Abstract
Objective:
To evaluate the effect of an intensivist-model of critical care delivery on the risk of death following injury.
Summary Background Data:
An intensivist-model of ICU care is associated with improved outcomes and less resource utilization in mixed medical and surgical ICUs. The process of trauma center verification assures a relatively high standard of care and quality assurance; thus, it is unclear what the effect of a specific model of ICU care delivery might have on trauma-related mortality.
Methods:
Using data from a large multicenter (68 centers) prospective cohort study, we evaluated the relationship between the model of ICU care (open vs. intensivist-model) and in-hospital mortality following severe injury. An intensivist-model was defined as an ICU where critically ill trauma patients were either on a distinct ICU service (led by an intensivist) or were comanaged with an intensivist (a physician board-certified in critical care).
Results:
After adjusting for differences in baseline characteristics, the relative risk of death in intensivist-model ICUs was 0.78 (0.58–1.04) compared with an open ICU model. The effect was greatest in the elderly [RR, 0.55 (0.39–0.77)], in units led by surgical intensivists [RR, 0.67 (0.50–0.90)], and in designated trauma centers 0.64 (0.46–0.88).
Conclusions:
Care in an intensivist-model ICU is associated with a large reduction in in-hospital mortality following trauma, particularly in elderly patients who might have limited physiologic reserve and extensive comorbidity. That the effect is greatest in trauma centers and in units led by surgical intensivists suggests the importance of content expertise in the care of the critically injured. Injured patients are best cared for using an intensivist-model of dedicated critical care delivery, a criterion that should be considered in the verification of trauma centers.
We report the mortality benefit among trauma patients cared for in an intensivist model ICU compared with an open unit. This model of critical care delivery was associated with a 45% reduction in mortality among the elderly (age ≥55 years), with no apparent benefit in the young.
High quality supportive care in an intensive care unit is a key factor impacting survival following severe injury and is second only to initial hemorrhage control in its importance. As a result, there is much to be gained by optimizing the critical care of the severely injured patient, a point not lost on those seeking careers in trauma who frequently seek advanced training in surgical critical care.
The delivery of surgical critical care services currently falls into 2 broadly defined models. In one, the operating surgeon assumes primary responsibility for postoperative care, including the provision of critical care services. This approach is in keeping with the ethical standards of the American College of Surgeons under which surgeons are responsible for the postoperative care of their patients. In this context, the ICU is only a location where advanced monitoring and organ support are available. The surgeon continues with clinical responsibilities outside the intensive care unit while also caring for his/her critically ill patients. This organizational approach is typically referred to as an “open” intensive care unit.1,2
The second model is referred to as a closed ICU, or an intensivist-model of critical care delivery.2 In this construct, physicians certified in critical care by one of several specialty boards (Internal Medicine, Anesthesiology, or Surgery) assume responsibility for delivery of intensive care. It is assumed that the intensivist has no other clinical care responsibilities outside the ICU and is primarily available to the critically ill patient. If the intensivist is a surgeon, it is expected that he or she perform no other duties that limit their availability to the patients in the ICU (eg, operating room or clinic attendance).
We have previously evaluated the principal organizational structure of critical care delivery in almost 300 trauma centers across the United States.3 In that sample, 77% of centers reported intensivist involvement in the care of the critically injured, either in the form of collaborative care or the transfer of care to an intensivist. Level II centers were less likely to adopt this approach, with almost 40% reporting that the operating or trauma surgeon was responsible for critical care delivery. Respondents from centers with open ICUs cited the potential for loss of continuity of care, lack of intensivists, and loss of autonomy as the most frequent reasons for not seeking the involvement of an intensivist.
Reports from medical and surgical intensive care units have suggested improved outcomes in patients cared for in closed or intensivist-model ICUs compared with an open unit, with reductions in hospital mortality of 30% to 40%.4–7 As a result of these data, the Leapfrog Group, a coalition of more than 170 public and private U.S. organizations that provide healthcare benefits to more than 36 million persons, has made ICU physician staffing an important quality indicator for its beneficiaries.8 However, the American College of Surgeons Committee on Trauma (ACS COT) criteria used for the verification of trauma centers suggests that the surgeon assuming initial responsibility for the care of the injured patient should maintain that responsibility throughout the acute care phase of hospitalization, including the ICU.9 While this standard might be interpreted as in direct conflict with the quality standards put forth by the Leapfrog Group recommendations, there are no data supporting any particular approach for severely injured patients.
We set out to fill this gap in knowledge using data derived from a large prospective cohort study of injured patients cared for in a wide variety of both trauma centers and nondesignated centers throughout the country. Our principal objective was to evaluate whether an intensivist model of critical care delivery offered a survival benefit compared with an open model of ICU care.
METHODS
Study Design
This is a cohort study with the exposure of interest being care within an intensivist-model critical care unit. The cohort is derived from centers participating in a large prospective study evaluating the effects of trauma center care on outcome.10 The primary endpoint was in-hospital mortality.
