Abstract
Objective:
This study examines donation after cardiac death (DCD) practices and outcomes in liver transplantation.
Summary Background Data:
Livers procured from DCD donors have recently been used to increase the number of deceased donors and bridge the gap between limited organ supply and the pool of waiting list candidates. Comprehensive evaluation of this practice and its outcomes has not been previously reported.
Methods:
A national cohort of all DCD and donation after brain-death (DBD) liver transplants between January 1, 2000 and December 31, 2004 was identified in the Scientific Registry of Transplant Recipients. Time to graft failure (including death) was modeled by Cox regression, adjusted for relevant donor and recipient characteristics.
Results:
DCD livers were used for 472 (2%) of 24,070 transplants. Annual DCD liver activity increased from 39 in 2000 to 176 in 2004. The adjusted relative risk of DCD graft failure was 85% higher than for DBD grafts (relative risk, 1.85; 95% confidence interval, 1.51–2.26; P < 0.001), corresponding to 3-month, 1-year, and 3-year graft survival rates of 83.0%, 70.1%, and 60.5%, respectively (vs. 89.2%, 83.0%, and 75.0% for DBD recipients). There was no significant association between transplant program DCD liver transplant volume and graft outcome.
Conclusions:
The annual number of DCD livers used for transplant has increased rapidly. However, DCD livers are associated with a significantly increased risk of graft failure unrelated to modifiable donor or recipient factors. Appropriate recipients for DCD livers have not been fully characterized and recipient informed consent should be obtained before use of these organs.
This study examines deceased donation practices and outcomes after liver transplantation using grafts from donation after cardiac death (DCD) donors in the United States. DCD livers were used for 472 (2%) of 24,070 transplants. The relative risk of DCD graft failure was 85% higher than that of brain-dead donor grafts (P < 0.001).
The large and persistent imbalance between the supply of donor organs for liver transplantation and the pool of potential liver transplant recipients continues to fuel efforts to maximize utilization from existing donors, increase the overall number of donors, and identify new donor sources. Until recently, deceased donor liver transplantation was largely confined to donors with ongoing cardiac activity and diagnosed with brain death. Donation after brain death (DBD) donors continue to supply about 95% of all livers used for transplantation in the United States.1 In recent years, donation of right hepatic lobes by living persons has been reported.2–4 These adult-to-adult living donor liver transplants place a healthy donor at risk for serious complications and death,5–7 and the number of such transplants has not had a major influence on overall liver donor availability.1
Recently, a number of transplant programs have begun to perform transplants using livers from deceased donors whose hearts have stopped beating. Procedures using such donor livers are termed donation after cardiac death (DCD) liver transplants, and they offer the potential to increase the number of available organs. Early reported experiences with DCD liver transplants were, not surprisingly, limited to single centers and modest numbers of cases. The number of DCD liver transplants continues to increase and more centers are now performing these procedures. We thought it timely, therefore, to comprehensively examine national practices regarding DCD liver transplantation and outcomes of liver transplants using DCD livers. These topics are the subject of this report.
METHODS
Data Sources
Data regarding donor characteristics, recipient characteristics, and transplant outcomes were from the Scientific Registry of Transplant Recipients (SRTR) database, as submitted by members of the Organ Procurement and Transplantation Network. The SRTR database includes information on all wait-listed candidates and transplant recipients in the United States, and is supplemented by mortality information from the Social Security Death Master File.8
National data on all DCD and DBD liver transplants in the U.S. between January 1, 2000 and December 31, 2004 were used in the analysis. Multiple-organ transplants were excluded from the analysis. Data from the entire study period listed above were analyzed to characterize donation and transplant practice patterns. A subset of the cohort used for evaluation of posttransplant outcomes included only transplants that occurred before November 1, 2003, to allow for a minimum of 1 year of follow-up on all transplant recipients. Analyses that included Model for End-stage Liver Disease (MELD) and Pediatric End-stage Liver Disease (PELD) scores were restricted to a subset of the cohort beginning at the time of mandatory MELD and PELD reporting on September 1, 2002.
Analytic Methods
To compare the outcomes of DCD and DBD transplants, time to graft failure was evaluated using Cox regression models. Time to graft failure was defined as the period between transplantation and graft loss due to the earlier of retransplantation or recipient death. All available posttransplant follow-up data were used in the analysis.9 Patients were followed for at least 1 year after transplantation. The median follow-up time was 2 years.
