Abstract
Objective:
To determine the prevalence and proximal extent of gastroesophageal reflux (GERD) in patients awaiting lung transplantation.
Background:
GERD has been postulated to contribute to accelerated graft failure in patients who have had lung transplantations. However, the prevalence of reflux symptoms, esophageal motility abnormalities, and proximal esophageal reflux among patients with end-stage lung disease awaiting lung transplantation are unknown.
Methods:
A total of 109 patients with end-stage lung disease awaiting lung transplantation underwent symptomatic assessment, esophageal manometry, and esophageal pH monitoring (using a probe with 2 sensors located 5 and 20 cm above the lower esophageal sphincter).
Results:
Reflux symptoms were not predictive of the presence of reflux (sensitivity, 67%; specificity, 26%). Esophageal manometry showed a high prevalence of a hypotensive lower esophageal sphincter (55%) and impaired esophageal peristalsis (47%) among patients with reflux. Distal reflux was present in 68% of patients and proximal reflux was present in 37% of patients.
Conclusions:
These data show that in patients with end-stage lung disease: 1) symptoms were insensitive and nonspecific for diagnosing reflux; 2) esophageal motility was frequently abnormal; 3) 68% of patients had GERD; 4) in 50% of the patients with GERD, acid refluxed into the proximal esophagus. We conclude that patients with end-stage lung disease should be screened with pH monitoring for GERD.
Gastroesophageal reflux disease has been cited as a cause of accelerated graft failure in patients who have had lung transplantations. Among 109 patients awaiting lung transplantation, the prevalence of distal reflux was 68% and the prevalence of proximal reflux was 37%.
Chronic allograft dysfunction due to obliterative bronchiolitis remains the main complication limiting long-term graft survival following lung transplantation. Gastroesophageal reflux disease (GERD) has been implicated in causing the bronchiolitis obliterans syndrome (BOS).1–6 It is postulated that, in some patients, the reflux extends into the upper esophagus and causes microaspiration.6–11 Analysis of bronchoalveolar lavage fluid from 120 posttransplant patients showed that, in those with BOS, levels of bile acids were high compared with specimens from patients without BOS. Furthermore, the onset of BOS (early vs. late) correlated with the amount of bile acid in the fluid.6 There is evidence that, in lung transplant patients with GERD, early fundoplication can improve allograft function and prevent obliterative bronchiolitis.4,5
These findings have stimulated fresh interest in the relationship of reflux to severe pulmonary disease in adults. At this point, little is known about esophageal function and the prevalence of proximal reflux, a better predictor of respiratory complications of GERD.7–10
The aims of this study were to determine the prevalence of GERD, the clinical presentation of GERD, and the manometric and reflux profile among patients with end-stage lung disease awaiting lung transplantation.
PATIENTS AND METHODS
A total of 109 patients with end-stage lung disease who were being evaluated for transplantation at the University of California San Francisco were referred to the UCSF Swallowing Center for esophageal manometry and 24-hour ambulatory pH monitoring. They were identified by cross-referencing the list of 762 patients evaluated for lung transplantation with the Swallowing Center database of 4385 preoperative patient evaluations. This yielded 109 patients, 104 of whom were studied after esophageal studies were made a routine component of the pretransplant evaluation in July 2003. Five additional patients were studied before that time. Patients who were studied after a lung transplant or who had combined heart-lung transplantation were not included.
The patients were divided into the following lung disease subgroups: restrictive, obstructive, mixed, pulmonary hypertension, and bronchoalveolar carcinoma, according to the principle reason for referral for transplantation. For patients with more than one of these conditions, the most severe one was chosen for the group assignment. For example, patients with scleroderma were classified as restrictive or pulmonary hypertension depending on which predominated. Patients in the restrictive group were further divided into idiopathic pulmonary fibrosis (IPF) or one of the following interstitial lung diseases: nonspecific interstitial pneumonia, hypersensitivity pneumonitis, scleroderma, or sarcoidosis. Specific diagnoses in the obstructive group included chronic obstructive pulmonary disease (COPD), bronchiectasis, alpha-1 antitrypsin deficiency (AAT), and cystic fibrosis. Patients in the mixed group had both restrictive and obstructive ventilatory deficits, including eosinophilic granuloma and lymphangiomyomatosis (LAM). Pulmonary hypertension included primary pulmonary arterial hypertension and secondary pulmonary hypertension (due to interstitial lung diseases or some other process). One patient had bronchoalveolar carcinoma. The patient's classification was determined by the transplant pulmonologists (J.G., S.H., and L.L.).
Symptomatic Evaluation
The subjects underwent standardized interviews with the Swallow Center physician or technician. We used a nonvalidated, standard questionnaire in place for 16 years. They estimated the severity of their symptoms (heartburn, regurgitation, dysphagia, cough) using a 5-point scale ranging from 0 (no symptom) to 4 (disabling symptom). They were also questioned about the use of acid-reducing medications (H2-blocking agents and proton pump inhibitors [PPIs]).
