivals for Patients With Noncolorectal Nonendocrine Liver Metastases From Individual Primary Tumor Histologies
Analysis of Prognostic Factors
Univariate analysis identified 16 factors associated with overall survival (Table 4). These were categorized into 4 groups: patient factors, primary tumor factors, hepatic metastases factors, and hepatectomy factors. To determine which of these factors was independently associated with outcome, a Cox proportional hazards model was used. A total of 974 of the 1452 study patients (67%) with complete data for all factors were entered into the model. This analysis determined that 10 factors were independently associated with poor outcome (ie, shortened overall survival time). These factors included:
TABLE 4. Prognostic Factors Identified in Univariate and Multivariate Outcomes Analysis for Study Patients With Noncolorectal Nonendocrine Liver Metastases Treated With Hepatic Resection
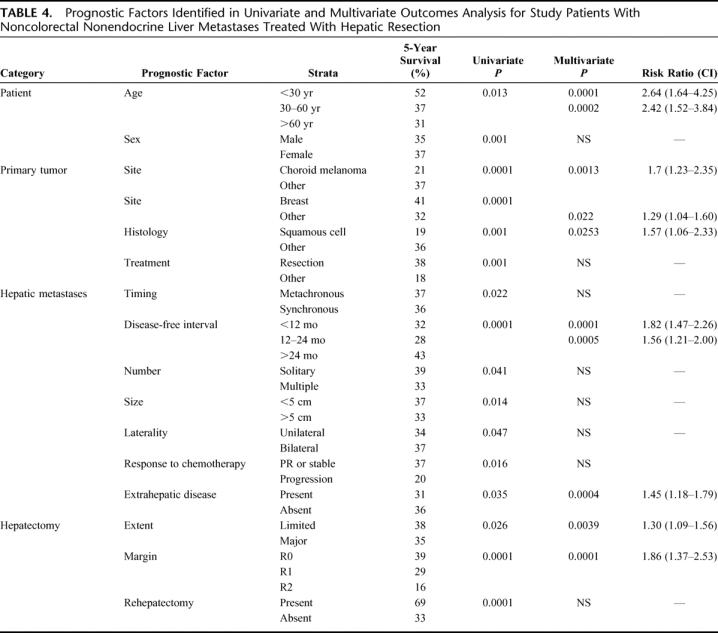
Patient Factors
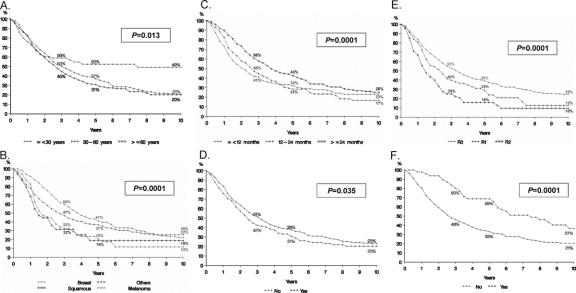
Age greater than 60 years and age greater than 30 years (Fig. 3A).
FIGURE 3. Survivals for individual prognostic factors based on univariate and multivariate analysis. A, Age. B, Primary tumor site and histology. C, Disease-free interval from treatment of primary tumor to diagnosis of liver metastases. D, Extrahepatic disease. E, Resection margin. F, Repeat hepatectomy for hepatic recurrence.
Primary Tumor Factors
Tumors other than breast, squamous histology and choroid melanoma (Fig. 3B).
Hepatic Metastases Factors
Disease-free interval between treatment of primary tumor and diagnosis of metastasis less than 12 months, less than 24 months (Fig. 3C) and the presence of extrahepatic disease prior to or at the time of hepatectomy (Fig. 3D). For the 42% of patients who received prehepatectomy chemotherapy, tumoral response was associated with outcome. Compared with patients with stable disease or partial response during therapy, patients with disease progression experienced lower 5-year survival rates (37% vs. 20%, univariate P = 0.016).
Hepatectomy Factors
R2 resection (Fig. 3E) and need for a major hepatectomy (≥3 segments). In univariate analysis, repeat hepatectomy for intrahepatic recurrence was associated with significantly higher 5-year survival rates than single hepatectomy (69% vs. 33%, P = 0.0001, Fig. 3F). However, this factor was not found to be significant in multivariate analysis.