To isolate the impact of DCD livers on posttransplant outcomes, models were adjusted for relevant donor, recipient, and transplant factors. Donor variables that were used in the model included age, cause of death, race, gender, and height. Recipient variables included age, race, gender, diabetes, diagnosis, medical condition at transplant, status 1 (fulminant hepatic failure, primary nonfunction, or hepatic artery thrombosis), pretransplant dialysis, previous liver transplant, need for pretransplant life support, body mass index (BMI), serum creatinine, inotropic blood pressure support, and history of portal vein thrombosis, malignancy, previous abdominal surgery, hepatitis B, or hepatitis C. Transplant-related factors included blood type compatibility, partial/split liver, cold ischemia time, and origin of the donor organ beyond the recipient's listing organ procurement organization (shared organ).
To evaluate whether or not the effect of DCD on graft failure varied by other risk factors, we sought statistically significant interactions between DCD and selected donor, recipient, and transplant risk factors. The following factors were tested: donor age, cause of death, recipient age, diagnosis, creatinine, BMI, MELD/PELD, and cold ischemia time.
Comparisons among transplant centers that performed DCD transplants between January 1, 2000 and October 31, 2004 were evaluated using 2 methods. First, unadjusted survival curves among the 10 centers that had performed the largest numbers of DCD liver transplants were compared by the log-rank test. The small numbers of DCD transplants and observed events precluded an adjusted comparison among centers. Second, we tested for associations between transplant center experience with DCD liver transplants (or overall transplant center volume) and graft failure among DCD liver transplants. Transplant center experience with DCD transplants was measured by the number of DCD liver transplants that the center had performed during the above time interval, and was grouped into one of the following categories: 1 to 9, 10 to 19, and 20 or more DCD transplants. Overall transplant center volume was assessed by volume tercile over the study period.
All analyses were performed using SAS version 9.1.
Human Subjects Protection
The study was approved by the HRSA SRTR project officer. HRSA has determined that this study satisfies the criteria for the institutional review board exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)5 and HRSA Circular 03.
RESULTS
Study Population Characteristics
