Abstract
Background/Objective:
While the Cox-Maze procedure remains the gold standard for the surgical treatment of atrial fibrillation (AF), the use of ablation technology has revolutionized the field. To simplify the procedure, our group has replaced most of the incisions with bipolar radiofrequency ablation lines. The purpose of this study was to examine results using bipolar radiofrequency in 130 patients undergoing a full Cox-Maze procedure, a limited Cox-Maze procedure, or pulmonary vein isolation alone.
Methods:
A retrospective review was performed of patients who underwent a Cox-Maze procedure (n = 100), utilizing bipolar radiofrequency ablation, a limited Cox-Maze procedure (n = 7), or pulmonary vein isolation alone (n = 23). Follow-up was available on 129 of 130 patients (99%).
Results:
Pulmonary vein isolation was confirmed by intraoperative pacing in all patients. Cross-clamp time in the lone Cox-Maze procedure patients was 44 ± 21 minutes, and 104 ± 42 minutes for the Cox-Maze procedure with a concomitant procedure, which was shortened considerably from our traditional cut-and-sew Cox-Maze procedure times (P < 0.05). There were 4 postoperative deaths in the Cox-Maze procedure group and 1 in the pulmonary vein isolation group. The mean follow-up was 13 ± 10, 23 ± 15, and 9 ± 10 months for the Cox-Maze IV, the pulmonary vein isolation, and the limited Cox-Maze procedure groups, respectively. At last follow-up, freedom from AF was 90% (85 of 94), 86% (6 of 7), and 59% (10 of 17) in the in the Cox-Maze procedure group, limited Cox-Maze procedure group, and pulmonary vein isolation alone group, respectively.
Conclusions:
The use of bipolar radiofrequency ablation to replace Cox-Maze incisions was safe and effective at controlling AF. Pulmonary vein isolation alone was much less effective, and should be used cautiously in this population.
This study evaluated the efficacy of a full Cox-Maze IV procedure, a limited Cox-Maze procedure, and pulmonary vein isolation for the treatment of atrial fibrillation. Ablation lines using bipolar radiofrequency energy were used to replace many of the incisions of the original Cox-Maze procedure.
Atrial fibrillation (AF) is the most common sustained arrhythmia in the United States. Adults over the age of 40 years have a 25% risk of developing AF during their lives.1,2 Because AF has detrimental sequelae, including symptoms from tachyarrhythmias, hemodynamic compromise, and thromboembolic complications, many treatment strategies have been developed to alleviate the disease. Antiarrhythmic drugs, catheter-based ablation, and surgery have all been used with varying efficacies. Unfortunately, medical therapy has significant limitations and antiarrhythmic drugs have recurrence rates of AF of more than 60% in some series.3,4 Catheter ablation has reported short- and mid-term cure rates of 59% to 91%, respectively, at variable follow-up.5 The surgical treatment of AF has been most successful with the Cox-Maze III procedure, which has long-term cure rates over 90%.6,7
Although the Cox-Maze III procedure had excellent long-term efficacy,6 the cut-and-sew technique was technically challenging to carry out and few surgeons routinely performed the operation. Recent technologic advancements in ablation devices have provided surgeons with the possibility of performing a modified Cox-Maze procedure by replacing some or all of the traditional incisions with lines of ablation. At our institution, excellent laboratory results using a bipolar radiofrequency (RF) ablation clamp8,9 led to the development of a modification of the original cut-and-sew procedure, which was first performed in 2002. In this procedure, termed the Cox-Maze IV, many of the lesions of the conventional version are replaced with linear lines of ablation using bipolar RF energy.10 Early experience with the Cox-Maze IV procedure recorded comparable success rates to the Cox-Maze III procedure with freedom from AF at last follow-up of greater than 90%.11
The Cox-Maze procedure involved the creation of a myriad of lesions on the atria originally designed to prevent macro-reentrant circuits that were felt to be responsible for the initiation and/or maintenance of AF.12 Speculation concerning which lines of conduction block were essential for treating AF led to the development of several different operations involving limited lesion sets. These have included only left atrial lesions, right atrial lesions, pulmonary vein isolation alone, or a combination of different lesions.13,14 Using mapping techniques, some groups have found that many cases of AF initiate from ectopic beats at foci in the pulmonary veins.15–17 The use of catheter-based RF ablations to isolate the pulmonary veins and treat AF has shown short-term success rates near 80%.5 As a result, it has been suggested that pulmonary vein isolation by catheter-based RF ablation be considered as a first-line treatment of AF.18,19 Surgeons have also attempted to treat AF with fewer lesions on the atria than the traditional Cox-Maze procedure, but reports of long-term success have been scarce.
The purpose of this study was to examine results using bipolar RF ablation in 130 patients undergoing a Cox-Maze IV procedure, a limited Cox-Maze procedure, or pulmonary vein isolation alone and to compare them to a historical cohort of patients undergoing a “cut-and-sew” Cox-Maze III procedure.
PATIENTS AND METHODS
Study Design and Endpoints
A retrospective review was performed of patients who underwent surgery utilizing bipolar RF ablation to complete a Cox-Maze IV procedure (n = 100), pulmonary vein isolation alone (n = 23), or a limited Cox-Maze procedure (n = 7). Follow-up was available on 129 of 130 patients (99%). The primary endpoints of this study were: freedom from recurrence of AF, pulmonary vein isolation as evaluated by intraoperative pacing thresholds, presence of postoperative atrial arrhythmias, and continued use of antiarrhythmic medications. Secondary endpoints were hospital length of stay, postoperative atrial arrhythmias, postoperative permanent pacemaker insertions, and major postoperative events (myocardial infarction, bleeding, stroke, thromboembolism, or death). Follow-up was obtained at 1, 3, 6, and 12 months and then annually. Persistent AF was defined as continuous AF, permanent AF was defined as persistent AF of more than 6 months’ duration that had failed electrical or chemical cardioversion. Paroxysmal AF was defined as sinus rhythm with intermittent episodes of AF.
The patients undergoing these ablation-assisted procedures were compared with a historical cohort of patients undergoing a Cox-Maze III at our institution between January 1988 and January 2002.6
Patient Accrual
A total of 130 consecutive patients were enrolled after informed consent was obtained for their procedures, in accordance with the ethical standards of the Human Studies Committee at our institution. The specific procedure was determined by the operating surgeon. All patients who presented with AF as the primary indication for surgery underwent a full Cox-Maze procedure. Patients that had AF as a secondary indication for surgery, and who either had paroxysmal AF and a small left atrium (<5 cm) or who were deemed too high risk for a full CMP underwent PVI alone. A left atrial procedure was rarely performed (n = 2) in patients with isolated left atrial pathology. Patients with isolated right heart pathology (usually due to congenital heart disease and/or tricuspid regurgitation) had only the right-sided lesions done. All patients who underwent a Cox-Maze procedure, limited Cox-Maze procedure, or pulmonary vein isolation procedure were included in this study.