Analysis of Outcomes Based on the Time Period of Inclusion
With regard to the time period of inclusion, hepatic resections performed during the most recent period (2000–2004) were associated with 5-year survivals of 45% (n = 586 patients). In contrast, lower 5-year survivals were seen following hepatectomies performed during the period of 1995 to 1999 (36%, n = 486 patients) and following hepatectomies performed prior to 1995 (33%, n = 350). However, these differences did not achieve statistical significance (P = 0.28).
Development of a Risk Model
Based on the results of multivariate prognostic factor analysis, a risk model was created. Numeric scores were given for 6 sets of variables. One point was assigned for the presence of each of the following factors: extrahepatic metastases present prior to or at the time of hepatectomy, major hepatectomy, and R2 resection. Absence of these factors resulted in an assignment of 0 points for these categories. Two factors (patient age and length of disease-free interval between treatment of the primary tumor and diagnosis of metastases) were stratified on a 3-point scale. Patient's less than 30 years of age were assigned 0 points while those 30 to 60 years of age were assigned 1 point and patients older than 60 years were assigned 2 points. Patients with a disease-free interval greater than 24 months were assigned 0 points while those with a disease-free interval between 12 and 24 months were assigned 1 point and patients with a disease-free interval less than 12 months were assigned 2 points. Finally, the primary tumor characteristics (site and histology) were stratified on a 4-point scale. Patients with breast primary tumors were assigned 0 points, patients with squamous histology were assigned 2 points, and patients with melanoma were assigned 3 points. All other patients were assigned 1 point.
Based on this system, a scale of 0 to 10 points was obtained with an estimated 5-year survival decreasing from 69% for patients with 0 points to 0% for patients with 10 points (Table 5). Estimated 5-year survival was >30% for patients having 0 to 3 points, 10% to 30% for those having 4 to 6 points, and <10% for those having >6 points. Based on this analysis, patients were then grouped by combining patients with 0 to 3 total points (n = 456 patients), 4 to 6 total points (n = 679 patients), and 7 to 10 total points (n = 35 patients). This stratification was strongly associated with outcome. Patients with 0 to 3 points had a 5-year survival of 46% (range, 36%–69%) while patients assigned 4 to 6 points had an intermediate 5-year survival of 33% (range, 5%–46%) and patients with more than 6 points experienced a 5-year survival of only 2% (range, 0%–11%) (P = 0.0001) (Fig. 4).
TABLE 5. Estimated Survivals Following Hepatic Resection for Noncolorectal Nonendocrine Liver Metastases as a Function of Risk Model Score
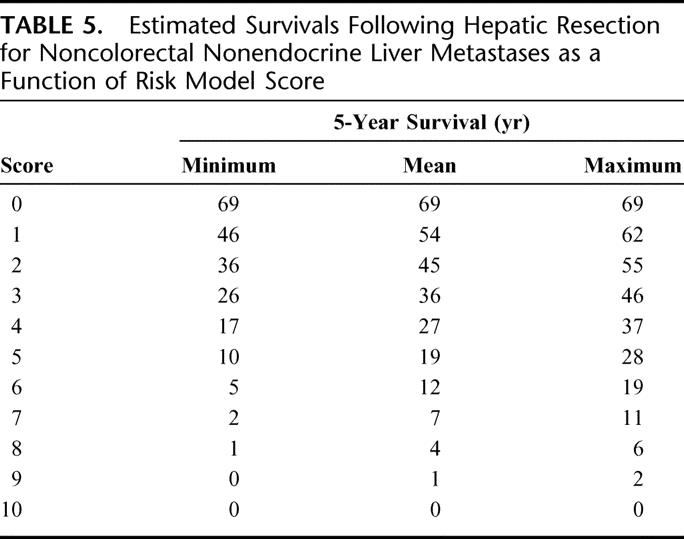
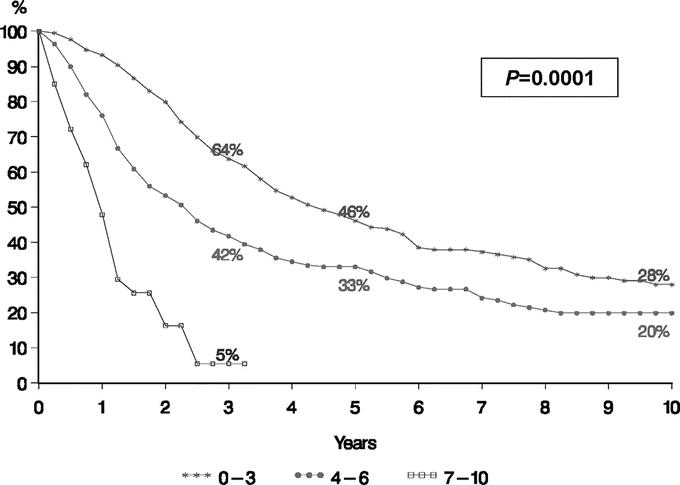
FIGURE 4. Analysis of survivals based on a risk model for patients with noncolorectal nonendocrine liver metastases. Points assigned as follows: extrahepatic metastases present prior to or at the time of hepatectomy = 1 point; major hepatectomy (>2 segments) = 1 point; R2 resection = 1 point. Absence of these factors resulted in an assignment of 0 points for these categories. Patient age less than 30 years = 0 points; 30–60 years = 1 point; greater than 60 years = 2 points. Patient with a disease-free interval greater than 24 months = 0 points; 12–24 months = 1 point; less than 12 months = 2 points. Patient with breast primary tumor = 0 points; squamous primary tumor histology = 2 points; choroids melanoma primary tumor = 3 points; all other primary tumor sites and histologies = 1 point.
