Abstract
Objective:
The objective of this study was to evaluate the impact of newer therapies on the highest risk patients with congenital diaphragmatic hernia (CDH), those with agenesis of the diaphragm.
Summary Background Data:
CDH remains a significant cause of neonatal mortality. Many novel therapeutic interventions have been used in these infants. Those children with large defects or agenesis of the diaphragm have the highest mortality and morbidity.
Methods:
Twenty centers from 5 countries collected data prospectively on all liveborn infants with CDH over a 10-year period. The treatment and outcomes in these patients were examined. Patients were followed until death or hospital discharge.
Results:
A total of 1569 patients with CDH were seen between January 1995 and December 2004 in 20 centers. A total of 218 patients (14%) had diaphragmatic agenesis and underwent repair. The overall survival for all patients was 68%, while survival was 54% in patients with agenesis. When patients with diaphragmatic agenesis from the first 2 years were compared with similar patients from the last 2 years, there was significantly less use of ECMO (75% vs. 52%) and an increased use of inhaled nitric oxide (iNO) (30% vs. 80%). There was a trend toward improved survival in patients with agenesis from 47% in the first 2 years to 59% in the last 2 years. The survivors with diaphragmatic agenesis had prolonged hospital stays compared with patients without agenesis (median, 68 vs. 30 days). For the last 2 years of the study, 36% of the patients with agenesis were discharged on tube feedings and 22% on oxygen therapy.
Conclusions:
There has been a change in the management of infants with CDH with less frequent use of ECMO and a greater use of iNO in high-risk patients with a potential improvement in survival. However, the mortality, hospital length of stay, and morbidity in agenesis patients remain significant.
A total of 1569 patients with diaphragmatic hernia were seen in 20 centers over 10 years. Patients with diaphragmatic agenesis had a 54% survival compared with 89% in the patients without agenesis. Use of ECMO declined and use of nitric oxide and permissive hypercapnea increased with no change in survival in patients with agenesis.
Congenital diaphragmatic hernia (CDH) occurs in between 1 in 2500 to 1 in 4000 live births.1,2 The first successful repair in an infant under 24 hours of age was performed by Gross in 1946.3 Since then, advances in neonatal anesthesia, surgical techniques, and neonatal intensive care have allowed sicker patients to survive transfer to tertiary hospitals. Mortality for patients with CDH remained around 50% in the 1970s and 1980s.4,5 A significant number of newer therapies for infants with respiratory failure became widely available in the late 1980s and 1990s. These therapies included fetal intervention, extracorporeal membrane oxygenation (ECMO), high frequency oscillatory ventilation (HFOV), exogenous surfactant, and inhaled nitric oxide (iNO). Application of these newer therapies to infants with CDH has been based on anecdotal case reports and retrospective reviews. While survival to hospital discharge appears to have improved, some authors dispute these conclusions.6,7 Indeed, some of the above-mentioned therapies, such as hyperventilation and fetal therapy, have either not been proven beneficial or have been shown to be harmful.8–10
While CDH has been viewed by some as a homogeneous disease process, there are clear differences in outcomes between certain groups of patients. Infants with larger defects have been shown to have a worse survival compared with those with smaller defects11,12 It has been difficult to demonstrate differences in outcome as the anomaly is uncommon and any single center cannot accrue enough high-risk patients to draw meaningful conclusions. The purpose of this study is to evaluate outcomes and management changes over a 10-year period in high-risk infants with CDH using a large, multicenter database.
METHODS
The Congenital Diaphragmatic Hernia Study Group (CDHSG) was formed in 1995 to compile data on liveborn babies with CDH at participating institutions with the goal of assessing therapies and improving outcomes. The CDH Study Group consists of tertiary referral centers that voluntarily provide data to a central registry (Appendix). Data on all infants with CDH who are born at or transferred to a participating center are entered into the database. Data were collected prospectively on all liveborn patients with CDH between January 1995 and December 2004 in participating hospitals. Information on delivery and subsequent hospital care (including surgery where applicable) until death or hospital discharge was collected. Institutions were included in this analysis if they had at least 10 consecutive years of data submission. The data from the registry forms were entered into a Microsoft Access database and were cross-checked against the original forms. Patient demographics, birth information, Apgar scores, treatments received, size of the defects, and outcome were recorded. Therapeutic interventions including the use of ECMO, iNO, ventilatory strategy (ie, permissive hypercapnea, hyperoxia, hyperventilation, etc), surfactant, intravenous vasodilators, neuromuscular blockade, and sedation were recorded.