Bipolar RF Ablation
The bipolar RF system consisted of the ablation sensing unit and the Atricure Isolator (Atricure, Inc., Cincinnati, OH), or the Medtronic Cardioblate BP Surgical Ablation System (Medtronic Inc., Minneapolis, MN). For the Atricure device, the energy was applied at 75 W and 750 mA between the jaws of the instrument. The generator continuously monitored voltage, current, temperature, time, and conductance. Tissue temperature was measured 1 mm from the electrode edge. Two seconds after conductance fell below 0.025 Siemens, an indicator light flashed and an audible tone was heard, signifying full thickness coagulation and termination of the ablation. Total ablation time and maximum tissue temperature were recorded for every lesion.
The Medtronic clamp used was an irrigated bipolar RF surgical ablation device, which consisted of a hand piece with embedded bipolar electrodes, and a RF generator. The device was constantly irrigated with saline solution, which acted as a conductor for the delivered energy. The generator delivered RF energy while an online system monitored tissue impedance, current, voltage delivered, and the duration of ablation in real time. The device operated on the principal that tissue is fully ablated when impedance reached a stable plateau. Initially, moderate power was applied to the tissue. Impedance (Z) was measured continuously, and the derivative of impedance (dZ/dt) was calculated every 200 ms. When impedance achieved a stable plateau, the algorithm logic determined that maximum ablation at this power level was complete. The power was then increased by a step function of 5 W. If the plateau in impedance was not sustained, then the algorithm determined that transmurality had not been achieved and ablation continued until another plateau in impedance was detected. This process was repeated until an impedance plateau was sustained after an increase in power. At this point, the microprocessor determined that transmurality had been achieved and the generator provided a signal to the user.
Operative Technique
After either median sternotomy or right thoracotomy, patients underwent a pericardotomy, and were placed on cardiopulmonary bypass. If patients were not in normal sinus rhythm, intraoperative direct-current cardioversion was performed. The right and left pulmonary veins were carefully dissected and isolated using a blunt technique. Pacing thresholds from the right and left pulmonary veins were obtained by performing bipolar epicardial pacing. The bipolar RF clamp was then placed such that a rim of atrial tissue surrounding the pulmonary veins was ablated. After ablation, electrical isolation was confirmed by bipolar pacing at 20 mA from both the superior and inferior pulmonary veins. If atrial capture was present, the ablation was repeated until electrical isolation was achieved. In 23 patients, pulmonary vein isolation alone was performed as the sole treatment of AF, based upon the operating surgeon's judgment. This procedure generally was chosen for patients who were felt to be too high a risk for a complete Cox-Maze IV, or who had paroxysmal AF of short duration with a small left atrium.
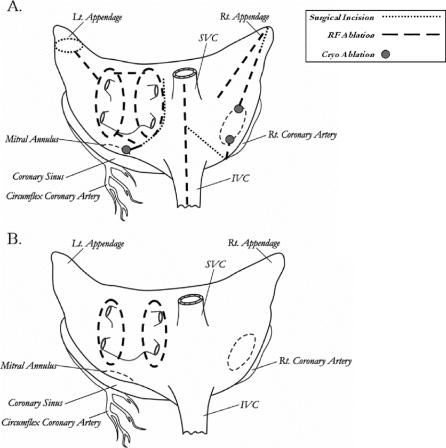
The remainder of the operation has been described in a prior publication.11 In summary, the right-sided lesions were created by making a simple atriotomy, which extended from the intra-atrial septum, up to near the atrioventricular groove at the acute margin of the heart. All the other incisions of the traditional cut-and-sew method were replaced with bipolar RF ablation lines (Fig. 1). Two cryolesions were placed at the tricuspid anulus using a linear cryoprobe cooled to −60°C for 2 minutes. In 5 of the 7 patients that underwent a limited Cox-Maze procedure, only the right-sided lesions were done (Fig. 1). These patients had isolated right heart pathology usually due to congenital heart disease or tricuspid regurgitation.
FIGURE 1. Lesion sets. A, Cox-Maze IV procedure. B, Pulmonary vein isolation procedure.
For those patients that had a full Cox-Maze procedure (n = 100) or limited Cox-Maze procedure including only the left-sided lesions (n = 2), only a single left atriotomy was performed. This incision was extended onto the dome of the left atrium and inferiorly around the orifice of the right inferior pulmonary vein. It intersected the encircling right pulmonary vein ablation. A connecting ablation lesion was performed from the inferior aspect of the left atrium into the left inferior pulmonary vein. In atria larger than 5 cm in diameter, a second connecting ablation was placed from the superior aspect of the incision into the left superior pulmonary vein. Finally, a bipolar RF ablation line was performed from the inferior end of the incision down to the mitral anulus at a point in between the circumflex and right coronary circulations. A cryolesion was placed at the mitral anulus with a 15-mm bell probe (Frigitronics, CCS200, Trumbull, CT) cooled to −60°C for 3 minutes. The left atrial appendage was amputated and a bipolar RF ablation was performed between the left atrial appendage and the left superior pulmonary vein. The left atrial appendage was oversewn (Fig. 1).
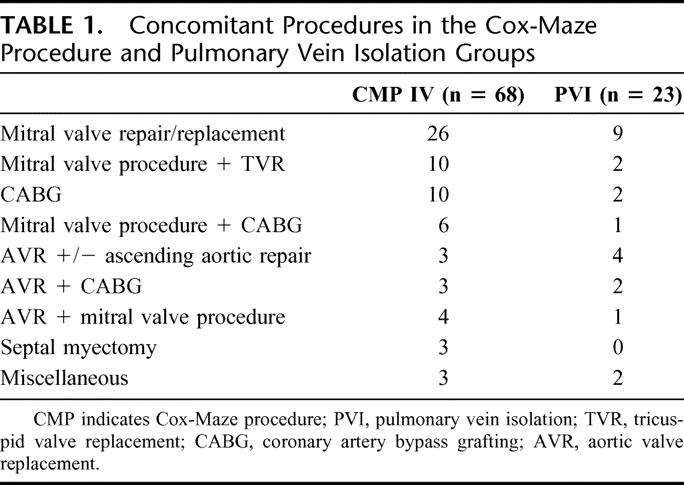
Concomitant procedures performed in this study included coronary artery bypass grafting, mitral valve repair/replacement, aortic valve replacement, tricuspid valve replacement, patent foramen ovale closure, atrial septal defect repair, left atrial reduction, septal myectomy, and resection of intracardiac tumor (Table 1).