National Study on the Costs and Outcomes of Trauma
The National Study on the Costs and Outcomes of Trauma (NSCOT) was a multicenter prospective cohort study designed to compare the long-term outcomes and cost-effectiveness of trauma center care compared with that in nondesignated centers.10 Patients were recruited from 18 level I trauma centers and 51 nondesignated centers in 15 regions defined by contiguous Metropolitan Statistical Areas located in 12 states over the interval July 2001 to November 2002. Hospitals were identified as level I trauma centers if designated by a state or regional authority or verified by the American College of Surgeons. Large nondesignated hospitals were neither designated nor verified as trauma centers at any level and treated at least 25 major trauma patients (ISS >15) annually. The study was approved by the institutional review boards of each of the participating hospitals.
Inclusion criteria for the NSCOT were age 18 to 84 years and the presence of at least one moderate to severe injury (Abbreviated Injury Scale (AIS) score ≥3). Patients who presented with no vital signs and pronounced dead within 30 minutes of arrival were excluded, as were patients who delayed treatment greater than 24 hours; patients 65 years or older with a first listed diagnosis of hip fracture; major burns; patients who were either non-English or non-Spanish speaking; non-U.S. residents; and individuals incarcerated or homeless at the time of injury.
A quota sampling strategy was used with the goal of enrolling approximately 3000 and 1200 patients ages 18 to 64 and 65 to 84, respectively, evenly distributed across trauma centers and nondesignated centers and within stratum defined by global injury severity and principal body region injured. This sample size was selected to allow for estimates of trauma center effectiveness. The rationale for the sampling approach was to avoid a disproportionate selection of patients selected from higher-volume trauma centers. Each in-hospital death was included, but only a random sample of patients discharged alive to accomplish the principal objectives of the NSCOT, yielding a total of 5191 enrolled subjects. As a result of this sampling strategy, it was necessary to weight each enrolled patient to the original population of 15,400 eligible patients. Of this population, there were 951 patients who received care at a non-NSCOT hospital before transfer to a participating center. These patients were excluded, leaving 14,489 patients. An extensive description of the sampling strategy and weighting scheme is provided in the parent study.10
For the purpose of evaluating ICU care in this sample, we limited the analyses to subjects admitted to an intensive care unit and excluded patients with a gunshot wound to the head. The rationale for the latter exclusion was because of the potential variability in transfer of perceived unsalvageable patients to an intensive care unit. These exclusions resulted in a sample size of 2599 patients. Using the sampling and weighting approach described above, the reference population to which inferences can be made consisted of 6789 subjects. A single nondesignated center did not admit any trauma patients to the ICU; thus, the analysis is based on 68 centers.
Model of Critical Care Delivery
Investigators at each participating center were required to fill out an extensive questionnaire outlining their resources. Included within this questionnaire was a series of questions evaluating the physical and human resources pertaining to critical care and the process of critical care delivery. While there is no standard definition for a closed or intensivist model of critical care delivery, we used a fairly inclusive definition to evaluate the effect of exposure to an intensivist on outcome following injury. An intensivist was defined as a physician board-certified in critical care. Thus, a patient was considered to have been exposed to an intensivist model of critical care if as a matter of routine in that center, critically ill trauma patients were either on a distinct ICU service (led by an intensivist) or were comanaged with an intensivist. For the sake of clarity, we use the term intensivist-model and closed ICU interchangeably.
Data Abstraction
Medical record abstraction was performed by nurses trained specifically for NSCOT and certified in AIS scoring by the Association for the Advancement of Automotive Medicine.11 Patient socio-demographic characteristics and coexisting diseases were abstracted from the medical record. The latter were weighted such that a Charlson comorbidity score was derived with the additional inclusion of obesity and coagulopathy given their strong association with mortality.12–14
Injuries were characterized by their mechanism, anatomic severity, and degree of physiologic derangement. Included in the latter were measures of systolic blood pressure in the emergency department, GCS motor score (with identification of those with and without pharmacologic paralysis), and pupillary responses. Both the ISS and the NISS were used as summary measures of injury severity.15,16
Data Analysis
Multiple imputation techniques using 10 datasets were used to impute missing covariates.17 Estimates and standard errors were computed using Rubin's combining rules.18 Robust standard errors were computed to account for clustering within hospitals. All analyses were performed using data weighted to the population of eligible patients.