The size of the diaphragm defect was determined by the surgeon at the time of repair and coded as “agenesis” if the entire diaphragm or most of the diaphragm was absent (based on surgeons' reports and/or operative notes with findings of “absent or missing rim of diaphragm” or repair requiring “suturing the patch to the ribs anteriorly and posteriorly”). All patients with diaphragm agenesis required a patch to repair the defect. In those patients without agenesis, either the defect could be repaired primarily or a patch was required to close the defect. A fourth group of patients never underwent operation; most had a combination of either severe other anomalies or were thought to have fatal pulmonary hypoplasia. Since this group has been shown to have the highest risk of death, patients coded as diaphragm agenesis were compared with the remainder of the patients who underwent operation.
Statistical Analysis
Death prior to hospital discharge was the primary outcome variable. The length of stay in the hospital and duration of mechanical ventilation were secondary outcomes. In an effort to determine if there had been a significant change in management from the start of the registry, data from the first 2 years were grouped and compared with the last 2 years to compare outcome and types of therapies used. These data were analyzed using a χ2 analysis or unpaired t test where appropriate. A P value of 0.05 was considered significant. Patients who did not undergo repair were not included in the final analysis.
The CDHSG registry was approved by the University of Texas-Houston Institutional Review Board. Participating centers filed a Waiver of Consent for data submission or signed a Data Use Agreement for a Limited Data Set. The analyses were conducted using the NCSS (version NCSS 2004; March 2004; NCSS, Kaysville, UT) statistical software package.
RESULTS
Between 1995 and 2004, a total of 3278 patients were entered in the registry from 90 centers. To determine changes over time, we only used the data from centers who had continuously submitted information for the entire 10-year time period. There were 20 centers that met these criteria, and they contributed 1569 patients to the database. Demographic data for these patients are shown in Table 1. The overall survival for all patients was 68%.
TABLE 1. Demographic Data for the Patients
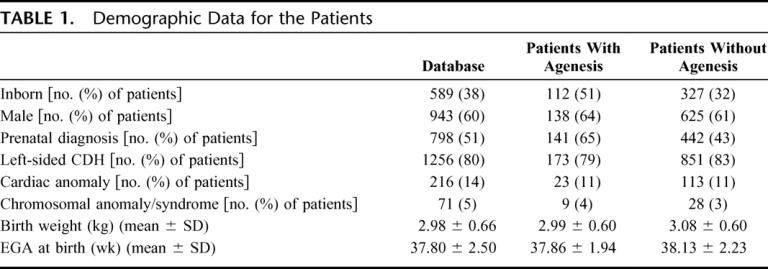
A total of 280 patients (18%) never underwent surgical repair and all died; 67 of the nonrepaired patients (24%) were noted to have severe associated anomalies (major cardiac, syndromal, or chromosomal). The rest of the patients were thought to have fatal pulmonary hypoplasia based on hemodynamic instability or blood gas values.
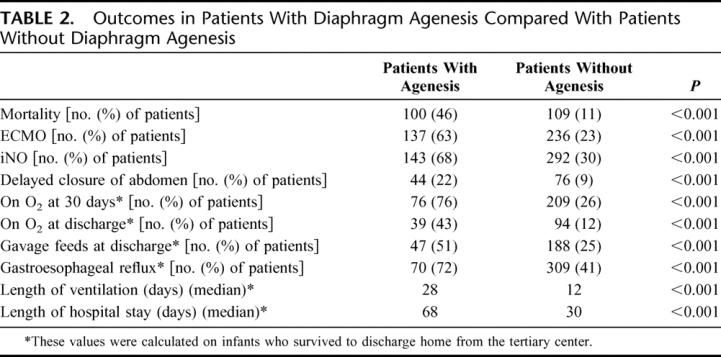
During this time period, 1247 patients underwent repair and had the defect size measured. There were 218 patients coded as having agenesis of the diaphragm. The survival to hospital discharge for this group was markedly lower than that of the 1029 patients without agenesis who underwent repair (54% vs. 89%, (P < 0.001) (Table 2).