TABLE 1. Concomitant Procedures in the Cox-Maze Procedure and Pulmonary Vein Isolation Groups
A description of the Cox-Maze III procedure has been published previously.20 The cryolesions were done on the valvular anulus in a similar manner for both the Cox-Maze III and IV procedures. The lesion made through the atrial septum in the Cox-Maze III procedure was not completed in the Cox-Maze IV surgery, as it was traditionally done for access only and was not considered a necessary part of the lesion set. The results for these Cox-Maze III patients have been reported previously.6
Postoperative Care and Patient Follow-up
After the operation, all patients were monitored continuously for arrhythmias. Perioperative AF was treated with antiarrhythmic drugs, usually amiodarone. If these drugs were not tolerated, patients were managed with rate control medication and underwent elective cardioversion at 2 to 6 weeks. Anticoagulation with Coumadin was used in patients for the first 3 months, unless there was a specific contraindication.
Patients had follow-up visits scheduled for 1, 3, 6, and 12 months and then annually. At all visits, a history and physical examination and an electrocardiogram were obtained. In patients with symptoms, palpitations, or other evidence of atrial arrhythmias, Holter monitoring or event recorders were obtained.
Data Analysis
Analysis was conducted after data entry into a confidential patient database. All continuous data were expressed as mean ± SD. Categorical data were expressed as counts and proportions. Comparisons were done with paired, 2-tailed t tests for means of normally distributed continuous variables and the Wilcoxon rank-sum tests for skewed data. χ2 or Fisher exact test tests were used to analyze differences among the categorical data. Statistical analysis of data was conducted with the SPSS system for statistics (SPSS Inc., version 11, Chicago, IL).
RESULTS
Patient Enrollment and Demographics
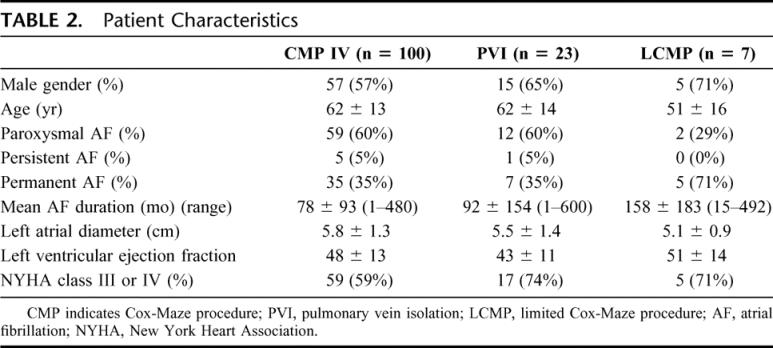
Between January 2002 and October 2005, 130 consecutive patients were enrolled in this study at Barnes-Jewish Hospital, St. Louis, MO. Follow-up was completed at 1, 3, 6, and 12 months, and annually thereafter. Mean follow-up was 13 ± 10, 23 ± 15, and 9 ± 10 months for the Cox-Maze IV, the pulmonary vein isolation, and the limited Cox-Maze procedure groups, respectively. The clinical, electrocardiographic, and operative characteristics of each group are outlined in Table 2.
TABLE 2. Patient Characteristics
Perioperative Results
Electrical isolation of the pulmonary veins was confirmed with bipolar epicardial pacing in 129 of 130 patients. A single patient could not be cardioverted into normal sinus rhythm; therefore, isolation could not be tested in that patient. There were 4 operative deaths (4%) in the patients that underwent a full Cox-Maze IV procedure, and 1 death (4%) in the pulmonary vein isolation group, and none in the limited Cox-Maze group. The median hospital length of stay was 11 days (range, 4–61 days) for the Cox-Maze IV patients, and 11 days (range, 3–54) for the pulmonary vein isolation patients, and 7 days (range, 4–8 days) for the limited Cox-Maze patients.
Perioperative complications for the patients in the Cox-Maze IV group included pulmonary embolism in 1 patient (1%), stroke in 1 patient (1%), reoperation for bleeding in 10 patients (10%), and permanent pacemaker placement in 10 patients (10%). Perioperative complications for the pulmonary vein isolation group included pulmonary embolism in 1 patient (4%), stroke in 3 patients (13%), reoperation for bleeding in 3 patients (13%), and no postoperative permanent pacemaker insertions. There was no reoperation for bleeding, embolism, stroke, or permanent pacemakers placed in the limited Cox-Maze procedure group. Postoperative atrial tachyarrhythmias occurred in 60 (60%) of the Cox-Maze IV group, 16 (70%) of the pulmonary vein isolation group, and 1 (14%) in the limited Cox-Maze procedure group. In a previous study by our group, the occurrence of postoperative atrial tachyarrhythmias was demonstrated to be unrelated to long-term freedom from arrhythmias.21
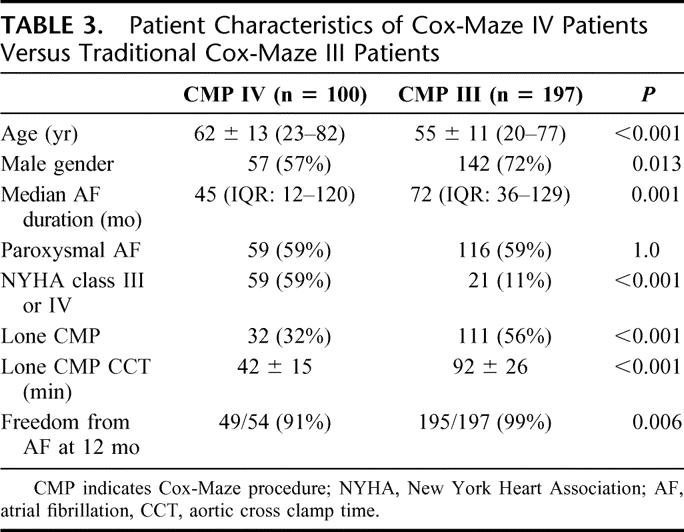
Cross-clamp time in the lone Cox-Maze IV procedure patients was 44 ± 21 minutes, while it was 104 ± 42 minutes for the Cox-Maze IV procedure with a concomitant procedure. The mean cross-clamp time in patients undergoing pulmonary vein isolation was 85 ± 30 minutes, and it was 99 ± 5 minutes for the 2 patients requiring bypass in the limited Cox-Maze procedure group. For patients undergoing a lone Cox-Maze IV procedure, the cross-clamp time was shortened considerably from our traditional lone cut-and-sew Cox-Maze procedure times (92 ± 26 minutes, P < 0.05). Patient characteristics comparing Cox-Maze III surgical patients versus Cox-Maze IV patients can be seen in Table 3.