There were 472 DCD liver transplants among 24,070 deceased donor liver transplants that took place during the study period. The annual number of DCD liver transplants increased from 39 (0.9%) in 2000 to 176 (3.2%) in 2004 (Table 1). The number of transplant programs that have performed DCD liver transplants each year has also increased (Table 1). Descriptive statistics for donor, recipient, and transplant factors by deceased donor type (DCD or DBD) are shown in Tables 2 to 4. The deceased donor type was significantly associated with donor age (P = 0.0002), race (P < 0.0001), and cause of death (P < 0.0001) (Table 2). Septuagenarian DCD donors were rare. DCD donors were more commonly white, and the cause of death was much less commonly stroke. Overall, recipients of DCD liver transplants were significantly older; recipients aged 55 years or older comprised 42.0% of DCD liver transplants compared with 33.8% of DBD liver transplants (Table 3). DCD recipients were also significantly more often white, had higher BMI, and were less often in the ICU or hospitalized at the time of transplant than recipients of non-DCD liver transplants. There were no significant differences between DCD and DBD transplants with respect to local or shared donor origin, ABO compatibility, or cold ischemia time (Table 4).
TABLE 1. Liver Transplants Using DCD Donors and Number of Liver Transplant Programs That Performed DCD Liver Transplants by Year (January 1, 2000 to December 31, 2004)
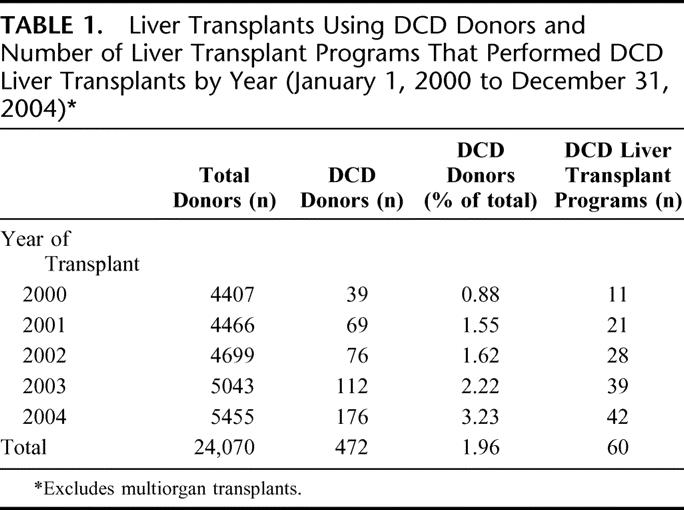
TABLE 2. Donor Characteristics by Deceased Donor Type (DCD vs. DBD) (January 1, 2000 to December 31, 2004)
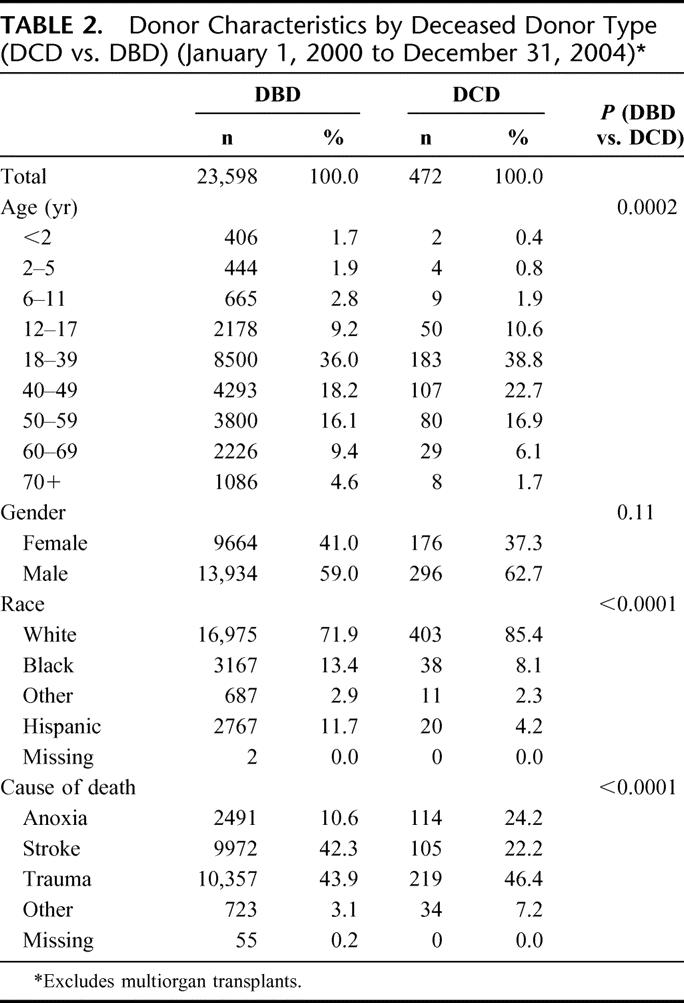
TABLE 3. Recipient Characteristics by Deceased Donor Type (DCD vs. DBD) (January 1, 2000 to December 31, 2004)
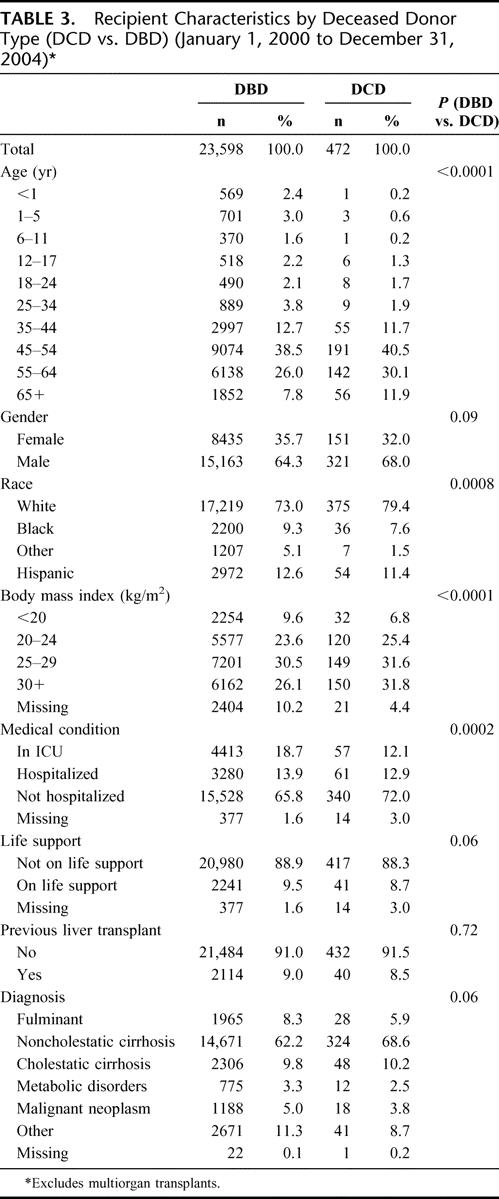
TABLE 4. Transplant Characteristics by Deceased Donor Type (DCD vs. DBD) (January 1, 2000 to December 31, 2004)
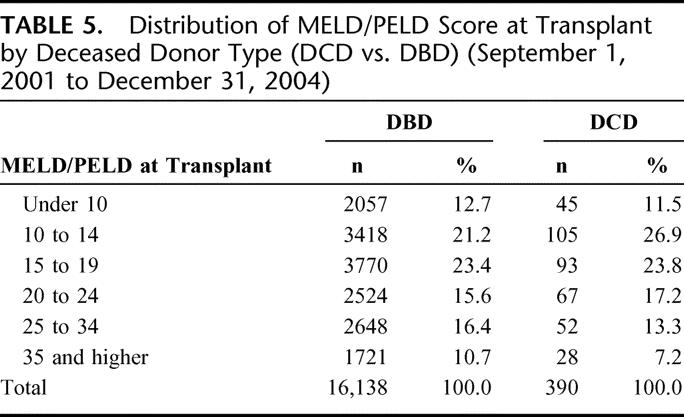
Table 5 shows the distribution of the recipient calculated laboratory MELD/PELD scores at the time of transplant by deceased donor type. The mean laboratory MELD/PELD score at transplant was modestly but statistically significantly higher for recipients of DBD compared with DCD transplants (19.6 ± 10.0 vs. 18.5 ± 8.6, respectively; P = 0.02). Excluding recipients who were granted an exception MELD/PELD score from the comparison revealed a larger difference in mean MELD/PELD scores between DBD and DCD recipients (21.7 ± 9.9 vs. 19.6 ± 8.7, respectively; P < 0.0001).