Triage of patients to centers with and without intensivist-model intensive care units might be dependent on several patient characteristics that might confound the relationship between the type of ICU care received and outcome. To address this potential confounding, we used the inverse probability of treatment weighted or marginal structural approach, in which each subject is further weighted by the probability of receiving their type of care (closed or open ICU), given their demographic and injury characteristics listed in Tables 1 and 2. The validity of this approach relies on 1) the assumption that there are no unmeasured confounders and 2) correct specification of a model for the probability of receiving an intensivist-model of care given the known demographic and injury characteristics (ie, propensity score).19
TABLE 1. Patient Characteristics in Open and Intensivist-Model ICUs
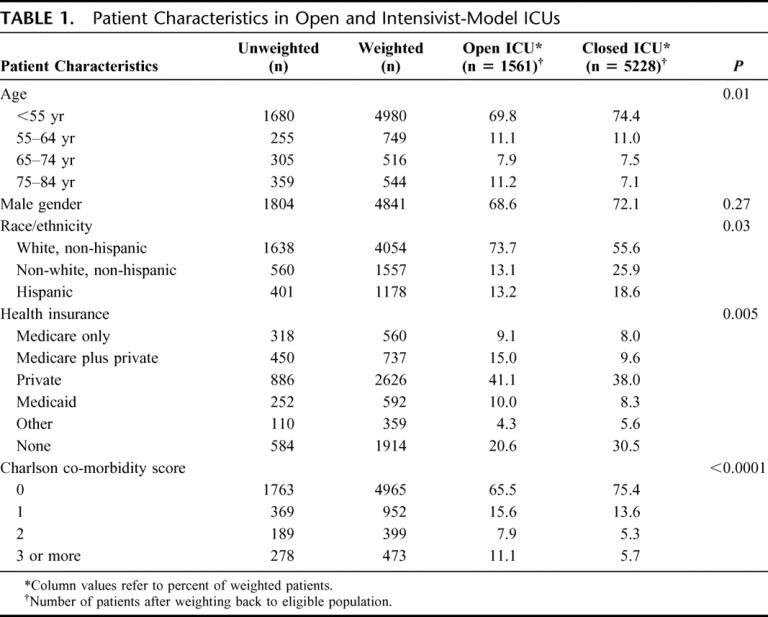
TABLE 2. Distribution of Injury Characteristics in Open Versus Intensivist-Model ICUs
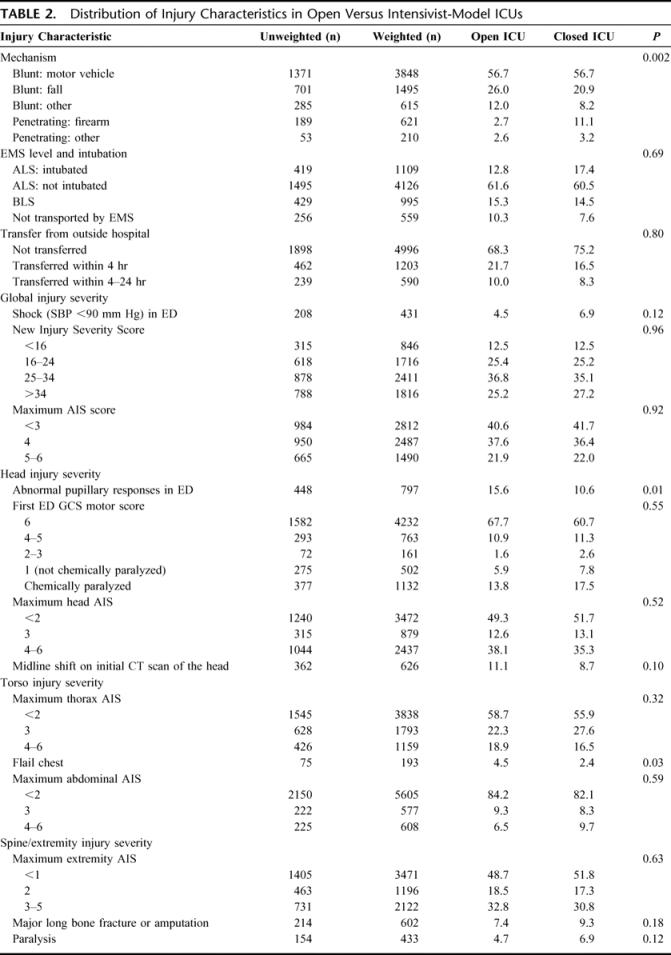
We also explored whether the effect of a closed ICU varied with certain patient characteristics including age (<55, ≥55 years), severity of head injury (head AIS <3, head AIS ≥3), global injury severity (ISS <25, ISS ≥25), and mechanism of injury (blunt, penetrating). Additionally, we considered the possibility that the effect of a closed ICU might vary with the hospital environment (trauma center, nondesignated center) or the expertise of the physician director (surgical intensivist, other). These relationships were evaluated by means of a Wald test of the null hypothesis of no difference between relative risks across specified subgroups, a similar approach to the evaluation of interaction terms in a multivariate model.
Data are presented as the adjusted risk of death along with the 95% confidence intervals in centers with intensivist-model ICUs compared with those without. All analyses were performed using SAS 9.1 and R 2.1.1.