TABLE 3. Patient Characteristics of Cox-Maze IV Patients Versus Traditional Cox-Maze III Patients
Late Results
The mean follow-up was 13 ± 10, 23 ± 15, and 9.0 ± 10 months for the Cox-Maze IV, the pulmonary vein isolation, and the limited Cox-Maze procedure groups, respectively. Of the 100 patients who underwent the Cox Maze IV procedure, 74 of 83 (89%), 63 of 72 (88%), and 49 of 54 (91%) were free from AF at 3, 6 and 12 months, respectively. The number of patients free from AF was 78 of 94 (83%) and 71 of 94 (76%), at time of discharge and 1 month, respectively. At last follow-up, 85 of 94 (90%) were free from AF. Overall antiarrhythmic usage was 38% (27 of 72) and 33% (18 of 54) at 6 and 12 months, respectively (Figs. 2, 3). Of patients who suffered from permanent or persistent AF, 96% were free from atrial arrhythmias at last follow-up in the Cox-Maze IV group. Of patients with paroxysmal AF, 93% were free from atrial arrhythmias at last follow-up in the Cox-Maze IV group (Table 4).
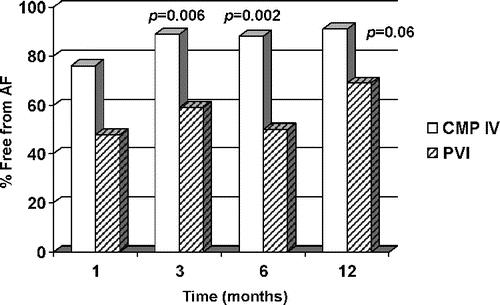
FIGURE 2. Patients free from atrial fibrillation recurrence after procedure. CMP IV, Cox-Maze IV procedure; PVI, pulmonary vein isolation procedure.
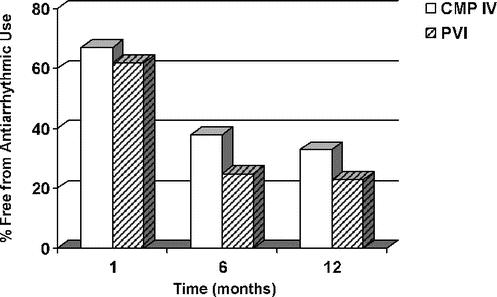
FIGURE 3. Patients who remained on antiarrhythmic medications after surgical intervention. CMP IV, Cox-Maze IV procedure; PVI, pulmonary vein isolation procedure.
TABLE 4. Freedom From Atrial Fibrillation at 12-Month Follow-up and at Last Follow-up
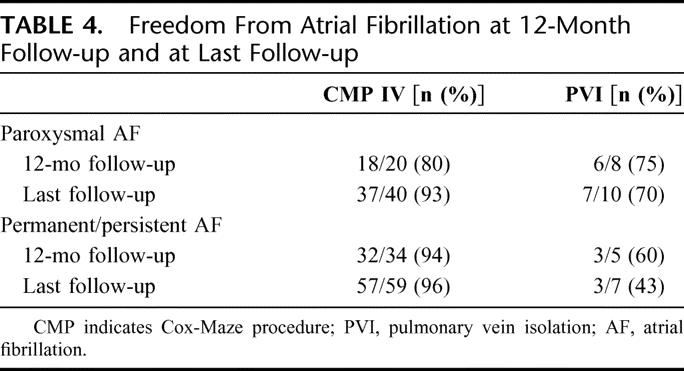
In the pulmonary vein isolation group, freedom from AF was 10 of 21 (48%), 10 of 17 (59%), 8 of 16 (50%), and 9 of 13 (69%) at 1, 3, 6, and 12 months, respectively (Fig. 2). This was significantly less than the Cox-Maze IV group at 3 and 6 months. At 12 months, the difference between both groups showed a strong trend toward significance (P = 0.06). Ten of 17 (59%) patients were free from AF at last follow-up (P = 0.003 vs. the Cox-Maze IV group). Overall antiarrhythmic usage was 25% (4 of 16) and 23% (3 of 13) at 6 and 12 months, respectively (Fig. 3). Of patients who suffered from permanent or persistent AF, 43% were free from atrial arrhythmias at last follow-up in the PVI group. Of patients who suffered from paroxysmal AF, 70% were free from atrial arrhythmias at last follow-up (Table 4).
In comparison to our historical Cox-Maze III cohort, patients undergoing the Cox-Maze IV procedure were significantly younger (55 ± 11 vs. 62 ± 13 years, P < 0.001). They also had a shorter preoperative duration of AF (45 months vs. 72 months, P = 0.001), and had more patients in NYHA class III or IV heart failure (59% vs. 11%, P > 0.001). More patients in the Cox-Maze IV group underwent concomitant procedures (68% vs. 44%, P < 0.001). Freedom from AF at 12-month follow-up was 91% for patients undergoing a Cox-Maze IV procedure versus 99% for patients undergoing the traditional Cox-Maze III procedure (P = 0.006, Table 3).
Of the 7 patients that underwent a limited Cox-Maze procedure, 6 of 7 (86%) were free from AF at the last follow-up. The recurrence of AF in this patient occurred within 1 month following surgery.
In the patients that had a CT or MRI after their Cox-Maze IV procedure, 10 of 10 (100%) were free from pulmonary vein stenosis. Mean follow-up for these imaging studies was 3.2 ± 2.0 months.
DISCUSSION
The traditional Cox-Maze procedure has been the gold standard for the surgical treatment of AF for nearly 20 years. The results of this procedure have been excellent, with greater than 90% freedom from AF reported by our group and others.6,21–23 However, the traditional cut-and-sew technique has several limitations. Foremost, it was a technically challenging procedure and few surgeons would perform the surgery in addition to valve or coronary surgery. The procedure added significantly to cardiopulmonary bypass and cross-clamp time. Because of its complexity, its application to the general population of patients with AF was limited. Recent technologic advances have the potential to make this surgery less difficult and hence more accessible to patients.
For any emerging technology to adequately replace the surgical incisions of the Cox-Maze procedure, it must be able to create discrete transmural lesions to assure conduction block. Various energy sources have been used to replace the surgical incisions, including RF, microwave, laser, high frequency ultrasound, and cryoablation.24–28 Many of these devices have limitations, particularly in their ability to consistently and rapidly create transmural lesions, and to avoid collateral injury to cardiac and extra cardiac structures.29–32 Bipolar RF energy has the potential to overcome these shortcomings. Using an algorithm based on changes in tissue conductance, this technology reliably created transmural ablations on the atrium in the laboratory.8,9,33,34 Moreover, 5- to 6-cm lesions were created in a matter of seconds. Collateral injury to surrounding structures was impossible because the energy was confined to tissue within the clamp.8,9 These characteristics make bipolar RF ablation an attractive alternative to the cut-and-sew lesions of the original Cox-Maze procedure.