TABLE 5. Distribution of MELD/PELD Score at Transplant by Deceased Donor Type (DCD vs. DBD) (September 1, 2001 to December 31, 2004)
The adjusted relative risk of graft failure following liver transplants that used DCD grafts was 85% higher than that after DBD transplants (relative risk [RR], 1.85; 95% confidence interval [CI], 1.51–2.26; P < 0.001). Corresponding point estimates for adjusted graft survival at 3 months, 1 year, and 3 years after DBD and DCD transplant are given in Table 6 and are depicted graphically in Figure 1 up to 4 years after transplantation. By 1 year after transplantation, the difference in graft survival between DCD and DBD grafts was 13 percentage points (70% vs. 83%, respectively).
TABLE 6. Adjusted Graft Survival at 3 Months, 1 Year, and 3 Years Posttransplant for DCD and DBD Transplants (January 1, 2000 to 10/31/2003)
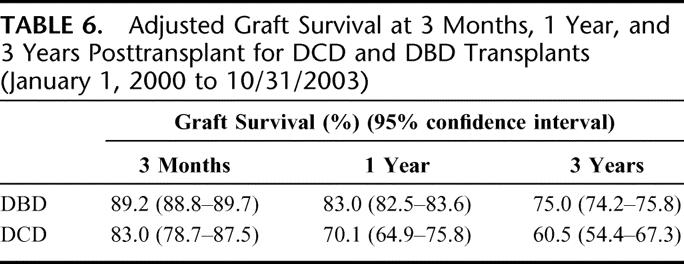
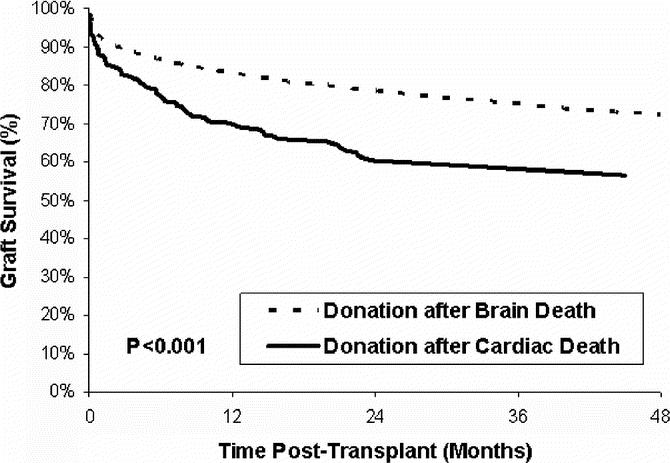
FIGURE 1. Adjusted graft survival for DCD and DBD liver transplants (transplants between January 1, 2000 and October 31, 2003).
We tested for possible associations between deceased donor type and the effects of a variety of factors on graft outcome by using statistical interaction terms. Among donor age, cause of death, recipient age, diagnosis, creatinine, BMI, MELD/PELD score at transplant, and cold ischemia time, the only variable that appeared to have a significantly different effect on graft outcome for DCD versus DBD grafts was recipient diagnosis (P = 0.04). However, the diagnosis group primarily responsible for the significant result (malignant neoplasms) comprised only 6 DCD transplants, 5 of which failed. Thus, these findings do not provide strong evidence for a meaningful interaction between the effects of deceased donor type and recipient diagnosis or any other tested variable. The lack of statistical significance for the interaction tests between deceased donor type and the other tested variables shows that the adverse effect of DCD donor on graft failure was not significantly different for any subgroups of these variables.