TABLE 2. Outcomes in Patients With Diaphragm Agenesis Compared With Patients Without Diaphragm Agenesis
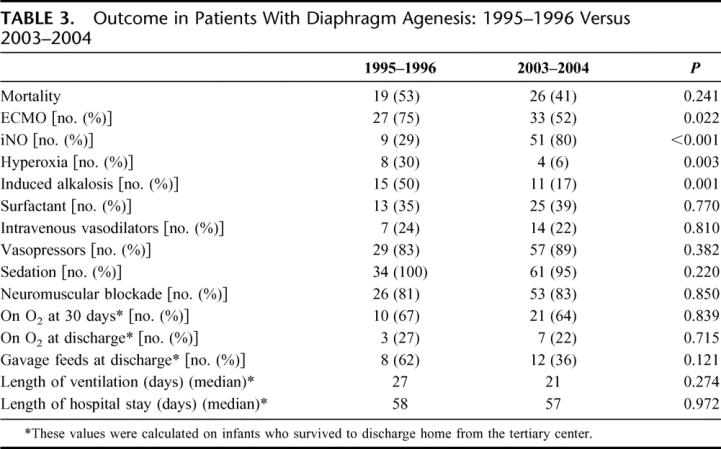
When analyzing the agenesis patients alone, there was a significant change in management over the 10 years of this study (Table 3). There was a significant decrease in the use of ECMO from 75% in the first 2 years to 52% of patients in the last 2 years. Conversely, there was a notable increase in the use of iNO to where 80% of patients with agenesis were treated with iNO in the last 2 years of the study compared with 30% in the first 2 years. Data regarding the primary respiratory strategy were not recorded until 2001 and were therefore not available for the first years of the study; however, by the last 2 years of this study, permissive hypercapnea had become the most widely used ventilation goal, with over 90% of patients having this as their strategy for ventilation. There was a significant decrease in the use of hyperoxia and induced alkalosis as a management strategy over the course of the study (P < 0.01) with no change in other management strategies (Table 3). There was a trend toward improved survival in the last 2 years of the study versus the first 2 years. Survival improved from 47% to 59% in patients with agenesis, although this did not reach statistical significance (P = 0.24).
TABLE 3. Outcome in Patients With Diaphragm Agenesis: 1995–1996 Versus 2003–2004
Morbidity in infants with CDH was high, but infants with agenesis had significantly greater morbidity when compared with the overall CDH population. As shown in Table 3, the median length of hospital stay for survivors with agenesis was much longer than for patients without agenesis. Some type of delayed closure of the abdomen, such as creation of a ventral hernia or patch abdominoplasty, was more commonly used in the patients with agenesis. Gastroesophageal reflux was also more prevalent in the patients with agenesis (Table 2). Similarly, the patients with agenesis had a significantly higher rate of being discharged while still requiring tube feeding as well as home oxygen.
DISCUSSION
CDH remains a significant cause of death in the newborn. In the past, survival rates for liveborn infants with CDH were often quoted in the 50% range, but as noted in this report, survival appears to have improved.13,14 This apparent increase in survival may be due to several causes, which may include newer therapies to support infants with respiratory failure that have been introduced in the past 2 decades. These therapies include ECMO, the use of exogenous surfactant, iNO, and high-frequency ventilation. Since infants with CDH often have severe respiratory failure, most of the therapies were rapidly adopted without the support of well-conducted trials. However, subsequent reports suggest that some of these therapies may have no proven benefit or may actually be harmful when compared with “standard” interventions. Examples include the previously mentioned widespread adoption of hyperventilation for infants with CDH, which has been shown to worsen survival, and the use of exogenous surfactant, which has not been shown to be efficacious.10,15,16
An essential issue in evaluating outcome data in patients with CDH is the size of the hernia defect. This study and others have shown that the size of the diaphragmatic defect correlates well with survival as well as morbidity in liveborn infants with CDH.11 While defect size is likely a marker for the degree of pulmonary hypoplasia, it may also correlate with the severity of pulmonary hypertension.