DISCUSSION
In the English-language literature, there are more than 80 studies documenting outcomes in patients with noncolorectal liver metastases treated with hepatic resection. Mostly, these studies report single-institution experiences with a wide variety of primary tumor types distributed over a small number of patients.6–23 The ability to draw strong conclusions from these studies is further limited by the frequent inclusion of patients with liver metastases from endocrine tumors, who are now known to have a favorable prognosis. In total, only 8 studies can be identified that focus on more than 10 patients with only noncolorectal, nonendocrine metastases treated with hepatectomy.16–23
Although the reported 5-year survival rates following hepatic resection for noncolorectal nonendocrine liver metastases are similar to those achieved following resection of colorectal liver metastases,1–3 there are several important differences in these 2 patient groups. First, it is likely that the patients with noncolorectal nonendocrine metastases are more selected. Second, whereas it is nearly impossible for patients with colorectal liver metastases to achieve 5-year survivals without hepatic resection, the specific survival benefit of surgical therapy for noncolorectal nonendocrine metastases is difficult to differentiate from that of medical therapy, or from the natural history of the disease. Therefore, the efficacy of resection for noncolorectal nonendocrine metastases remains unproven.
Our study assessed outcomes for 1452 patients treated at 41 centers. This analysis determined that the overall survival following hepatic resection was 36% at 5 years with 209 actual 5-year survivors and 23% at 10 years with 46 actual 10-year survivors. The disease-free survivals at these same time points were 21% and 15%, respectively. Given that hematogenous dissemination of tumor cells is responsible for the majority of liver metastases in patients with noncolorectal nonendocrine primaries, a 36% 5-year survival rate following hepatic resection is notable. These data demonstrate that hepatic resection offers as many as many as a third of selected patients with noncolorectal nonendocrine liver metastases the possibility of long-term survival.
Certainly, there are limitations to a retrospective analysis conducted on patients treated over a long time interval. Despite the large number of patients included in the study, this was a highly selected cohort, representing only a fraction (the “surgical” fraction) of the total number of patients with liver metastases from noncolorectal nonendocrine primary tumors diagnosed during the study period. However, by including patients treated at 41 different surgical centers, our results may be less subject to selection bias than previously reported single-institution experiences and may better represent common practice. In addition, the long time period of the study allowed us to document the impact of the significant advances that have been made in both hepatic surgery and medical oncology during this interval. Patients operated before 1995 experienced lower 5-year survivals (33%) compared with patients operated between 1995 and 2000 (36%). And those patients operated after 2000 experienced even better survivals (45%).