Neither the level of transplant program experience with DCD liver transplants nor overall transplant center volume was significantly associated with posttransplant graft outcome. Unadjusted graft survival of transplants performed at centers that performed fewer than 10 DCD liver transplants during the study period was not different from that experienced by recipients at centers that performed a larger number of DCD transplants (log-rank test P = 0.977). Similarly, there was no significant association found between the level of transplant program experience with DCD transplantation and adjusted risk of graft failure. DCD transplants performed in centers that performed 1 to 9 (RR, 1.27; CI, 0.73–2.22; P = 0.40) and 10 to 19 DCD transplants (RR, 1.03; CI, 0.612–1.75; P = 0.90) were not associated with significantly different outcomes than for the reference group of centers that performed 20 or more DCD transplants (RR, 1.00).
DISCUSSION
There is a limited range of strategies to increase the supply of donor organs for liver transplantation. These include: 1) increasing the number of deceased donors among decedents who are brain dead (ie, DBD donors), 2) increasing the proportion of existing DBD donors from whom livers are procured, 3) applying DBD split liver transplantation to 2 adult recipients, 4) using adult living liver donors, and 5) increasing use of livers from DCD donors. All of these strategies have been pursued to some degree. Herculean efforts have been invoked in recent years to increase the total number of DBD donors, particularly since the creation of the National Organ Donation Breakthrough Collaborative.10 Between 2000 and 2004, for example, the total number of DBD liver donors increased by 23%.1 The fraction of DBD donors from whom livers are procured has leveled off at about 90%,1 so it is unlikely that many incremental liver donors will be forthcoming from this approach. Split liver transplantation, where a single adult liver is divided to produce 2 transplantable lobes, has been applied in a very limited way to 2 adult recipients, but the modest parenchymal mass of the left lobe precludes its use except for small adult patients.11 Adult living donor liver transplantation places a healthy volunteer at risk for major complications or death,5 and, perhaps because of a small number of highly publicized donor catastrophes, this procedure has yet to gain wide acceptance.1 Despite all these strategies, there are still over 17,000 candidates awaiting liver transplantation in the United States, and about 2000 candidates die on the waiting list each year without the opportunity to receive a liver transplant.1
The use of livers from DCD donors is not a new idea. The first successful liver transplants, reported in 1967, used livers from DCD donors.12 Following the development of criteria for the diagnosis of brain death by the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death in 1968,13 nearly all deceased donor organ procurement transitioned to DBD donation. More recently, as the number of potential liver transplant candidates progressively eclipsed the number of available DBD donors, DCD liver donation was again pursued.
The annual number of DCD livers used for transplant has increased rapidly. However, DCD livers are associated with a significantly increased risk of graft failure unrelated to obviously modifiable donor or recipient factors. We have not identified any particularly noxious combinations of DCD liver donors with other donor or recipient characteristics. Nor have we identified situations where DCD liver donation is not a significant risk factor for graft failure.
Among DCD donors, the distribution of characteristics aside from the DCD status itself was in the direction expected to be associated with better, rather than worse, outcome, when compared with DBD donors. For example, DCD donors were younger than DBD donors. They had more commonly died of cerebral trauma and less commonly died of stroke. The differences in underlying demographic and other characteristics between DCD and DBD donor highlights the importance of adjustment for these factors, as was done in the analyses reported here, in determining, other things being equal, the independent effect of DCD status.
It is likely that more DCD liver donations will occur in the future. However, moving forward with a DCD donation in instances where a potential donor is likely to progress to brain death is undesirable, since DCD liver donation is associated with a much higher probability of graft failure. In addition, the average number of organs procured per donor is much lower in DCD than DBD donors.1 While there are algorithms that can assess a potential donor's likelihood of progressing to cardiac death after cessation of mechanical ventilation,14 it would also be useful to have a means to determine the probability that an irretrievably brain-injured individual being considered for DCD donation will progress to brain death.
One limitation of the current study with respect to identification of particularly suitable or unsuitable DCD liver recipients is the possibility of a type II error related to the relatively small number of DCD liver donations that have been done to date in the United States. In addition, the emerging use of DCD livers for transplant is likely to be associated with continued evolution in practice. A recent national conference was convened to bring together experts in the field to examine many of the clinical issues currently facing the field of DCD-based organ donation,15 and further improvements in selection, management, and outcomes of DCD donation are expected in the future. For example, the acceptable limit of warm ischemia for DCD livers, defined as the time between donor extubation and the initiation of cold perfusion, may be shorter than the 60 minutes customarily used for DCD kidney transplantation. For DCD livers, more than 30 minutes of warm ischemia may be associated with a higher risk of posttransplant biliary stricture.16
Notwithstanding these limitations, a potentially more important consideration is the comparison of the expected lifetimes with and without a DCD liver transplant. Thus, the expected lifetime of a liver transplant candidate offered a DCD liver could be compared with the expected lifetime of that candidate if they were to turn down the DCD offer and continue to wait for a DBD liver. The latter expected lifetime calculation is sensitive to the progression of the underlying liver disease and to the likelihood of a subsequent DBD offer. Analyses of this type have been carried out with respect to expanded criteria donor kidney (ECD) transplantation to determine appropriate recipients for ECD kidneys17 but will have to await a larger national experience to be feasible for DCD liver transplantation. In the meantime, clinical judgment will need to guide the counseling of liver transplant candidates regarding the advisability of their accepting a DCD liver. Recommendations by transplant teams and decisions by liver transplant candidates should be predicated on full disclosure of the known risks and potential benefits of DCD liver transplantation.