RESULTS
Hospital and Critical Care Unit Characteristics
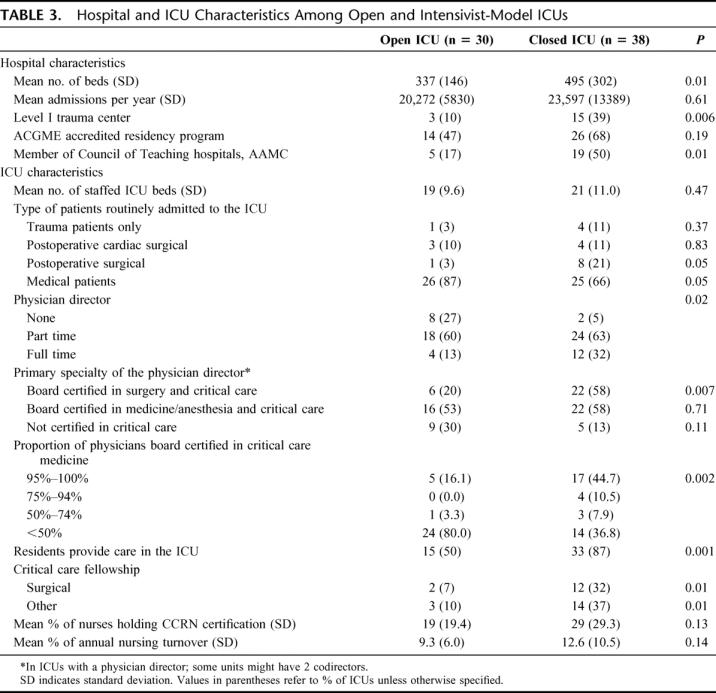
Critical care units in 38 (56%) of the centers met the criteria for an intensivist-model ICU. In 18 (47%), patients were cared for on a distinct ICU service while in the remainder there was a comanagement model such that patients were cared for both by an intensivist-led team and the primary surgical service. The ICU service had the sole authority to write orders in only 8 (21%) of these units. These 38 centers cared for 77% of patients meeting inclusion criteria. Institutions with closed ICUs were more likely to be a level I trauma center, had a greater number of hospital beds, and were more likely to be a teaching hospital compared with centers without (Table 3). Leadership differed across the 2 types of units, with full-time medical directors being more common in closed units. Additionally, there were a disproportionate number of such units in whom the director was board certified in both surgery and critical care. Consistent with their leadership, closed units had a greater proportion of physicians responsible for the delivery of care who were board certified in critical care. These units were also more likely to have residents involved in patient care and critical care fellowship programs. The only remarkable difference in patient composition across type of unit was an increased frequency of routine admission of postoperative surgical patients to intensivist-model ICUs and medical patients to open units.
TABLE 3. Hospital and ICU Characteristics Among Open and Intensivist-Model ICUs
Patient and Injury Characteristics
There were some differences in baseline patient characteristics across unit type, although these differences were relatively small. Specifically, patients in open units were older, less racially and ethnically diverse, more likely to have some form of insurance, and had a greater comorbidity burden than injured patients admitted to closed units (Table 1). With the exception of a higher incidence of firearm-related injury in closed units, the distribution of injury characteristics was quite similar (Table 2). Global injury severity as measured by NISS was no different across units, although there was an insignificantly higher rate of hypotension at presentation in centers with closed units. Conventional measures of head injury severity were no different across units (GCS motor score, head AIS score), yet the proportion of patients with abnormal pupillary responses in the ED was higher in centers with closed units and there was a tendency for a higher rate of midline shift on the initial CT scan of the head. Similarly, severity of torso and extremity injuries was similar with the exception of an excess of flail chest injuries in open units and an insignificant increased use of paralytics among patients cared for in closed units.
Mortality Outcomes
There was a significantly lower crude mortality rate in intensivist-model units (10.1%) compared with open units (13.9%), yielding a crude relative risk of death of 0.72 (0.62–0.84) in the former. To address the potential for confounding we used the inverse weighting approach as described in Methods using many of the parameters in Tables 1 and 2. The propensity score model included age, race, gender, insurance status, comorbidities including obesity and coagulopathy, mechanism of injury, shock status, pupillary responses, GCS motor score, NISS, ISS, maximum AIS score, maximum head AIS, midline shift on CT scan, major long bone fracture or amputation, maximum AIS of the thorax, abdomen, and extremities, the presence of flail chest, paralysis, and prehospital intubation status. Further, we have previously demonstrated a strong mortality benefit associated with trauma center care.10 As it was, our objective to evaluate the impact of an intensivist-model of ICU care independent of the effect of trauma center care, we also included a term in the model representing trauma center status.
The adjusted relative risk of death in intensivist-model ICUs compared with open units was 0.78 (0.58–1.04) (Table 4). However, the effects were nonuniform across several patient and institutional characteristics specified in Methods. Specifically, the relationship between an intensivist-model ICU and mortality was modified by age, with only the elderly (age >55 years) demonstrating a significant (45%) reduction in mortality. While the strength of the association between a closed ICU and outcome across high and low ISS strata was relatively similar, there was a tendency for patients with less severe head injuries to derive greater benefit, with a 36% reduction in the risk of death.