When evaluating those patients with large defects or agenesis of the diaphragm there has been a trend to improved survival, but the outcome remains poor. Since these patients are the sickest of the population of infants with CDH, it is not surprising that some of the advanced therapies are more frequently used in this group of patients. ECMO is one example of the application of advanced therapies without clear evidence of benefit.17 The initial use of ECMO for infants with CDH was often in patients with severe barotrauma, while currently, it is used primarily to assist in preoperative stabilization and avoid ventilator induced lung injury. Despite a decline in ECMO utilization in the high-risk patient, over half of these infants were still managed with ECMO during the last 2 years of the study. While it is possible to place an infant with CDH and fatal pulmonary hypoplasia on ECMO immediately upon delivery, there may be no therapeutic options available to reverse the problem, which is pulmonary hypoplasia. ECMO is best used as a supportive therapy for cardiopulmonary failure until this can be reversed or corrected. If an infant has fatal pulmonary hypoplasia, there are no available proven therapeutic options beyond lung transplantation, and there are no good metrics to accurately predict the degree of pulmonary hypoplasia today. The appropriate role for ECMO in these patients remains unclear.18
When iNO has been used in the newborn with respiratory failure as a rescue therapy, it has been shown to be useful in several randomized trials.19–21 Analysis of the subgroup of CDH patients in these trials, however, has failed to show benefit when used after the development of hypoxemic respiratory failure.22 Despite these data, the majority of high-risk CDH patients are being treated with iNO, with a marked increase in utilization in the past few years. While the indications and method of use were not captured in the registry form, it is likely that the current use of iNO represents a different strategy than that evaluated during the clinical trials. Increasingly, iNO has been used early as an adjunct to manage the pulmonary hypertension and right heart dysfunction commonly seen in patients with CDH as opposed to being used as a rescue therapy to avoid ECMO support.23 While it is now clearly a routine therapy in the high-risk CDH patient, there are no well designed trials to demonstrate optimal use of iNO in this population.
The CDH Registry was formed to allow collection and analysis of data on treatment and outcomes of infants with CDH from a large number of centers. While registries have proven to be a good way to collect information on various disorders, care should be taken in interpreting the data. The database is observational and conclusions about therapies should be made cautiously. The data are collected from institutions that differ significantly in their patient referral base and their criteria for accepting an infant with CDH for admission. Criteria for “nonsalvageable” patients vary between centers and some patients in the repaired group in one center may not have undergone operation in another. Many infants with severe cardiac and chromosomal anomalies did not undergo repair making the true impact of these factors difficult to determine. There are also variations in surgical practice that determine whether a patch is used. Similarly, the definition of agenesis is likely to vary somewhat between centers.
In addition to the high mortality, infants with CDH have significant in-hospital morbidity and many are discharged home requiring considerable further care. The length of stay in the hospital for patients with agenesis is quite long, with over half of the patients staying over 60 days. Importantly, these data only represent outcome to hospital transfer or discharge; both long-term mortality and morbidity are likely to be worse.24,25 As survival for these high-risk infants improves, many will require careful long-term follow-up for hernia recurrence, hearing deficits, and other problems.26,27
CONCLUSION
Infants with CDH and diaphragm agenesis continue to have a significant mortality rate despite the application of advanced therapies. Use of these advanced therapies such as ECMO and iNO has changed notably over the past decade, but in-hospital morbidity has remained high. As survival of these patients improves, a focus on long-term issues will become increasingly important.
APPENDIX
APPENDIX. Members of the CDH Study Group Registry

APPENDIX. (Continued)
APPENDIX. (Continued)
REFERENCES
- 1.Wenstrom KD, Weiner CP, Hanson JW. A five-year statewide experience with congenital diaphragmatic hernia. Am J Obstet Gynecol. 1991;165:838–842. [DOI] [PubMed] [Google Scholar]
- 2.Langham MR Jr, Kays DW, Ledbetter DJ, et al. Congenital diaphragmatic hernia: epidemiology and outcome. Clin Perinatol. 1996;23:671–688. [PubMed] [Google Scholar]
- 3.Gross RE. Congenital hernia of the diaphragm. Am J Dis Child. 1946;71:579–592. [DOI] [PubMed] [Google Scholar]