ACKNOWLEDGMENTS
The authors thank Miles P. Finley for editorial assistance.
Discussions
Dr. Goran B. Klintmalm (Dallas, Texas): It is a privilege to have been asked by Dr. Merion and his co-authors to comment on their presentation on donation after cardiac death as a strategy to increase deceased liver donor liver availability. Due to the success of liver transplantation as treatment for all liver failure, more and more patients are being referred to transplant centers for this procedure. The number of liver donors in the United States was stable for many years and in spite the 23% increase in liver donors between 2000 and 2004, the number of patients waiting transplantation grows faster.
There have been a number of attempts to increase the donor supply through the use of split livers, living donors, and xenotransplantation. This report is focused on going back to where we started. Organ transplantation was dependent on cardiac death for organ donors during the infancy of transplantation. After Congress accepted brain death in 1972, it became the norm for organ donation.
Recently, as the disparity between the numbers of patients waiting on the list and the number of donors has been increasing, several institutions around the country have started to look into practices of the past in order to find more donors. The premise is that many patients with catastrophic brain damage still have brain stem function and consequently are not brain dead. However, if allowed to experience cardiac death, many more organs would be available for donation.
Dr. Merion and his co-authors have made a huge effort in clarifying the impact with the use of these donors. I am privileged to have received the manuscript ahead of time and perused through its many details. I have a few questions for Dr. Merion.
First, more DCD donors were used for patients with a MELD score of less than 14. With the new UNOS rules disfavoring the transplantation of such patients, should these donors be used for high-risk patients instead? How will this affect the outcome? Will it be worse?
Second, the study shows that there was no difference in DCD donor results regardless of the volume and experience with such donors. Have you looked at the institutional experience with liver transplantation overall? Or does it matter at all if you have any experience or not?
Thirdly, the study has found that the DCD donors are more likely to be perfect donors. That means that there were in the age group of 18 to 49, they were white, have suffered from anoxic or trauma death, and were less likely stroke victims than brain dead donors. Thus, it seems like the DCD donors are the more ideal. I would like to hear Dr. Merion's speculations for why this is.
Finally, maybe the most important finding of this study was that you were able to show a significantly lower 1- and 3-year survival rate with a DCD donor. In effect, you could show that the relative risk for graft failure was 85% higher than grafts from brain dead donors. And this leads to my final and most important question and concern. There are already anecdotal reports from experienced surgeons and centers that potential donors suitable for multiple organ donation based on brain death criteria are being “converted” into cardiac death donors, which results in making a perfect donor with perfect organs into a marginal donor with substandard kidneys and livers. And in the process, donation of hearts, lungs, and intestines was made impossible. For many institutions and others involved this is an easier way out by providing a simpler process for the donor institutions and the organ banks. I would like to hear your comments on this potentially serious adverse outcome by the reintroduction of old techniques and how we can prevent this from happening.
Dr. Robert M. Merion (Ann Arbor, Michigan): Thank you, Dr. Klintmalm. As always, you raise important and vexing and not always easily answered questions. But let me give it a shot.
Your first question asked about what I think will happen to the outcomes if DCD livers that are currently being placed into patients with low MELD scores are shifted as a result of recent policy changes to individuals that have high MELD scores. I think the short answer is that the adjusted risk of failure of a DCD organ wouldn't really change because we accounted for that in the model. In terms of the absolute survival of those individuals, because patients who have higher MELD scores have a higher risk of death regardless of the donor type than do individuals who have low MELD scores, we would expect to see worse absolute outcomes in that case.
Your second question had to do with the relative balance between the experience of a center with DCD transplantation vis-a-vis their overall experience with liver transplantation. That is a very good question. We did not actually look at overall center volume as a potential confounding variable in the model, and that is something that we could look at.
With respect to why DCD donors tend to have characteristics that are more commonly associated otherwise with more ideal donors, for example, younger age and other attributes, I would say that this is principally due to the mechanism and etiology of death in those individuals that lead to conditions that don't result typically in brain death. But they do result in devastating cerebral injury and make the donor suitable for donation after cardiac death.
And that really leads into your fourth question, which is whether pursuit of DCD donation is going to be at the expense of brain dead donors, which can provide many more organs on average than a DCD donor. And that, I think, is the most vexing question.