TABLE 4. Adjusted Relative Risk of Death in Intensivist-Model ICUs
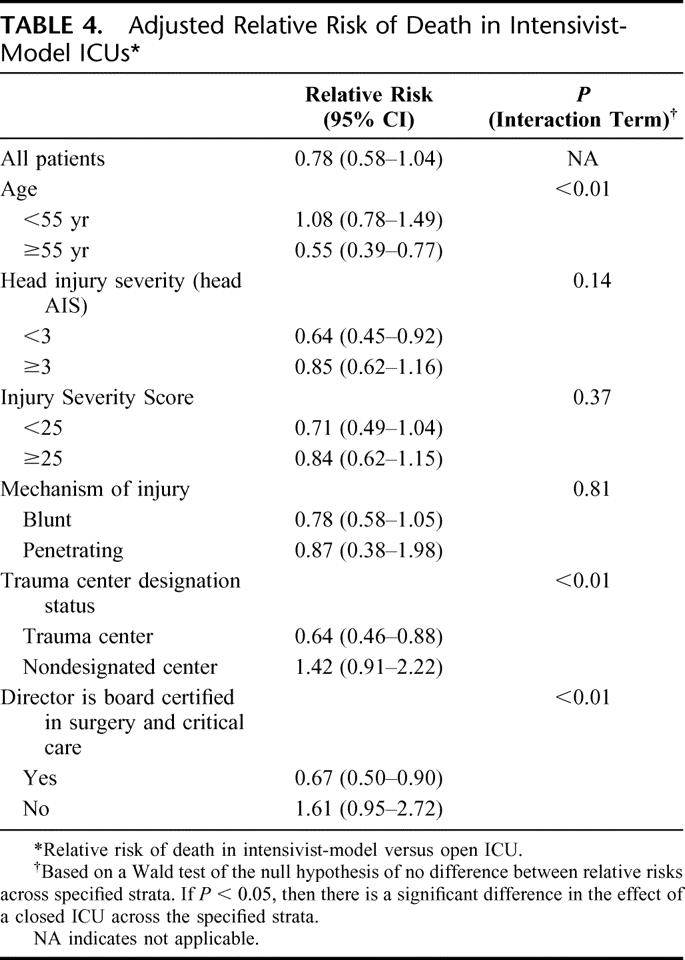
There was also a significant relationship between trauma center status and an intensivist-model ICU. Care in an intensivist-model ICU in a trauma center was associated with a 36% reduction in the risk of death. By contrast, there was no benefit and a tendency toward harm when patients in nondesignated centers were cared for in intensivist-model ICUs. To gain insight into what might explain this effect, we evaluated whether the relationship between an intensivist-model ICU and outcome was modified by the specialty and focus of the ICU director. There was clear evidence of a beneficial effect of an intensivist-model ICU with a director board certified in surgical critical care. However, like the effect seen in nondesignated centers, there was no benefit and potential for harm in a closed unit run by either nonsurgeons or those not certified in critical care.
DISCUSSION
We have demonstrated a reduction in mortality associated with the involvement of board-certified intensivists in the care of the critically injured trauma patient. In a national survey of trauma centers, we previously reported that 77% of level I trauma centers and 60% of responding level 2 centers routinely involve an intensivist in the routine care of trauma patients, either through a model of collaborative care with the trauma surgeon or through transfer of care to an intensivist, who may or may not be a surgeon. In the centers studied in the current report, 83% of level I centers and only 35% of nondesignated centers fulfill this criterion of a closed ICU model. While these level I centers are representative of level I centers throughout the United States, the nondesignated centers were selected because of their annual volume of major trauma (>25 cases per annum) and were larger and more likely to be teaching hospitals than the average nondesignated center in the United States.10 While the number of cases cared for by each of these centers annually is relatively small, these types of nondesignated centers care for approximately 30% of major trauma patients in the United States.20 Thus, while many but not all level I trauma centers already meet this criterion, there is substantial room for improvement in the critical care of the severely injured patient.
We estimated a relative risk of mortality of 0.78 (0.58–1.04) associated with care in an intensivist-model ICU. The width of the confidence interval is a reflection of the limited sample size and the robust standard error estimates necessary to account for clustering of patients within centers, a methodologic limitation often ignored in many multicenter studies.21 Nevertheless, there is a marked clinical and statistically significant benefit in certain subgroups of patients. For example, the risk of death in the elderly is reduced by almost 50% with the involvement of an intensivist. This effect might relate to a greater understanding of the impact and care of coexisting diseases in this population. Alternatively, dedicated intensivists might simply have more available time to care for patients who require more frequent evaluation and more careful titration of therapy due to their limited physiologic reserve. There is a lesser effect in patients with severe head injury and those with an ISS ≥25, as their outcome might be more dependent on the severity of injury and less so on variations in the quality of care.
To provide greater insights into the mechanisms by which intensivists might play a role in the care of the critically injured, we evaluated the impact of an intensivist-model ICU in trauma centers compared with nondesignated centers. While intensivists had a marked beneficial effect in the former, there was no observable benefit and perhaps a potential for harm in the latter. As there is a strong association between trauma center status and the expertise of the critical care director,3 we stratified centers by whether the director was board certified in surgical critical care. In this latter analysis, an intensivist-model ICU whose director (or a codirector) was board certified in surgical critical care was associated with a marked benefit, while this ICU structure without such leadership offered no benefit. These data speak to the value of time, attention to detail, and content expertise in caring for these patients. Specifically, ICU care might not be generic and the application of a particular body of knowledge and experience relevant to the care of these patients is necessary for optimal outcomes.
This analysis has several limitations that might impact on the interpretation of these data. First, the data on the model of critical care delivery was self-reported by institutions and was not subject to any form of external validation. However, the data were collected as part of an extensive questionnaire designed to evaluate hospital resources, and there is no reason to believe there was a systematic bias in the interpretation of the questions or their responses by the respondents. It is unlikely there are biases introduced in outcome assessment, as we selected mortality given its objective nature and its imperviousness to other factors (eg, bed availability) compared with complications and length of stay, respectively.