Although over half of the patients were treated with a major hepatectomy, the 60-day operative mortality was only 2.3%. This is a relatively low mortality rate considering that multicenter studies commonly report higher mortality rates than smaller, single-center studies.6,8,19,20 In addition, our analysis showed a progressive increase over time in the number of patients with noncolorectal nonendocrine liver metastases treated with resection. Therefore, it appears that the risk of hepatic resection has diminished even as the indications have expanded.
By analyzing the outcomes following hepatic resection, the first question that our study addressed was: What is the efficacy of hepatic resection for patients with noncolorectal nonendocrine liver metastases? The results from our study that support the use of hepatic resection include data on the safety of resection and the overall survivals for patients in our cohort. The survival benefit observed in association with repeat hepatectomy for recurrence (5-year survivals: 69% vs. 33%) further supports the inclusion of surgical therapy in the multidisciplinary care of these patients. Finally, for the majority of tumors types analyzed, the outcomes observed in patients selected for hepatic resection were better than those achieved with currently available nonsurgical therapies, suggesting that hepatic resection may provide an independent survival benefit.
The second critical question that our study addressed was: For which tumors, within the broad range of noncolorectal nonendocrine tumors that metastasize to the liver, is hepatic resection most useful? To answer this question, we examined outcomes based on patient, primary tumor, liver metastases, and hepatectomy variables, and developed a risk model to help further stratify outcomes.
In terms of primary tumor factors, outcomes analysis indicated that individual tumor sites and histologies can be grouped into 3 main categories (favorable, intermediate, and poor) based on 5-year survivals. Patients with urologic and gynecologic primary tumors faired well with 5-year overall survivals of 48% and 42%, respectively. Within these 2 groups, patients with adrenal, testicular, ovarian, uterine, and renal cancer metastases all experienced 5-year survivals greater than 30%. These primary tumor groups with favorable outcomes were joined by patients with metastases from breast cancer, who experienced 5-year and 10-year survivals of 41% and 22%, respectively. Given these favorable outcomes and the large number of patients with breast cancer metastases in the study (n = 460), the presence of a breast primary tumor became an important factor in our risk model.
In contrast, patients with liver metastases from gastrointestinal primary tumors generally experienced intermediate outcomes following hepatic resection (5-year survivals from 15% to 30%). Patients with metastases from the stomach and duodenum experienced 5-year survivals between 15% and 30%, but patients with esophageal and gastroesophageal junction tumors experienced 5-year survivals less than 15%. Patients with metastases from pancreatic adenocarcinoma who were submitted to hepatic resection had a lower survival than those with metastases from ampullary primary tumors (5-year survival, 20% vs. 46%). Patients with melanoma primary tumors, who represented 10% of all of the patients entered into the study, also experienced intermediate outcomes, with 5-year survivals for patients with choroid and cutaneous melanoma of 21% and 22%, respectively. Finally, patients with primary tumors from the head, neck, and lungs (mainly squamous cell histologies) consistently experienced poor 5-year survivals (<15%).
In addition to these tumor-specific factors, multivariate analysis identified several other factors independently associated with poor outcome following hepatectomy. These included patient-related factors, such as patient age with 2 significant breakpoints (>30 years and >60 years), and hepatic metastases-specific factors, including a disease-free interval between treatment of the primary tumor and diagnosis of the metastases with 2 significant breakpoints (<12 months and <24 months) and the presence of extrahepatic disease. Finally, 2 hepatectomy specific factors, a margin-positive resection and the need for major hepatectomy, were independently associated with poor outcomes.
Although the identification of 10 independent prognostic factors greatly adds to our understanding of the surgical treatment of these diseases, it may be difficult to assimilate all of these data for a given patient. Therefore, we aggregated the most significant of these factors into a prognostic scoring system. The risk model proved to distinctly separate patients based on outcomes with median 5-year survivals of 46% for low-risk patients, 33% for intermediate-risk patients, and only 5% (at 3-years) for high-risk patients. For example, an older patient with significant intrahepatic tumor burden from a nonbreast primary tumor, particularly of squamous histology, is likely to have a high recurrence rate and experience poor survivals following hepatic resection. In contrast, a young patient with limited metastases from a breast primary adenocarcinoma can expect significant benefits from hepatic resection in terms of both recurrence-free and overall survivals.