There has been one publication from a Dutch center that suggested that as DCD transplantation increased in Holland, the increase was at the expense of brain dead donation for multiple organs. We have looked at data from the Scientific Registry of Transplant Recipients, and at least in the United States it would appear that in fact the opposite is the case. Those organ procurement donor service areas that have seen the biggest percentage increases in DCD donation have also had the biggest increases in brain death donations as well.
We know, however, that this is a big country, and there are certainly isolated reports from various parts of this country that would suggest that a countervailing practice is actually occurring where DCD is occurring at the expense of donation after brain death. So I think we need to watch this carefully. And we certainly don't want to promote DCD donation to increase the donor pool if it is going to be at the expense of the overall pool.
Dr. James F. Burdick (Baltimore, Maryland): This certainly was an excellent work, very nicely presented. And it brings up some very critical points.
I think it is important to note that there has been a remarkable improvement in organ donation in the country in the last couple of years, an unprecedented over 20% increase in organ donation, probably largely as a result of the organ donation collaborative thing nationally. And this includes an emphasis on the use of DCD, but not the use of organs that are not going to work. Rather, the principle being that every organ that can be transplanted successfully be able to be transplanted. There has been a lot of encouragement that DCD could contribute to this.
And the conundrum here and the first question relates to the fact that for kidneys it is clear that they do well at one year compared to non-DCD donor organs. And even for lungs now there are programs that feel that DCD are equivalent or even better on average from donors after brain death. So why the problem with the livers? Is it a real problem specifically? Would it be possible, first question, to go back and look at these donors and be sure that the other organs that we know don't seem to have this difficulty really did do well? Presuming that is the case, the bile duct and late bile duct problems are one of the target areas. Is that something that you were able to look at? Or is that in your thinking about this something that should be examined in a more organized way?
Many of these patients would get retransplanted. Do you have any sense that that goes well? Related to that, since it was lower MELD scores for the DCD donors, are these patients who have cancer and are getting fitted into the overall pattern, because you were using the non-exception MELD. I think, when you did this, so is there a pattern of overall how this works that you could say more about?
Dr. Robert M. Merion (Ann Arbor, Michigan): Dr. Burdick, in his role as the director of the Division of Organ Transplantation at the Health Resources and Services Administration, oversees the project that he mentioned briefly, the Organ Transplant Breakthrough Collaborative. And I can't emphasize enough how important that has been in increasing organ donation generally. Clearly, we are very much in agreement with the Collaborative that we want to encourage donation of organs that will work. And really, it is studies like this that hopefully will help us over time to understand which will work and which will not.
We have not specifically looked at how the other organs procured from DCD liver donors have fared. In the vast majority of instances DCD liver donors have also been the donors of other organs, usually kidneys. And we do know from looking very carefully at the cohort of kidney transplant patients that DCD kidney donation does not appear to be a significant risk factor for graft failure. So there does appear to be something that is a little special about the liver.
We know that early on, as I demonstrated, there is a higher risk of early failure. That is clearly not due to bile duct problems. But increasingly there are single-center reports of intrahepatic ischemic-type biliary strictures that result in late graft loss for some patients.
So I think that ultimately what we need to do is to figure out how to do DCD liver donations smarter rather than to do it less.
Dr. Nancy L. Ascher (San Francisco, California): Dr. Merion, you and SRTR are to be congratulated for the incredible power that you have given the transplant community with the robust data that you have. I have 3 questions.
The first, we have all heard data from SRTR about the poorer results and the risk of using live donor liver transplant. But over time that risk has decreased. So I would like to you comment about that as it relates to the DCD. Might we expect DCD results to improve over time?
The second question relates to the distribution of DCD to the higher MELD or more gravely ill patients. It is clear that high MELD does not dictate post-transplant outcome but probably does dictate post-transplant cost. So I would estimate that the use of the DCD would increase the total cost for transplant. I wonder if you have any cost data on that.
Finally, the whole concept of DCD is actually part of an end-of-life management ideology of how we are managing patients at the end of their life. This is an alternative way to manage patients for the intensivist. Can you comment on this approach to end of life care?
Dr. Robert M. Merion (Ann Arbor, Michigan): With respect to your first question, we did look at year of transplant, and at least within the context of this study we were not able to find a significant effect of year of transplant on outcome. But we are seeing only the beginning stages of DCD liver transplantation practice. And my own hunch would be that over time as we understand the consequences of DCD liver donation, we will probably get better at it.
The publication recently in the American Journal of Transplantation of the proceedings of the National Conference on DCD Organ Donation certainly expressed a consensus that the permissible amount of time from removal of ventilatory support until declaration of death for a DCD donor, which is typically 60 minutes for a DCD kidney donor, may simply be too long for the liver to tolerate, and I think increasingly the consensus is that probably should be more like 30 minutes. So as those kinds of practice pattern changes filter out through the community, I would expect that we would see some salutary effects.