With only 68 centers participating, we acknowledge the limitations in sample size, which might have 2 effects: a higher likelihood of type II error and potential limited generalizability. Both of these are a reflection of the fact that NSCOT was neither powered adequately nor designed specifically to evaluate ICU care. We have provided estimates of uncertainty in the form of confidence intervals to allow the reader to gauge the importance of the relative risk reduction. In our assessment of overall effect, the upper limit of the confidence interval just exceeds unity. Further, in many subpopulations (elderly, less severe head injury), the effect is both clinically and statistically significant. The extent to which these data are generalizable to a broader spectrum of trauma centers is less clear. It is likely that the participating level I trauma centers are representative of all U.S. level I trauma centers given the relatively similar standards applied in most regions of the country. By contrast, the participating nondesignated centers differ in their annual trauma volume, size, and teaching status compared with other U.S. hospitals.10 At a minimum, it is likely they are providing superior care to the average nondesignated center.
These data have significant practical and policy implications. They emphasize the importance of continuing to provide critical care training to surgeons interested in pursuing a career in trauma. This combination of expertise in the critical care director is associated with a significant mortality benefit. It is likely that the influence of the director is critical in the development of policy and protocols, quality assurance, and in the selection of physicians most capable of providing care in the ICU. While we did not specifically ask the extent to which intensivists are dedicated to the ICU (ie, relieved of other operative or clinic responsibilities during their attendance in the unit), this aspect of an intensivist-model unit is thought to be important in assuring availability of the physician to address the immediate needs of the patient and is implicit in the concept of an ICU service. Thus, a distinct ICU service allowing for a dedicated team to care for the patient is critical to success. Given the high standard set by the American College of Surgeons Committee on Trauma for the verification of trauma centers and the accruing data supporting this approach, the Committee should consider adding a dedicated ICU team as an essential criterion for institutions caring for the critically injured.
Discussions
Dr. Anthony A. Meyer (Chapel Hill, North Carolina): This is an excellent study from a group of investigators who have established a track record of quality outcomes research especially focusing on rural trauma centers and care of the injured. The study was designed to investigate whether a traditional structure of ICU care of trauma patients has a direct impact on mortality and outcome. The study used the National Study on Cost and Outcomes Trauma data set in the experimental design had reasonable exclusion criteria data elements. And all such studies have limitations, but the quality of this data set appears sound.
The data provide several interesting findings. First, that there was a benefit, an approximately 22% improvement in mortality when adjusted in closed versus open units managing trauma patients in level 1 and level 2 trauma centers. Although the confidence limits ranged from .78 to 1.74, this is a worthwhile finding. The relationship becomes statistically significant in non-head-injured patients, and especially elderly patients, with confidence limits that do not cross 1.0.
The most important finding, I believe, is that closed ICUs with surgeon intensivists had the best outcomes while closed ICUs with non-surgeon intensivists had no significant advantages and even a tendency toward harm. An important collateral finding was that in non-trauma centers the closed units had a tendency toward worse outcome. I have a few questions and some comments.
You described the closed or intensivist type unit as 2 different types, those of which there is collaborative or co-managed care as you describe, that is with orders written by both primary service and by the ICU team, as well as the more traditionally described closed units where orders are written only by the ICU team. This is an enormous difference and one that has far-reaching implications for training and financing of surgery programs. Do you have an analysis of collaborative versus truly closed units? This directly relates to your findings in the non-trauma center and entities where surgeons are involved in the team providing ICU care.
Second, there were some baseline differences in your patients, with patients in the open units more likely to be insured and older and gunshot wounds to be more prevalent in the closed unit trauma centers. Is this because there were more private hospitals or centers in the open unit rather than public institutions in the closed unit trauma center group?
Third, what do you have in your ICU? How many of your intensivists are Board certified in critical care and how many are surgeons? And what percent of the time is the ICU team attending a non-surgeon?
Fourth, what about non-trauma patients? This study focused on trauma centers, but what is your thought about with other groups of patients such as transplant, cardiothoracic, neurosurgery or more complex general surgery patients that make up significant surgical ICU populations?
I believe this is a very important paper not only because of its findings but because subsequent studies will follow because of it. It is extremely important to understand, however, that there are individuals with positions at closed units, especially those units where intensivists write all orders and are not surgeons would argue that this proves that their closed system is superior to a collaborative unit. Your data does not suggest this nor do you recommend this in your discussion. However, the use of the word “closed” is a description that for both collaborative and truly closed units will likely be taken by some as substantiation for their particular agenda.
What is particularly interesting in your data is that it suggests that a non-surgeon directed closed unit actually has a tendency toward harm compared to an open or clinical unit, possibly because of type 2 error or possibly because there are so many other important variables. This is not statistically significant, but the interpretation of the results is clear to me.