- 4.Mishalany HG, Nakada K, Woolley MM. Congenital diaphragmatic hernias: eleven years' experience. Arch Surg. 1979;114:1118–1123. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JM, Lund DP, Lillehei CW. Congenital diaphragmatic hernia—a tale of two cities: the Boston experience. J Pediatr Surg. 1997;32:401–405. [DOI] [PubMed] [Google Scholar]
- 6.Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–535. [DOI] [PubMed] [Google Scholar]
- 7.Javid PJ, Jaksic T, Skarsgard ED, et al. Canadian Neonatal Network. Survival rate in congenital diaphragmatic hernia: the experience of the Canadian Neonatal Network. J Pediatr Surg. 2004;39:657–660. [DOI] [PubMed] [Google Scholar]
- 8.Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. [DOI] [PubMed] [Google Scholar]
- 9.Cass DL. Fetal surgery for congenital diaphragmatic hernia: the North American experience. Semin Perinatol. 2005;29:104–111. [DOI] [PubMed] [Google Scholar]
- 10.Kays DW, Langham MR Jr, Ledbetter DJ, et al. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg. 1999;230:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh SJ, Cummins GE, Cohen RC, et al. Adverse outcome of congenital diaphragmatic hernia is determined by diaphragmatic agenesis, not by antenatal diagnosis. J Pediatr Surg. 1999;34:1740–1742. [DOI] [PubMed] [Google Scholar]
- 12.Tsang TM, Tam PK, Dudley NE, et al. Diaphragmatic agenesis as a distinct clinical entity. J Pediatr Surg. 1994;29:1439–1441. [DOI] [PubMed] [Google Scholar]
- 13.Boloker J, Bateman DA, Wung JT, et al. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–366. [DOI] [PubMed] [Google Scholar]
- 14.Azarow K, Messineo A, Pearl R, et al. Congenital diaphragmatic hernia—a tale of two cities: the Toronto experience. J Pediatr Surg. 1997;32:395–400. [DOI] [PubMed] [Google Scholar]
- 15.Van Meurs KP. The Congenital Diaphragmatic Hernia Study Group. Is surfactant therapy beneficial in the management of the term newborn with congenital diaphragmatic hernia? J Pediatrics. 2004;145:312–316. [DOI] [PubMed] [Google Scholar]
- 16.Anonymous. Surfactant does not improve survival in preterm infants with congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:829–833. [DOI] [PubMed] [Google Scholar]
- 17.Khan AM, Lally KP. The role of extracorporeal membrane oxygenation in the management of infants with congenital diaphragmatic hernia. Semin Perinatol. 2005;29:118–122. [DOI] [PubMed] [Google Scholar]
- 18.Rothenbach P, Lange P, Powell D. The use of extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia. Semin Perinatol. 2005;29:40–44. [DOI] [PubMed] [Google Scholar]
- 19.Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn: Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–474. [DOI] [PubMed] [Google Scholar]
- 20.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term (Cochrane Review). Cochrane Database Syst Rev. 2001;4:CD000399. [DOI] [PubMed] [Google Scholar]
- 21.Kinsella JP, Truog WE, Walsh WF. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131:55–62. [DOI] [PubMed] [Google Scholar]
- 22.Neonatal Inhaled Nitric Oxide Study Group (NINOS). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics. 1997;99:838–845. [DOI] [PubMed] [Google Scholar]
- 23.Downard CD, Jaksic T, Garza JJ, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg. 2003;38:729–732. [DOI] [PubMed] [Google Scholar]
- 24.Davis PJ, Firmin RK, Manktelow B, et al. Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr. 2004;144:309–315. [DOI] [PubMed] [Google Scholar]
- 25.Cortes RA, Keller RL, Townsend T, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg. 2005;40:36–45. [DOI] [PubMed] [Google Scholar]
- 26.Trachsel D, Selvadurai H, Bohn D, et al. Long-term pulmonary morbidity in survivors of congenital diaphragmatic hernia. Pediatr Pulmonol. 2005;39:433–439. [DOI] [PubMed] [Google Scholar]
- 27.West SD, Wilson JM. Follow up of infants with congenital diaphragmatic hernia. Semin Perinatol. 2005;29:129–133. [DOI] [PubMed] [Google Scholar]
Discussions
Dr. Charles J. Stolar (New York, New York): Early this morning we heard Dr. Warshaw comment that most surgical challenges can be solved. Diaphragmatic hernia, however, remains a significant neonatal challenge, which is an important subset of patients that do succumb to this disease. Defining this population either antenatally or postnatally continues to be a very vexing challenge.
The good news is that individual neonatal centers, as you allowed, Dr. Lally, such as those in New York, Boston, Ann Arbor, Gainesville and others are now reporting single institution survivals in large series between 80% and 90% and were clearly improving morbidity. I think we are all finally beginning to do better than, if that is possible.
Dr. Lally to his credit has motivated a lot of people to amass a lot of information from a lot of diaphragmatic hernia patients from a lot of neonatal centers in an effort to learn from a collective experience. He is to be congratulated for taking the initiative virtually single-handedly, along with his wife, and following through on it.