With respect to the cost question, we don't have any specific cost data. So in the interest of time I will just say we don't have an answer to the cost question. I am sure there will be more cost.
Finally, with respect to end-of-life care, for individuals who are dying of devastating brain injury without brain death (principally in trauma units), DCD donation offers to those individuals and to their families an opportunity to glean something positive from what is otherwise a tragic situation. And certainly my own personal experience with DCD donation is that it is a very positive end-of-life experience when it can be accomplished.
Dr. Jean C. Emond (New York, New York): From an ethical point of view, the equipoise state would be achieved if the recipient has a higher chance of dying or suffering harm by waiting longer than by accepting DCD as a paradigm of a whole group of donors that seem to confer increased risk.
Since scarcity is in fact not uniformly distributed across the 50 or so waiting lists across the country, would you agree or think that as a matter of policy extended criteria donors of all types might be more appropriately used into populations that have a higher experience of scarcity?
Dr. Robert M. Merion (Ann Arbor, Michigan): Dr. Emond, very good question. And the answer to that question lies in the analysis of as many of the factors contributing to the balance between the risk of death in the absence of a transplant versus the risk of death with a transplant, modifying that calculated risk by all sorts of factors, including things like whether the donor is a DCD and other donor risk factors that we and others have identified.
One of those factors is that for a patient with a given level of severity, you are absolutely correct, that in differing parts of the country the amount of time waiting, and more specifically the access to transplantation, the transplant rate, given a certain MELD score, which is the principal arbiter of allocation, those rates vary widely from region to region around the country. So individuals who happen to reside in an area where their access to a transplant may be impaired for whatever reason might actually consider more strongly accepting a donor organ that has a higher risk of failure because their access otherwise to transplantation in general is impaired. So I think that is a dynamic that may change over time and certainly has major impact in terms of the geographic location of the potential recipient.
Footnotes
Supported by Contract No. 231-00-0116 from the Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services.
The views expressed herein are those of the authors and not necessarily those of the U.S. Government.
Reprints: Robert M. Merion, MD, Scientific Registry of Transplant Recipients, 315 West Huron, Suite 320, Ann Arbor, MI 48103. E-mail: merionb@umich.edu.
REFERENCES
- 1.Department of Health and Human Services, Health Resources and Services Administration. 2005 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry for Transplant Recipients: Transplant Data 1995–2004. Ann Arbor, MI: Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, 2006. [Google Scholar]
- 2.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. [DOI] [PubMed] [Google Scholar]
- 4.Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transplantation. 2000;6:296–301. [DOI] [PubMed] [Google Scholar]
- 5.Middleton PF, Duffield M, Lynch SV, et al. Living donor liver transplantation—adult donor outcomes: a systematic review. Liver Transpl. 2006;12:24–30. [DOI] [PubMed] [Google Scholar]
- 6.Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transplantation. 2002;8:110–117. [DOI] [PubMed] [Google Scholar]
- 7.Trotter JF, Talamantes M, McClure M, et al. Right hepatic lobe donation for living donor liver transplantation: impact on donor quality of life. Liver Transplantation. 2001;7:485–493. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Commerce. Social Security Administration Death Master File. Springfield, VA: Federal Computer Products Center, National Technical Information Service, U.S. Department of Commerce. [Google Scholar]
- 9.Wolfe RA, Schaubel DE, Webb RL, et al. Analytical approaches for transplant research. Am J Transplant. 2004;4(suppl 9):106–113. [DOI] [PubMed] [Google Scholar]
- 10.Marks WH, Wagner D, Pearson TC, et al. Organ donation and utilization, 1995–2004: entering the collaborative era. Am J Transplantation. 2006;6:1101–1110. [DOI] [PubMed] [Google Scholar]
- 11.Humar A, Khwaja K, Sielaff TD, et al. Technique of split-liver transplant for two adult recipients. Liver Transplantation. 2002;8:725–729. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Marchioro TL, Porter KA, et al. Homotransplantation of the liver. Transplantation. 1967;5:(suppl):790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205:337. [PubMed] [Google Scholar]
- 14.Lewis J, Peltier J, Nelson H, et al. Development of the University of Wisconsin donation after cardiac death evaluation tool. Prog Transplant. 2003;13:265–273. [DOI] [PubMed] [Google Scholar]
- 15.Bernat JL, D'Alessandro AM, Port FK, et al. Report of a national conference on donation after cardiac death. Am J Transplant. 2006;6:281–291. [DOI] [PubMed] [Google Scholar]
- 16.Abt P, Crawford M, Desai N, et al. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659–1663. [DOI] [PubMed] [Google Scholar]
- 17.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726–2733. [DOI] [PubMed] [Google Scholar]