The safety and efficacy of this technology was documented in this study. The bipolar RF clamp was effective at isolating the pulmonary veins in every patient who was tested. Furthermore, the Cox-Maze IV procedure significantly shortened cross-clamp times when compared with the Cox-Maze III procedure. This was not surprising as the majority of incisions were replaced with ablation and the necessity of extensive surgery was eliminated. The efficacy of the Cox-Maze IV procedure was manifested by a freedom from AF of 91% at 1 year. While less effective than our historical results with the Cox-Maze III group, these 2 populations were not comparable due to significant differences in age, AF duration, NYHA heart failure class, and percentage of concomitant cardiac surgery. Because of its simplicity, this technology is now used in all patients with AF undergoing concomitant cardiac surgery at our institution. This has expanded the operation to older patients with larger left atria, and more complex cardiac disease, making a direct unmatched comparison to our previous results with the Cox-Maze III procedure difficult. Our opinion is that the over 90% freedom from AF at 1 year justifies the use of this enabling new technology. Moreover, a recent study from our group in which propensity analysis was used to match Cox-Maze III to Cox-Maze IV patients showed no significant differences in 1-year freedom from AF between groups.35
In this initial clinical experience, bipolar RF ablation was safe. There were no device-related complications. In 10 patients, follow-up cardiac computed tomography (CT) or magnetic resonance imaging (MRI) documented no evidence of pulmonary vein stenosis. There were no instances of coronary, valvular, or esophageal damage in these 130 patients.
Recently, there have been many new more limited procedures proposed for the treatment of AF. There also have been a number of centers that have advocated performing only ablations on the left atrium. The success rates from these procedures have been reported between 44% and 95% at variable follow-up.36–40 One complication of performing only the left atrial lesions is postoperative atrial flutter, as reported in the literature with rates of 13% to 21% with 15- to 37-month follow-up.38,41 One of the most common modifications has been to perform pulmonary vein isolation alone. This has been supported by the fact that in a majority of patients with paroxysmal AF the triggers for the arrhythmia originated in the pulmonary veins.15–17 Addition of a lesion from the pulmonary vein island to the mitral isthmus decreased the incidence of postoperative AF in a large study from the Cleveland Clinic.42
In this study, patients undergoing pulmonary vein isolation alone had only a 59% freedom from AF at last follow-up. These results are understandable when considering that almost half of foci for AF have been shown to originate outside the pulmonary veins.17 These poor results have been supported by other studies in the literature on patients undergoing pulmonary vein isolation and concomitant cardiac surgery. Gaita et al randomized 105 patients undergoing AF and valve surgery to either PVI or more extensive left atrial ablation procedures.43 At a mean follow-up of 41 ± 17 months, only 29% of the PVI patients were in normal sinus rhythm. In a recent study from Japan, 101 patients undergoing concomitant surgery underwent PVI. At last follow-up, only 53% of patients were in normal sinus rhythm, only 25% were free from antiarrhythmic drug usage.44
In this series, a very limited number of patients received only the left- or right-sided lesions of the Cox-Maze procedure. The cure rate for this modified technique was similar to the results of the full Cox-Maze IV procedure. However, these patients were highly selected, most of whom had isolated right heart pathology, with congenital heart defects and/or isolated tricuspid regurgitation. Results from previous series lend credence to the idea that this surgery may be sufficient in this subset of patients.23 Only 2 patients underwent an isolated left atrial procedure, and this experience is too small to draw any significant conclusions.
Limitations of Study
Bipolar RF ablation technology has some limitations. As opposed to our laboratory experience in animals, multiple applications were often required to isolate the pulmonary veins. This could have been due to the thicker human atria, the presence of extensive fat around the pulmonary veins, and the bunching large amounts of tissue within the clamp. The algorithm to determine lesion transmurality was not 100% accurate in the clinical setting. Because of this, pacing is essential in all patients undergoing bipolar RF ablation to document conduction block following pulmonary vein isolation.
There are also difficulties in comparing our results with bipolar RF ablation, particularly the Cox-Maze IV with the Cox-Maze III procedure. There was shorter follow-up for the Cox-Maze IV versus the Cox-Maze III procedure. Over time, there is a risk that ablation technology may be less durable in creating conduction block than an incision. Long-term follow-up will be needed to document chronic efficacy. Also, there were significant differences in patient characteristics between the Cox-Maze III and IV patients, as the Cox-Maze IV patients had more organic heart disease, worse congestive heart failure, and more concomitant procedures. These disparities make direct comparisons inadequate without either a propensity analysis or a randomized trial comparing the 2 procedures.
There are also some difficulties in comparing the different lesion sets used in this study. The great majority of patients underwent a full Cox-Maze IV lesion set, with the majority of the incisions of the traditional Cox-Maze III replaced with bipolar RF ablation or cryoablation. This was due to our bias toward a full lesion set. Pulmonary vein isolation alone was reserved either for high-risk patients who were felt to not be candidates for the full lesion set or in patients with short durations of paroxysmal AF in which available evidence suggested that pulmonary vein isolation had a reasonable likelihood of success. Thus, there were significant differences in the demographics between these groups. However, the difference in success rate clearly suggests that a significant number of patients with AF and organic heart disease will not be cured by pulmonary vein isolation alone. It is possible that, if one selected a group of patients particularly with paroxysmal AF and small atria, the success rate would be higher for pulmonary vein isolation alone. The success rate for the limited right or left atrial lesion sets was interesting. However, the numbers in this series were too small to draw any conclusions.
It is a weakness of this study that we did not examine pulmonary vein isolation in patients who had lone AF. Further data are needed to evaluate the efficacy of this procedure in this group. However, our historical results with the cut-and-sew procedure had higher success rates in patients who had AF associated with concomitant cardiac pathology as opposed to those who had lone AF.
Finally, this study did not incorporate routine Holter monitoring to evaluate for atrial arrhythmias in the postoperative period. Thus, the true incidence of postoperative AF may be underestimated. However, our follow-up has been similar to most other reports in the surgical treatment of AF, and there are presently no large reports of surgical patients followed postoperatively with continuous monitoring.