He has told us that congenital diaphragmatic hernia is indeed a heterogeneous disease but in the collective experience agenesis diaphragm remains associated with a poorer prognosis than it might be if the diaphragm were wholly there, and that over time there has been a coincidental increase in the use of inhaled nitric oxide, changing respiratory care strategies, specifically the use of hyperventilation and the avoidance of hyperventilation with reduced alkalosis, also a decreased use of ECMO. Of interest in your aggregate, survival of 68% is telling in that often-quoted mortality rates for diaphragmatic hernias is often in excess of 68% as justification for fetal intervention.
I would like to ask your views on some general and some specific issues, Dr. Lally.
In general are you concerned about the heterogeneity of the data and the impact on your observations? For example, the wide range of respiratory care strategies that were used on this population. For example, the range of individuals making treatment decisions. Although we surgeons like to be see ourselves as wholly responsible for neonates, the fact of the matter is that taking care of a newborn in a modern neonatal intensive care unit is a collaborative experience. I am also worried about the range of indications for specific treatments such as ECMO, such as nitric oxide, such as oscillating ventilation and the like.
My specific questions, however, I would like to you comment specifically on the following. Although you do pay homage to the use of persistent hypercapnia and spontaneous respiration, I come from the institution that first described this in a paper written by Virginia Ephart and Stan James in 1975, and this strategy specifically forbids the use of muscle relaxants and muscle paralysis. But I note in the data in the manuscript that 80% to 83% of the patients had muscle paralysis. And while I understand that not everybody agrees to the same definition of hyperventilation due to alkalosis, this is experience as the strategy was originally described.
I am concerned about the indications for inhaled nitric oxide because in these several multicenter clinical trials inhaled nitric oxide is a very effective agent for prematurity, for idiopathic pulmonary hypertension. The one diagnosis for which inhaled nitric oxide has never been systematically shown to work is congenital diaphragmatic hernia, and I wonder if its broad applications just spill over from an experience with the premature baby.
I observed in your manuscript report that 12% to 43% of these patients went home on oxygen and a significant number are also on oxygen after 30 days. Many of us consider this prima facie evidence of either bronchial pulmonary dysplasia or persistent pulmonary hypertension. To what extent is this really patient disease and to what extent is it iatrogenic disease?
Dr. Kevin P. Lally (Houston, Texas): Regarding your concerns over the heterogeneity of the data and the range of indications, I think this is a reality of practice. These data represent 20 centers but the entire study group represents about 55 active centers today. This is just what we are dealing with.
It is possible in a single institution to get one person or several people managing a patient in the exact same fashion. However, this becomes difficult to translate to many other institutions. In an ideal world you would have a protocol-based, evidence-based method of approaching these patients. Even in my own institution, with a printed, agreed-upon, standardized approach to how we are going to manage the patient, I can still come in at 7:00 in the morning and go, “What went on last night?”
The Columbia version of permissive hypercapnia is obviously not widely embraced by everybody around the world. And I think that we need to get to the point, which we are evolving to but it will take time, to when we report our data where we are talking apples and apples. I cannot interpret individual center data because I don't know how many high risk patients exist in an individual center. And it is surprisingly variable. In the registry, it ranges from 5% to 35% of centers that manage patients that have agenesis, which can have a huge impact on overall survival. So until we get to the point we are comparing apples and apples, I don't know. But there are several centers that have and identical survival to Columbia that use neuromuscular blockade but they maybe have a different type of patient.
The indications for nitric oxide have been changing. Every randomized trial of inhaled NO show no benefit in CDH. All of them are designed as a rescue strategy for avoidance of ECMO and improving survival when used early in patients in respiratory failure. Most likely the current use of nitric oxide is in a different strategy altogether. Much like there has been an evolution in ECMO from a rescue strategy to an early stabilization strategy, the same is true with NO. I think that people generally avoid nitric oxide as a rescue strategy but use it much more in the management of cardiovascular failure and right heart failure in the high risk patients. It is only used in about 25% of the non-agenesis patients, so it is not applied to all patients with diaphragmatic hernia.
And lastly, the home oxygen patients—again, it is an observational database. I suspect that there is a subgroup of babies who still have significant iatrogenic lung disease, but some centers will frequently send patients home on oxygen with very little indication.
Dr. Keith Georgeson (Birmingham, Alabama): Just a couple of questions. First of all, congratulations on the tenacity to hang in there for over 10 years to get this data.