The complexity of these clinical situations is further increased by the need to integrate the response to chemotherapy in the decision-making process. Given the long time period of patient inclusion and the relatively recent emergence of effective therapies for most tumors, the prognostic value of chemotherapy response was not fully explored in our study. However, our analysis did indicate that chemotherapy response (diminution or stabilization) before hepatic surgery was associated with a higher 5-year survival compared with tumoral progression (37% vs. 20%). Therefore, it is critical that treatment decisions for patients with metastases from noncolorectal nonendocrine tumors be made by multidisciplinary treatment groups that include medical oncologists, radiation oncologists, and surgeons. The prognostic factors identified in our study and the risk model that was developed from these factors may help these groups predict the additive benefit of surgical treatment.
The results of this study suggest that the dogma that “there is no role for the surgical treatment of liver metastases from noncolorectal nonendocrine tumors” is no longer valid. However, in contrast to the treatment of colorectal liver metastases where surgery has the key role and chemotherapy acts as an adjuvant treatment, it is likely that the reverse situation is currently observed for noncolorectal nonendocrine metastases, where systemic chemotherapy plays the key role and surgery acts as an adjuvant therapy. As such, increased utilization of surgery in the multimodality treatment of patients with metastatic liver tumors will depend on continued progress in the development of active systemic treatments. In current practice, liver surgery for noncolorectal nonendocrine metastases should be considered only when the metastatic disease is well controlled or responding to systemic therapy. When applied in these situations, surgery may be able to offer selected patients a real benefit in long-term survival.
ACKNOWLEDGMENT
The authors thank Valerie Delvart for her expert contribution to data management and statistical analysis.
Discussions
Dr. Michael Choti (Baltimore, Maryland): Thank you, Dr. Adam, for the opportunity to review this manuscript and comment on this presentation. The authors are to be congratulated on by far the largest reported series of hepatic resection of non-colorectal, non-neuroendocrine liver metastases. And while this is a retrospective cohort study, it is nonetheless important as it provides of us with much needed help in managing these patients for which we are being asked to treat with increasing frequency.
Let me first comment on the surprisingly superior outcomes reported in this series, with overall 5-year survival of 36%. Even for resection of breast cancer metastases, you report a 5-year overall survival of 41%. These results are far better than other reports. In fact, they are comparable to long-term outcomes of hepatic colorectal metastases. Clearly, careful patient selection is an important reason for these excellent results. For example, there were 40 patients who had liver resection for metastatic adenocarcinoma of the pancreas. You find a 5-year survival rate of 25% and a median survival of 20 months, which is as good or better than many reports following resection of primary pancreatic cancer. Therefore, one needs to interpret these results with some caution. We still cannot definitively conclude how much the liver resection itself is contributing to the outcome and how much is from the favorable biology of highly selected patients who might have done well regardless.
I have 3 questions for you. The first is related to the prognostic score, and particularly the impact of age. Two negative points are given for with an age over 60; yet there is only one point if, for example, the primary tumor is of pancreatic or gastric origin, or the hepatectomy is an R2 resection. This seems at odds with our expectations. Can you comment on why age appears to be such a powerful prognostic factor in this analysis?
Second, as you and your group have reported in the past, response or progression to preoperative chemotherapy is emerging as an important prognostic factor following liver resection of colorectal metastases. Did you look at incorporating chemotherapy response or progression into your prognostic score for non-colorectal metastases?
Finally, can you comment on the role of tumor ablation in the management of patients with non-colorectal non-neuroendocrine liver metastases? Does it make sense to consider these less invasive approaches when treating patients who may have higher risk of distant recurrence or at least less clear benefit of local therapy, or should liver resection be the gold standard when possible even in these patients?