CONCLUSION
This study documented a large single-center experience with bipolar radiofrequency ablation used to replace the surgical incisions of the Cox-Maze III procedure. There was excellent follow-up in this series. In a group of 100 consecutive patients, the Cox-Maze IV procedure was effective in over 90% of patients. The majority of patients were also free from antiarrhythmic drugs. There were no device-related complications. These results strongly suggest that bipolar radiofrequency is an effective and safe technology for the surgical treatment of atrial fibrillation. The use of bipolar radiofrequency simplified the procedure, decreased cross-clamp times, and expanded our indications. However, our initial results with pulmonary vein isolation were not as positive. There was a higher success rate in those patients with paroxysmal atrial fibrillation undergoing concomitant surgery, but this did not approach the greater than 90% success rate seen with the full Cox-Maze procedure. In our opinion, the use of pulmonary vein isolation should be limited in patients undergoing concomitant surgery.
Discussions
Dr. Irving L. Kron (Charlottesville, Virginia): I am delighted to discuss this fabulous manuscript by Damiano and colleagues. As you probably have figured out, he is truly one of the pros in this field. You ought to pay attention to what he suggests.
This procedure was invented at Washington University. The Cox-Maze procedure has been and perhaps is still the gold standard. What Damiano and colleagues demonstrated today is that they can truly replace these lesion sets with radiofrequency ablation. They can do this with a cross-clamp time of 40 minutes, which is no small feat, and most importantly perhaps, that the pulmonary vein isolation procedure practiced by electrophysiologists and many surgeons is only effective in 60% of cases.
Now the difficulty for most surgeons is exactly what lesion sets need to be performed. What I need to ask first of all from Dr. Damiano is, which lesion sets have to be done, and when do you do pulmonary vein isolation, if ever?
The second question is more difficult. Everyone who does cardiac surgery is besieged by salesmen with multiple devices and different energy sources. They tell you they can do it using unipolar catheters and do everything that Damiano says he can do. Certainly that is not correct. So I would like to ask, Dr. Damiano, is a unipolar source ever better a bipolar source. What energy sources does he think are on the horizon, and exactly what should we be using?
Finally, most cardiologists who do this sort of procedure suggest an efficacy of 80%, which doesn't make any sense based on what he shows us with pulmonary vein isolation. I would like him to put this together for us. How does that compare to his procedure?
Dr. Ralph J. Damiano, Jr. (St. Louis, Missouri): Dr. Kron, thank you for your excellent questions, and I would like to acknowledge the work your group has done in this area.
Your first question is when to do which lesion set. And I will be the first to say that there is no one right answer to that question. The data clearly are not definitive because of the lack of any prospective, randomized trials. However, we do have decades of experience with the surgical treatment of atrial fibrillation at our institution, have performed mapping in over 50 patients who have come to the operating room with chronic atrial fibrillation. I think we can draw some conclusions, though I would have to say it is a field certainly in want of more rigorous data.
We are predisposed to perform a full lesion set on most patients who present with concomitant cardiac disease and chronic atrial fibrillation. This is because of our initial poor results with pulmonary vein isolation alone in this group, and the results of our intraoperative mapping in these patients, which have shown that only 40% to 50% of patients appear to have localized drivers for their atrial fibrillation in the left atrium. It is important to remember that these are surgical patients who are being referred mostly with large left atria and probably are not the same group of patients that they are reporting on in the cardiology literature.
In patients coming for valve or coronary surgery, we would generally perform the full lesion set, which would be the Cox-Maze 4. Exceptions to this are those patients with isolated right heart pathology. We have 5 patients upon whom we have performed just the ablation-assisted right atrial Maze, and have had excellent results in those patients with isolated tricuspid disease or right heart pathology due to congenital disease.
In terms of what are the present indications for pulmonary vein isolation, they are quite limited. We feel that for concomitant cardiac disease, it probably does not have much of a role until more data are available. In patients with lone atrial fibrillation, we think it is a reasonable option in those patients whose left atrial size is less than 5 cm, and who have paroxysmal atrial fibrillation. The basic science and electrophysiological literature would suggest that these patients would have the highest incidence of their atrial fibrillation being driven out of the pulmonary veins. We do inform patients that we only are getting about a 70% success rate for pulmonary vein isolation as opposed to over 90% with a Maze 4. We let them choose what operation they would like to have, knowing that you can do pulmonary vein isolation through very small incisions.
In terms of your next question regarding the benefits of unipolar versus bipolar ablation, I did not mention it, but we still do use unipolar ablation around the valve annuli. We favor cryosurgery in this area. The problem with the bipolar devices is you have to clamp to ablate, which is fine for the pulmonary veins but is limiting, as you know, for patients in whom you are trying to do a minimally invasive approach.
One of the problems in our literature now is the only minimally invasive operation people can do with these bipolar clamps is pulmonary vein isolation. This may not be sufficient for the kind of patients who eventually get referred for the surgical treatment of atrial fibrillation. So I think there is a big role for unipolar energy sources particularly in the minimally invasive lone atrial fibrillation population. We are awaiting further technological advances from industry in this area.
There also is a role in places you can not clamp easily. In our experience, this has been around the tricuspid mitral annulus where clamping is often difficult because of the thickness of the tissue and the possibility of damaging the valve leaflets. There are a number of very exciting energy sources on the horizon including laser and high frequency ultrasound. Unfortunately, experimental data are still very skimpy on these devices.
In terms of commenting on the efficacy of catheter-based ablation, I would just say that I think a great disservice has been their use of the term “pulmonary vein isolation.” In my opinion, what they do in the Electrophysiology Laboratory has nothing to do with pulmonary vein isolation. In the great majority of patients, they do not isolate the pulmonary veins. Numerous investigators have shown that pulmonary vein isolation does not predict either success or failure of the procedure.
What they are doing in the Electrophysiology Laboratory is ablating most of the back of the left atrium, which our data would suggest would be successful in a significant number of these patients who do not have organic heart disease and a small left atrium. So I do believe there are certain centers around the world that can get excellent success rates with catheter-based ablation. I personally feel that surgical pulmonary vein isolation would be less effective than catheter ablation because it doesn't ablate as much atrial tissue. In centers across the United States, success with first-time catheter-based ablation is probably approaching 60% or 70%.
Dr. Alden H. Harken (Oakland, California): Dr. Damiano, I would like to echo Dr. Kron's comments about your continuing pioneering work in this area, not just the clinical application of it but also our understanding of the basic electrophysiology.
You commented that some of the atrial fibrillation really has a benign outcome. But you have got to remember this provokes the use what I think is the most dangerous drug we ever use, and that is Coumadin. So I think you are really solving a major problem.
If you think about the basic pathophysiology of atrial fibrillation as being you can exacerbate it with an increase in atrial mass, if you decrease conduction velocity or increase refactoriness, all of those factors will make it more likely to have atrial fibrillation.