I wonder if you can break down the difference between survival of patients that are patch repaired and those that are primarily repaired.
Also, I would like to you speculate on the impact of therapeutic abortion. In some other papers, therapeutic abortion has been shown to be one of the reasons why our survival has improved, and I wonder what your thoughts are with this database.
Lastly, in our center now ECMO is primarily used as a last resort and most of these patients are dying. So I am a little surprised that the ECMO rate is still over 50%.
Dr. Kevin P. Lally (Houston, Texas): Regarding the survival for patch repair versus primary repair, we can actually grade these further by even large defects versus those defects with patch. We are working towards using that as a potential postoperative staging system that would enable us to compare patients with the same level of severity. The infants who undergo primary repair of the diaphragm, which is about 50% of the operative patients, have a 95%-plus survival. And I think that is going to ultimately be important to us because throwing all these advanced therapies at that low-risk population is probably not a good idea.
Regarding the impact of elective termination of pregnancy, I think that it probably does impact outcome. However, I don't think that those are necessarily terminated on the basis of appropriate prenatal stratification. So I think that they are terminating pregnancies on the basis of diagnosis alone, hence it probably includes both high and low risk groups. This represents only liveborn infants so I am not sure that it impacts on the data set per se.
As far as ECMO, very clearly, it is applied today as a stabilization device as opposed to rescue therapy. There is a whole list of other questions now as we evolve our use of ECMO and avoid iatrogenic lung injury, what is its benefit in these patients? And I remain very unclear about what ECMO's role is. A few patients, I am sure, are alive that wouldn't be without it, but there are several others where that is unclear.
Dr. George W. Holcomb, III (Kansas City, Missouri): In looking over the last ten years of data have you noticed an evolution of the type of patch that is used when a patch is required? And do you have any data to suggest that one type of patch has a lower recurrence rate than another?
Dr. Kevin P. Lally (Houston, Texas): We asked patch type in the first version of registry, and since it was overwhelming PTFE we quit asking. We may actually dial it into Version 3 of the registry again. But I think if you look specifically at the subgroup of agenesis patients it is impossible not to expect recurrence because chest wall has to grow as does the patient. And the issue of whether some other type of material is optimal or not, I don't know.
Dr. Thomas F. Tracy, Jr. (Providence, Rhode Island): Dr. Lally, congratulations to the Diaphragmatic Hernia Study Group especially for giving us the reality of the mortality figures. I have had a sense that we have come close to hubris because of the outstanding work that has been done at Columbia and subsequently Florida with the other papers that Dr. Stolar mentioned about outcomes.
However, the specter of fetal intervention is still alive. There is a current study that is going on through Eurofetus as a trial of late tracheal occlusion that may be more sensitive and specific for adequate prenatal lung development. You focused on a group of diaphragmatic agenesis patients, which can now be defined prenatally. Does your study look at any other parameters that we can look to besides other anatomical indicators similar to lung to head circumference ratios or anything else that would help refine that study to target a subset of fetal patients that would be best suited for this trial?
Dr. Kevin P. Lally (Houston, Texas): No. But we are hoping that a subset of the next group of the registry, because it has to be in consent form, is to dial in a prenatal component to attach to the postnatal outcome. This is a post-delivery outcome. There are no multi-centered, validated accurate predictors for outcome in prenatally diagnosed CDH. The group in Europe talks about patients with both the liver up in the chest and a low LHR and combined to come up with some outcomes for this. I think there is a subgroup of babies with CDH that we do not have an answer for today.
So I don't know if it is the specter of fetal intervention as much as we need to accurately and appropriately define a subgroup of babies where it's use can be more appropriately studies. We are clearly not there today.
Footnotes
This manuscript was prepared by the following investigators: Kevin P. Lally, MD,* Pamela A. Lally, MD,* Krisa P. Van Meurs, MD,† Desmond J. Bohn, MD,‡ Carl F. Davis, FRCS,§ Bradley Rodgers, MD,∥ Jatinder Bhatia, MD,¶ and Golde Dudell, MD**.
Supported in part by NIH Grant Nos. K24RR17050 and M01RR002558.
Reprints: Kevin P. Lally, MD, Department of Surgery, University of Texas Health Sciences Center at Houston, 6431 Fannin, Suite 5.258, Houston, TX, 77030. E-mail: kevin.p.lally@uth.tmc.edu.