Dr. Harold J. Wanebo (Providence, Rhode Island): I would also echo the compliments to the authors for collecting such a massive series of this unique patient group and for being able to analyze them in the way that you have.
I would like to ask you how we could put this kind of information into practical use. I would raise the question, in relation to patients that are receiving neoadjuvant therapy for cancers of the esophagus, stomach or pancreas, in which the surgeon finds an occult lesion in the liver at the time of resection of a responsive tumor primary. In patients with colorectal cancer we would take care of the liver tumor as part of the process, but if it is a cancer of the pancreas we are somewhat reluctant to do that. In fact, that is considered a “no-no.” The same thing appears to apply in patients with esophageal and gastric cancers, although we know that occasional patients have survived this approach. So how do you apply your data set to that kind of a question?
Dr. Rene Adam (Villejuif, France): First, I would like to thank Professors Choti and Wanebo for their thoughtful review of our work and their insightful questions. In response to Dr. Choti's comments concerning the favorable survivals experienced by patients with breast and pancreas primary tumors with liver metastases, I should say frankly that this has been a surprise for us as well. But the data are the data and so we must investigate further.
On one hand, it is clear that the patients reported in our study are a selected group from the total population of patients with non-colorectal and non-endocrine liver metastases. It is difficult to speak about the exact selection process because these are the results of 41 different centers, each probably with some variability in their selection process. On the other hand, by including so many centers, I think the data may correspond much more to common practice. I think that both sides of this issue should be taken into account when trying to determine the role of the surgeon for these 2 indications, for example. In addition, the favorable outcomes for patients with breast and pancreatic tumors may have emerged because of the increasing efficacy of chemotherapy.
With regard to this type of patient, probably the most important factor is not to operate on the patient without any previous chemotherapy, but to operate on this type of patient following the delivery of active chemotherapy associated with a tumoral response. Indeed, in this setting, surgery probably has much more of an adjuvant role to chemotherapy, in contrast to the relationship between surgery and chemotherapy for patients with colorectal metastases.
To address your first question regarding the relative weight of patient age in the prognostic score, these are the results of the statistical analysis which factored in the risk ratio and the number of patients with each factor. I think the statistics are strong enough for this large cohort of patients to demonstrate that age with a cutoff of 30 years and 60 years was indeed an important factor in predicting the survival of patients already selected for hepatic resection. Certainly, primary tumor type may be a more important factor than age for all patients with non-colorectal non-endocrine liver metastases.
With regard to the issue of response to preoperative chemotherapy, this data was requested from all the centers. However, given the long time period of the study, it was clear that effective chemotherapy regimens were not always available and so chemotherapy was not widely used. For the prognostic model we were obligated to select study factors that were present in a vast majority of patients. This having been said, the analysis of the subset of patients who did receive preoperative chemotherapy, even with the less active agents, demonstrated that a good response to chemotherapy influenced the survival following hepatic resection of liver metastasis from breast cancer. And this was also the case for patients with liver metastases from lung cancer. Certainly, the interaction between systemic therapies and surgical resection is based on the activity of the chemotherapy. We believe that response to chemotherapy is a critical factor.
With regard to your third question concerning the less invasive techniques to destroy tumors, similar to the approach for patients with colorectal liver metastases, I think that these methods should be reserved for those patients with non-resectable disease. For the patients with non-colorectal non-endocrine metastases it is common for additional intrahepatic disease to be missed on preoperative imaging. During laparotomy we frequently discover more lesions than expected from the preoperative imaging, and this circumstance is also an argument for surgical exploration and against percutaneous approaches with radiofrequency ablation.
With regard to the question posed by Dr. Wanebo, this is a very important point. How should we approach patients with synchronous disease, for example, from carcinoma of the pancreas or carcinoma of the stomach? In the case of patients who are treated with neoadjuvant chemotherapy for their primary tumor and are then found to have liver metastases when they are explored for resection of the primary, I think the approach depends on the response to chemotherapy of the primary tumor and the ease of resection of both areas. For patients responding very well to chemotherapy, given the low risk of mortality of hepatic surgery in this type of patient, I would advocate giving them the chance to be put in long-term remission with hepatic surgery combined with the surgery of the primary. Of course, for patients not exposed to preoperative chemotherapy who are found to have a synchronous liver metastasis, I would not advocate operating at the same time on both the primary tumor and the metastatic disease. If symptoms warrant resection of the primary tumor then this should be done and chemotherapy should follow. Future treatment of the metastatic disease would then depend on response to therapy. This approach allows us to learn about the biological process of the tumor. In general, if the morbidity of the procedures are acceptable and there is a response to systemic therapy I think we should give even patients with synchronous presentations a chance to be put into long-term remission.