I guess I am a little surprised at the success rate of just the pulmonary vein isolation alone. Why do you think the success rate is as high as 50%? And second, what is the status of the minimally invasive? Obviously if you can do this thoracoscopically and access many of the incisions that you describe, what is the likelihood of this being successful, knowing that you have got a heat sink flowing through the left atrium at a very high velocity? Again I commend you on continuing the pioneering work, and what is the next step?
Dr. Ralph J. Damiano, Jr. (St. Louis, Missouri): Why was pulmonary vein isolation as successful as we showed it to be? First of all, we did not have a control group. You have to remember that doing nothing has been shown to cure probably 20% to 30% of patients. So I would argue that a 50% success rate in patients coming for concomitant heart surgery, some of whom have had short durations of atrial fibrillation, is not a particularly good success rate.
The success rate is close to what we expected in this population. We have tended to use this operation more in paroxysmal atrial fibrillation patients, and our success rate is higher in this group. Several recent large studies have shown that in patients with paroxysmal atrial fibrillation, the arrhythmia seems to originate from in around the pulmonary veins in anywhere from 50% up to perhaps 70% of cases. This is close to our actual success rate. In fact, atrial fibrillation is a very delicate arrhythmia in some patients, and almost anything can perturb it.
In terms of what we need for a successful minimally invasive operation, this is an excellent question. Right now, we are hampered by the technology. We have proceeded headlong into minimally invasive procedures just because they are minimally invasive without any appropriate experimental testing as to whether the technology works or whether the operation may be appropriate.
Perhaps the boldest example of that has been the fact that with bipolar ablation devices, the only thing you can do is pulmonary vein isolation. So we have called that our minimally invasive procedure. The problem is that our experimental data and our early clinical results in patients with concomitant disease have shown this operation is not all that successful. Time will tell. One day we will be smart enough to be able to pick out the patients who will be cured by this operation. If we could pick out the 50% to 70% of patients in whom pulmonary vein isolation will work ahead of time, we could have 100% success. And that is probably where we are going.
The other problem is that with unipolar technology, which is easier to create the ablations in a minimally invasive way, virtually none of the energy sources seem to work well with the heart beating. Dr. Melby, who is one of our co-authors, has published very nice work with microwave energy, showing that it does not create transmural lesions in the beating heart, despite having been widely used for this purpose in minimally invasive pulmonary vein isolation. However, if you go on bypass, it works well. Perhaps our first step should be going on cardiopulmonary bypass and using some of this unipolar technology and doing more aggressive lesion sets.
In summary, I think a truly successful minimally invasive procedure would have to have 90%-plus success rates to play a large role versus a catheter-based approach. To achieve these high success rates, we will need both better preoperative diagnostics and technology.
Dr. Lawrence H. Cohn (Boston, Massachusetts): We have done several hundred of these procedures at the Brigham like you have. And one of the questions that comes up all the time is, how do you know you really cure atrial fibrillation? And we have had cardiologists say to us, “Well, you know, I'm not going to really believe that you have cured atrial fibrillation until we have a 2-week monitor 6 months to a year post-op.” What is the criteria in your mind, and what is the criteria for this study, that says atrial fibrillation is truly cured after these procedures?
Dr. Ralph J. Damiano, Jr. (St. Louis, Missouri): Well, I will try to be very short. That is an excellent question, Dr. Cohn, and one that doesn't lend itself actually to a short answer.
The easy thing, what were our criteria for success in this study? We obtained EKGs on all these patients at the follow-up intervals that I quoted to you. We had 99% follow-up, which I think is excellent for a surgical series. It is often hard to get EKGs on these patients. If they were asymptomatic and their serial EKGs showed no arrhythmia, we considered them cured.
Obviously they could be having periods of asymptomatic atrial fibrillation, which we wouldn't know about, and that is clearly a weakness of our study. Anybody with any symptoms or any evidence of palpitations or arrhythmias, we did get Holter monitoring or event recorders.
I agree with you that if we are going to be rigorous, we should do long-term monitoring in these patients. The problem is that we do not know what to do with that data. I think Dr. Harken very appropriately brought up the morbidity of Coumadin. When do you think it would be safe to stop the Coumadin? What if you are having atrial fibrillation 1% of the time? How about ten seconds a day? The problem I think is unfortunately most of the cardiologists would keep the Coumadin going. With our present follow-up, we virtually have seen no late strokes in these patients. In all the operations, we have amputated the left atrial appendage, so there is a low risk of stroke.
You are exactly right, we need to have better follow-up in these patients. We also need to develop some standards on how to interpret that data.
Footnotes
Supported in part by National Institutes of Health Grant Nos. R01-HL032257 and F32 HL078136-01. Dr. Damiano is a consultant for Atricure, Inc., and Medtronic, Inc.
Reprints: Ralph J. Damiano, Jr, MD, Department of Cardiac Surgery, Washington University School of Medicine, Barnes-Jewish Hospital, 660 S. Euclid Ave., Box 8234, St. Louis, MO 63110. E-mail: damianor@wustl.edu.