Surgeon intensivist critical care units provide superior outcomes for trauma patients compared to open units and non-surgeon directed closed units. This information, along with the confirmation in your present study as well as the previously cited study from the New England Journal that trauma centers independently improve the outcome of trauma patients makes this a study that will be cited regularly and hopefully lead to follow-up investigation by your group and others. I heartily commend you for the study and look forward to subsequent research along the same path.
Dr. Avery B. Nathens (Seattle, Washington): You asked whether we compared collaborative models of ICU care with true closed units. We have not looked at this in great detail. As a very rudimentary analysis, there appears to be a greater benefit if the ICU was truly closed, but we haven't evaluated this with appropriate risk adjustment. Unfortunately, as you start slicing and dicing the units to a greater extent, the results become less robust.
In our intensive care unit, we have surgeons rotating through the unit; all are board certified intensivists. However, we wear different hats at different times. When I am the surgical intensivist, I defer surgical decision-making to my colleague who is the trauma surgeon of the month. When I am the trauma surgeon, I expect the intensivist to defer surgical decisions to me. This appears to work very well, with each respecting the other's autonomy We occasionally have a non-surgical intensivist; typically an anesthesiologist or pulmonologist who rotates through the ICU. This occurs in only a handful of weeks annually. We don't notice a significant difference in care as our non-surgical colleagues have become accustomed to what we do.
You asked what the effects or a closed unit might be on non-trauma surgical patients. There are a few studies that look at the effects in critically ill surgical patients, one that Dr. Cioffi published several years ago, showing better outcome in closed units. There are studies now in major vascular surgery and major elective GI surgery showing similar benefits.
Dr. A. Brent Eastman (San Diego, California): Dr. Nathens, I congratulate you and your group for this paper, a provocative paper that I believe challenges yet again one of the sacred cows in the Optimal Care Document for verification of trauma centers. Hopefully these data and other evidence will continue to evolve that document.
I have 2 questions. As I recall, the MacKenzie paper that you quoted showed that the benefit of trauma centers was limited to the young and in fact the old did not fare as well. And yet you find the opposite in this. Secondly, with the acute care surgeon, which Dr. Jurkovich at your institution and others are so put vocally putting forward, with the acute care surgeon in a hospital where he or she was the only surgical intensivist, would they be an operating surgeon as well as running the ICU? Or would they do both?
Dr. Avery B. Nathens (Seattle, Washington): With regard to the National Study on the Costs and Outcomes of Trauma (NSCOT, MacKenzie et al, NEJM, 2006) and the impact on the elderly, it is true, the young benefited from care in a trauma center (compared to a non-designated center) to a greater extent than the elderly. However, this study evaluates something other than ICU care in trauma centers versus non-trauma centers, as there are intensivist model units in non-designated centers and open units in trauma centers.
You asked what the role of the acute care surgeon in the context of critical care within an institution. In a perfect world, the surgical intensivist would run the SICU. When attending to patients in the unit, the intensivist would not have operative responsibilities to distract him/her from patients in the ICU. We live in a world far from perfect and the solution is a collaborative care model with input from a surgical intensivist (who may or may not be the operating surgeon or who may or may not be the intensivist of the day), I think patients are likely to fare just as well as they would with surgical intensivists there 24 hours a day. Clearly there are manpower issues, and I think the onus is on us to train more surgical intensivists.
Dr. Douglas E. Wood (Seattle, Washington): Dr. Nathens, a very nicely presented paper. But I am a little puzzled by the strength of your conclusion, that is, that the difference is the closed ICU. Can you comment on whether the closed ICU in this case is a surrogate for other aspects of the institution that is caring for the patients that may be equally or maybe even more important than actually the construct of the ICU? I know you tried to correct for that in some ways. But it looks like there are many ways that that can't really be corrected. And I wonder whether there might be other institutional factors that are equally important.
This becomes important, for example, in cardiothoracic surgery where our pulmonary and critical care colleagues extrapolate from data like this–I think incorrectly. I might add that cardiothoracic surgeons shouldn't take care of their patients in the ICU, that there needs to be a closed ICU. So I am very interested in kind of the conclusions and whether they are completely supported by the data.
Dr. Avery B. Nathens (Seattle, Washington): I agree with your comments. This issue is complex in that closed ICUs tend to occur more frequently in hospitals that are larger, hospitals that have fellowship programs, and hospitals that likely see more trauma patients on an annual basis. They develop this infrastructure in their intensive care unit because of these other things. They have the manpower. They have the patients. So I believe these are inseparable.
It is important to note that in our analysis, we did adjust for trauma center status, which includes a lot of those other things including size, volume, teaching status, and the effect still remains. How that might into other ICU environments–cardiac surgery, or transplant, for example–is not clear. Clearly, having a closed ICU represents a lot of different things cannot be accounted for. So I encourage readers to take this information and use it as they see fit.
Dr. Joseph VanDeWater (Macon, Georgia): Did you see a difference in the placement and, more importantly, use of pulmonary artery catheters? Secondly, how about algorithms for treatment of the trauma patients? Were there algorithms in place and was there a difference?
Dr. Avery B. Nathens (Seattle, Washington): We are about to look at the variation in use of PA catheters and other interventions in these patients. We don't have any data on the use of algorithms these units. We have some process measures to ascertain how they care for patients but we don't actually have knowledge of whether they use particular algorithms.