Dr. Merril T. Dayton (Buffalo, New York): I, too, would like to commend Dr. Adam and his colleagues for what I think is a courageous study. Operating on liver metastases from these malignancies has been a surgical “no man's land” for many years. Despite the biases and the vagaries that one sees in a retrospective study from a multi-institutional setting like this, one has to be impressed with the survival data that has been presented here today.
One of the caveats, though, is that we need to have some sense of perspective with regards to the denominator. It is unlikely that you have any idea how many patients with metastases were refused surgery. However, until we have those numbers, we really won't know what the real contribution of this operation is for this subset of patients with this group of cancers. Would you comment on that concern and whether you have any idea what the denominator might be? I suspect you don't have that, but it would be very important to know.
Dr. Rene Adam (Villejuif, France): Thank you Dr. Dayton. Your point is very critical. It is difficult to know the denominator for a study like this. I can say, following my detailed review of all the data, that the percentage of all patients with non-colorectal non-endocrine metastases who may be candidates for liver resection is between 1% and 10%. Certainly, no more than 10%. If we consider, for example, breast cancer liver metastases, it is accepted that probably 3% to 5% will benefit from a liver resection. Across all of the primary tumors studied the proportion of patients eligible for this treatment is probably small.
Despite its limitations, I think that the important message to be taken from our study is that long-term survivals are possible for selected patients in these situations and, therefore, we should not miss the opportunity to offer them liver resection. As you know, many medical oncologists are currently reluctant to consider any liver surgery. Hopefully, this analysis will stimulate a change in our own thinking about these patients and also a change in the approach taken by our medical oncologists so that liver resection will be discussed more frequently in multidisciplinary treatment planning conferences and liver resection will be proposed when it may be, I would say, potentially curative.
Dr. Edward M. Copeland, III (Gainesville, Florida): My 2 questions can be combined into one and should read as such: Did you correlate the original TNM stage with survival after liver resection? As I recall for the early data on liver resection for colorectal metastases, the survival for patients with advanced local disease was better if they had a liver metastasis than if they did not. This, of course does not make much biological sense. It would be much like resecting a liver metastasis in someone with inflammatory breast cancer and expecting a better survival if they had never had the hepatic metastasis. Later it was shown that the original TNM stage of colorectal cancer did correlate with survival after liver resection. I do not see this correlative data in your report.
One thing I did not see in your list of prognostic indicators, if you will, was the biology of the original tumor. What was the stage of the original tumor? If you take stage 3 patients and resect biliary metastasis or you take stage 1 patients and resect biliary metastasis, you are going to get a different result. So I suspect that is true in your patient population based on the status of the original primary.
Dr. Rene Adam (Villejuif, France): Thank you for your questions Dr. Copeland. In order to determine the relative impact of primary tumor factors on survival we did indeed analyze many of these factors, and in particular the TNM classification. These factors did not, however, emerge as important in the multivariate statistical analysis. This is likely due to the fact that most patients in our series had metachronous disease with a long time interval from primary treatment to metastases diagnosis. In other words, patient selection may have lessened the influence of primary tumor factors such as TNM classification on outcomes.
At our center we address each patient individually. Certainly, primary tumor factors including nodal status and other markers of aggressiveness are considered, but ultimately control of extrahepatic disease, response of liver metastases to chemotherapy, and technical aspects of resectability are the factors we rely on to decide which patients to offer an operation.
Footnotes
Reprints: René Adam, MD, PhD, Paul Brousse Hospital, 12-14 Avenue P.V. Couturier, Villejuif Cedex, France 94804. E-mail: rene.adam@pbr.aphp.fr.