REFERENCES
- 1.Thom T, Haase N, Rosamond W, et al. Heart Disease and Stroke Statistics–2006 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. [DOI] [PubMed] [Google Scholar]
- 3.Fetsch T, Bauer P, Engberding R, et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004;25:1385–1394. [DOI] [PubMed] [Google Scholar]
- 4.Marcus GM, Sung RJ. Antiarrhythmic agents in facilitating electrical cardioversion of atrial fibrillation and promoting maintenance of sinus rhythm. Cardiology. 2001;95:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Cappato R, Calkins H, Chen S-A, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–1105. [DOI] [PubMed] [Google Scholar]
- 6.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. [DOI] [PubMed] [Google Scholar]
- 7.Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:2–14. [DOI] [PubMed] [Google Scholar]
- 8.Prasad SM, Maniar HS, Schuessler RB, et al. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002;124:708–713. [DOI] [PubMed] [Google Scholar]
- 9.Prasad SM, Maniar HS, Diodato MD, et al. Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: a chronic animal study. Ann Thorac Surg. 2003;76:836–841. [DOI] [PubMed] [Google Scholar]
- 10.Mokadam NA, McCarthy PM, Gillinov AM, et al. A prospective multicenter trial of bipolar radiofrequency ablation for atrial fibrillation: early results. Ann Thorac Surg. 2004;78:1665–1670. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. [DOI] [PubMed] [Google Scholar]
- 12.Cox JL, Boineau JP, Schuessler RB, et al. Successful surgical treatment of atrial fibrillation: review and clinical update. JAMA. 1991;266:1976–1980. [PubMed] [Google Scholar]
- 13.Nitta T. Surgery for atrial fibrillation. Ann Thorac Cardiovasc Surg. 2005;11:154–158. [PubMed] [Google Scholar]
- 14.Gillinov AM, McCarthy PM. Advances in the surgical treatment of atrial fibrillation. Cardiol Clin. 2004;22:147–157. [DOI] [PubMed] [Google Scholar]
- 15.Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt C, Ndrepepa G, Weber S, et al. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. 2002;89:1381–1387. [DOI] [PubMed] [Google Scholar]
- 18.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. [DOI] [PubMed] [Google Scholar]
- 19.Verma A, Natale A, Padanilam BJ, et al. Why atrial fibrillation ablation should be considered first-line therapy for some patients. Circulation. 2005;112:1214–1222. [DOI] [PubMed] [Google Scholar]
- 20.Cox JL, Boineau JP, Schuessler RB, et al. Electrophysiologic basis, surgical development, and clinical results of the maze procedure for atrial flutter and atrial fibrillation. Adv Cardiac Surg. 1995;6:1–67. [PubMed] [Google Scholar]
- 21.Ishii Y, Gleva MJ, Gamache MC, et al. Atrial tachyarrhythmias after the Maze procedure: incidence and prognosis. Circulation. 2004;110(11 suppl 1):II164–II168. [DOI] [PubMed] [Google Scholar]
- 22.Gammie JS, Laschinger JC, Brown JM, et al. A multi-institutional experience with the CryoMaze procedure. Ann Thorac Surg. 2005;80:876–880. [DOI] [PubMed] [Google Scholar]
- 23.Schaff HV, Daly RC, Orszulak TA, et al. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg. 2000;1:30–37. [DOI] [PubMed] [Google Scholar]
- 24.Ninet J, Roques X, Seitelberger R, et al. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. J Thorac Cardiovasc Surg. 2005;130:803–809. [DOI] [PubMed] [Google Scholar]
- 25.Damiano RJ Jr. Alternative energy sources for atrial ablation: judging the new technology. Ann Thorac Surg. 2003;75:329–330. [DOI] [PubMed] [Google Scholar]
- 26.Gillinov AM, Smedira NG, Cosgrove DM 3rd. Microwave ablation of atrial fibrillation during mitral valve operations. Ann Thorac Surg. 2002;74:1259–1261. [DOI] [PubMed] [Google Scholar]
- 27.Poa L. Thoracoscopic ablation for treatment of atrial fibrillation: a 2-port approach. Heart Surg Forum. 2006;9:590–592. [PubMed] [Google Scholar]
- 28.Khargi K, Hutten BA, Lemke B, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27:258–265. [DOI] [PubMed] [Google Scholar]
- 29.Gaynor SL, Byrd GD, Diodato MD, et al. Microwave ablation for atrial fibrillation: dose-response curves in the cardioplegia-arrested and beating heart. Ann Thorac Surg. 2006;81:72–76. [DOI] [PubMed] [Google Scholar]
- 30.Santiago T, Melo JQ, Gouveia RH, et al. Intra-atrial temperatures in radiofrequency endocardial ablation: histologic evaluation of lesions. Ann Thorac Surg. 2003;75:1495–1501. [DOI] [PubMed] [Google Scholar]
- 31.Doll N, Borger MA, Fabricius A, et al. Esophageal perforation during left atrial radiofrequency ablation: is the risk too high? J Thorac Cardiovasc Surg. 2003;125:836–842. [DOI] [PubMed] [Google Scholar]
- 32.Manasse E, Medici D, Ghiselli S, et al. Left main coronary arterial lesion after microwave epicardial ablation. Ann Thorac Surg. 2003;76:276–277. [DOI] [PubMed] [Google Scholar]
- 33.Hamner CE, Potter DD Jr, Cho KR, et al. Irrigated radiofrequency ablation with transmurality feedback reliably produces Cox Maze lesions in vivo. Ann Thorac Surg. 2005;80:2263–2270. [DOI] [PubMed] [Google Scholar]
- 34.Melby SJ, Gaynor SL, Lubahn JG, et al. Efficacy and safety of right and left atrial ablations on the beating heart using irrigated bipolar radiofrequency energy: a chronic animal study. J Thorac Cardiovasc Surg. In press. [DOI] [PubMed]
- 35.Lall SC, Melby SJ, Voeller RK, et al. The impact of ablation technology on surgical outcomes following the Cox Maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. In press. [DOI] [PubMed]
- 36.Doukas G, Samani NJ, Alexiou C, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA. 2005;294:2323–2329. [DOI] [PubMed] [Google Scholar]
- 37.Kottkamp H, Hindricks G, Autschbach RU, et al. Specific linear left atrial lesions in atrial fibrillation: intraoperative radiofrequency ablation using minimally invasive surgical techniques. 2002;40:475–480. [DOI] [PubMed] [Google Scholar]
- 38.Imai K, Sueda T, Orihashi K, et al. Clinical analysis of results of a simple left atrial procedure for chronic atrial fibrillation. 2001;71:577–581. [DOI] [PubMed] [Google Scholar]
- 39.Deneke T, Khargi K, Grewe PH, et al. Left atrial versus bi-atrial Maze operation using intraoperatively cooled-tip radiofrequency ablation in patients undergoing open-heart surgery: safety and efficacy. J Am Coll Cardiol. 2002;39:1644–1650. [DOI] [PubMed] [Google Scholar]
- 40.Benussi S, Nascimbene S, Agricola E, et al. Surgical ablation of atrial fibrillation using the epicardial radiofrequency approach: mid-term results and risk analysis. Ann Thorac Surg. 2002;74:1050–1056. [DOI] [PubMed] [Google Scholar]
- 41.Golovchiner G, Mazur A, Kogan A, et al. Atrial flutter after surgical radiofrequency ablation of the left atrium for atrial fibrillation. Ann Thorac Surg. 2005;79:108–112. [DOI] [PubMed] [Google Scholar]
- 42.Gillinov AM, McCarthy PM, Blackstone EH, et al. Surgical ablation of atrial fibrillation with bipolar radiofrequency as the primary modality. J Thorac Cardiovasc Surg. 2005;129:1322–1329. [DOI] [PubMed] [Google Scholar]
- 43.Gaita F, Riccardi R, Caponi D, et al. Linear cryoablation of the left atrium versus pulmonary vein cryoisolation in patients with permanent atrial fibrillation and valvular heart disease: correlation of electroanatomic mapping and long-term clinical results. Circulation. 2005;111:136–142. [DOI] [PubMed] [Google Scholar]
- 44.Isobe N, Taniguchi K, Oshima S, et al. Left atrial appendage outflow velocity is superior to conventional criteria for predicting of maintenance of sinus rhythm after simple cryoablation of pulmonary vein orifices. Circ J. 2005;69:446–451. [DOI] [PubMed] [Google Scholar]