Dr. Philip S. Barie (New York, New York): We have discussed the manpower and the training implications, but the fact remains that manpower projections by ICCM and other groups suggest that we don't have enough intensivists of any primary specialty now and that that shortage is only going to get worse. But even recognizing that, essentially every time this question has been examined in a scientifically valid manner the open ICU model has been shown to have significant shortcomings.
Are we making a promise here, a promise of better outcomes with an intensivist-led model that we can't keep? And are we at risk of not only (a) breaking our compact with our patients to provide optimal care, but (b) also creating a threat that non-surgeon intensivists will fill the manpower void and make it even more difficult for surgeons to care for their patients?
Dr. Avery B. Nathens (Seattle, Washington): I think the Optimal Resources Document has a critical role to play here. The effect was greatest in trauma centers. We already require that ICUs in trauma centers be led by surgical intensivists. With NSCOT (MacKenzie et al. NEJM, 2006), we have already identified the need for major trauma patients to be care for in designated centers. So, much of this as it pertains to trauma has already been addressed.
I really do not have an answer for non-trauma surgical ICU patients. I don't think that ignoring the data because there are manpower issues is appropriate. What I can say is that with a limited number of surgical intensivists, the default then becomes collaboration with a non-surgical intensivist. And I emphasize “collaboration” here. I don't think that a medical intensivist writing ventilator orders means the surgeon cannot care for his or her patients in the unit.
Footnotes
Funded by Grant No. R49/CCR316840 from the National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention and Grant No. R01/AG20361 from the National Institute on Aging of the National Institutes of Health.
Reprints will not be available from the authors.
Correspondence: Avery B. Nathens, MD, PhD, MPH, 325 9th Avenue, Box 359796, Seattle, WA 98104. E-mail: anathens@u.washington.edu.
REFERENCES
- 1.Haupt MT, Bekes CE, Brilli RJ, et al. Guidelines on critical care services and personnel: recommendations based on a system of categorization of three levels of care. Crit Care Med. 2003;31:2677–2683. [DOI] [PubMed] [Google Scholar]
- 2.Brilli RJ, Spevetz A, Branson RD, et al. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007–2019. [DOI] [PubMed] [Google Scholar]
- 3.Nathens AB, Maier RV, Jurkovich GJ, et al. The delivery of critical care services in US trauma centers: is the standard being met? J Trauma. 2006;60:773–784. [DOI] [PubMed] [Google Scholar]
- 4.Ghorra S, Reinert SE, Cioffi W, et al. Analysis of the effect of conversion from open to closed surgical intensive care unit. Ann Surg. 1999;229:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson CW III, Deutschman CS, Anderson HL III, et al. Effects of an organized critical care service on outcomes and resource utilization: a cohort study. Crit Care Med. 1999;27:270–274. [DOI] [PubMed] [Google Scholar]
- 6.Pronovost PJ, Jenckes MW, Dorman T, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281:1310–1317. [DOI] [PubMed] [Google Scholar]
- 7.Pronovost PJ, Angus DC, Dorman T, et al. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. [DOI] [PubMed] [Google Scholar]
- 8.Leapfrog Group. ICU Physician Staffing Factsheet. Washington, DC: Leapfrog Group, 2004. [Google Scholar]
- 9.American College of Surgeons Committee on Trauma. Resources for Optimal Care of the Injured Patient 1999. Chicago: American College of Surgeons, 1998. [Google Scholar]
- 10.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. [DOI] [PubMed] [Google Scholar]
- 11.Association for the Advancement of Automotive Medicine. The Abbreviated Injury Scale, 1990 revision. 1990. Des Plaines, IL: Association for the Advancement of Automotive Medicine. [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 13.Morris JA, MacKenzie EJ, Edelstein SL. The effect of pre-existing disease on hospital mortality in trauma patients. JAMA. 1990;263:1942–1946. [PubMed] [Google Scholar]
- 14.Neville AL, Brown CV, Weng J, et al. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch Surg. 2004;139:983–987. [DOI] [PubMed] [Google Scholar]
- 15.Osler T, Baker SP, Long W. A modification of the injury severity score that both improves accuracy and simplifies scoring. J Trauma. 1997;43:922–925. [DOI] [PubMed] [Google Scholar]
- 16.Baker SP, O'Neill B, Haddon W Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 17.Raghunathan TW, Lepkowski JM, Van Hoewyk J, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Biometrika. 2001;27:85–95. [Google Scholar]
- 18.Rubin DB. Multiple Imputation for Non-Response in Surveys. New York: Wiley, 1987. [Google Scholar]
- 19.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 20.Nathens AB, Jurkovich GJ, MacKenzie EJ, et al. A resource-based assessment of trauma care in the United States. J Trauma. 2004;56:173–178. [DOI] [PubMed] [Google Scholar]
- 21.Roudsari B, Nathens AB, Koepsell T, et al. Analysis of clustered data in multicentre trauma studies. Injury. 2006;37:614–621. [DOI] [PubMed] [Google Scholar]