REFERENCES
- 1.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases: Association Francaise de Chirurgie. Br J Surg. 1997;84:977–980. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 5.Stangl R, Altendorf-Hofmann A, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. [DOI] [PubMed] [Google Scholar]
- 6.Paineau J, Hamy A, Savigny B, et al. Resection of hepatic metastases from non colorectal cancers: our experience apropos of 20 cases. J Chir (Paris). 1995;132:1–6. [PubMed] [Google Scholar]
- 7.Berney T, Mentha G, Roth AD, et al. Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg. 1998;85:1423–1427. [DOI] [PubMed] [Google Scholar]
- 8.Lindell G, Ohlsson B, Saarela A, et al. Liver resection of noncolorectal secondaries. J Surg Oncol. 1998;69:66–70. [DOI] [PubMed] [Google Scholar]
- 9.Le Treut YP, Sebag F, Hardwigsen J. Surgery of liver metastases of non-colorectal origin. Ann Chir. 1998;52:88–91. [PubMed] [Google Scholar]
- 10.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187:487–493. [DOI] [PubMed] [Google Scholar]
- 11.Benevento A, Boni L, Frediani L, et al. Result of liver resection as treatment for metastases from noncolorectal cancer. J Surg Oncol. 2000;74:24–29. [DOI] [PubMed] [Google Scholar]
- 12.Hamy AP, Paineau JR, Mirallie EC, et al. Hepatic resections for non-colorectal metastases: forty resections in 35 patients. Hepatogastroenterology. 2000;47:1090–1094. [PubMed] [Google Scholar]
- 13.Buell JF, Rosen S, Yoshida A, et al. Hepatic resection: effective treatment for primary and secondary tumors. Surgery. 2000;128:686–693. [DOI] [PubMed] [Google Scholar]
- 14.van Ruth S, Mutsaerts E, Zoetmulder FA, van Coevorden F. Metastasectomy for liver metastases of non-colorectal primaries. Eur J Surg Oncol. 2001;27:662–667. [DOI] [PubMed] [Google Scholar]
- 15.Goering JD, Mahvi DM, Niederhuber JE, et al. Cryoablation and liver resection for noncolorectal liver metastases. Am J Surg. 2002;183:384–389. [DOI] [PubMed] [Google Scholar]
- 16.Harrison LE, Brennan MF, Newman E, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–632. [DOI] [PubMed] [Google Scholar]
- 17.Hemming AW, Sielaff TD, Gallinger S, et al. Hepatic resection of noncolorectal nonneuroendocrine metastases. Liver Transpl. 2000;6:97–101. [DOI] [PubMed] [Google Scholar]
- 18.Laurent C, Rullier E, Feyler A, et al. Resection of noncolorectal and nonneuroendocrine liver metastases: late metastases are the only chance of cure. World J Surg. 2001;25:1532–1536. [DOI] [PubMed] [Google Scholar]
- 19.Yamada H, Katoh H, Kondo S, et al. Hepatectomy for metastases from non-colorectal and non-neuroendocrine tumor. Anticancer Res. 2001;21:4159–4162. [PubMed] [Google Scholar]
- 20.Takada Y, Otsuka M, Seino K, et al. Hepatic resection for metastatic tumors from noncolorectal carcinoma. Hepatogastroenterology. 2001;48:83–86. [PubMed] [Google Scholar]
- 21.Karavias DD, Tepetes K, Karatzas T, et al. Liver resection for metastatic non-colorectal non-neuroendocrine hepatic neoplasms. Eur J Surg Oncol. 2002;28:135–139. [DOI] [PubMed] [Google Scholar]
- 22.Weitz J, Blumgart LH, Fong Y, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ercolani G, Grazi GL, Ravaioli M, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–466. [DOI] [PubMed] [Google Scholar]
- 24.Adam R, Chiche L. Chirurgie des métastases hépatiques de cancers non colo-rectaux non endocrine. 107er Congrés Français de Chirurgie. Paris, Arnette, 2005. [Google Scholar]
- 25.Couinaud C. Le Foie. Etudes Anatomiques et Chirurgicales. Paris: Masson, 1957. [Google Scholar]