Esophageal Manometry
Esophageal manometry was performed after an overnight fast using techniques previously described.12 Medications that might interfere with esophageal motor function (ie, calcium channel blocking agents, nitrates, and metoclopramide) were discontinued at least 48 hours before the study. Position, pressure, length, and relaxation of the lower esophageal sphincter (LES) were measured using the station pull through technique, with 0.5-cm increments between stations. Esophageal body function was recorded 3, 8, 13, and 18 cm above the upper border of the LES by giving 10 swallows of 5 mL of water at 30-second intervals. The amplitude of peristaltic waves was calculated independently for the distal esophagus (3 and 8 cm above the LES) and proximal esophagus (13 and 18 cm above the LES).8 Esophageal length was measured from the upper esophageal sphincter to the distal end of the LES. Ineffective esophageal motility was defined as distal esophageal amplitude <30 mm Hg or >30% simultaneous waves in the distal esophagus.13
Ambulatory pH Monitoring
Proton pump inhibitors (PPIs) were withheld for 14 days and histamine-2 blockers were withheld for 3 days before the study. The pH catheters were calibrated in a standard buffer solution at pH 1 and pH 7 before and after monitoring. A dual-channel pH catheter with 2 antimony sensors located 15 cm apart was placed transnasally, with the distal sensor 5 cm above the upper border of the manometrically determined LES. Patients were instructed to consume an unrestricted diet, refrain from taking any acid-suppressing medications, and to keep a diary of symptoms during the pH testing. The data were incorporated into a composite score (ie, DeMeester score), which takes into account 6 elements: 1) number of reflux episodes, 2) number of reflux episodes longer than 5 minutes, 3) duration of the longest reflux episode, 4) percentage of time the pH is <4.0 for the total duration of the study, and 5) in the upright and 6) supine positions. A score greater than 14.7 was set as abnormal based on the data obtained from 50 volunteers.14 Proximal reflux was defined as >1% total time pH <4 at the proximal sensor.15 The data were analyzed using a commercial software program (Gastrosoft, Medtronic Functional Diagnostic, Shoreview, MN).
Other Tests
Gastric emptying studies were performed in 17 patients. Other studies were performed in a nonsystematic manner, mostly in patients who were candidates for antireflux surgery. Ten patients had upper endoscopy and 15 patients had a barium swallow.
Statistical Analysis
Statistical analysis was performed using STATA Statistical Software: Release 9.1 (Stata Corp., College Station, TX). Nonparametric statistical analyses were performed. Differences between the GERD positive and negative groups were analyzed with the χ2 test for proportions or the Mann-Whitney U test for continuous variables. Comparisons between lung disease subgroups were performed with the Kruskal-Wallis test. Data are reported as number (percentage) from χ2 analysis or as median (interquartile range) for nonparametric tests. Spearman rank correlation was used to examine associations between continuous or ordinal variables.
The study protocol was reviewed and approved by the University of California San Francisco Committee on Human Research.
RESULTS
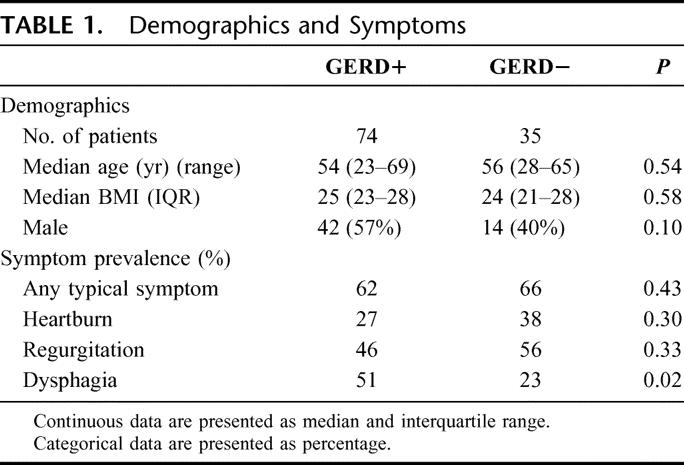
A total of 109 patients were evaluated with esophageal manometry and pH studies. Seventy-four patients had reflux (68%; DeMeester score >14.7, GERD+) and 35 had normal esophageal acid exposure (32%; DeMeester score <14.7, GERD−) (Table 1).
TABLE 1. Demographics and Symptoms
Symptomatic Evaluation
A total of 104 patients completed the symptom questionnaire. The main symptoms were respiratory. Seventy-two subjects (69%) reported having at least one typical symptom of GERD (heartburn, regurgitation, or dysphagia). There was no difference in the prevalence of heartburn or regurgitation among patients with and without reflux (Table 1). Although dysphagia was more common among GERD+ patients, only half of the GERD+ patients had dysphagia. Reflux was asymptomatic in 33% of those with distal reflux and in 38% of those with proximal reflux. There was no difference in the severity of heartburn (P = 0.23) and regurgitation (P = 0.26) between the 2 groups. The severity of typical symptoms did not correlate with the DeMeester score or the percentage of time the pH was <4.0 in the proximal esophagus (Spearman ranks r = 0.09 and −0.05, P = 0.35 and 0.61, respectively).
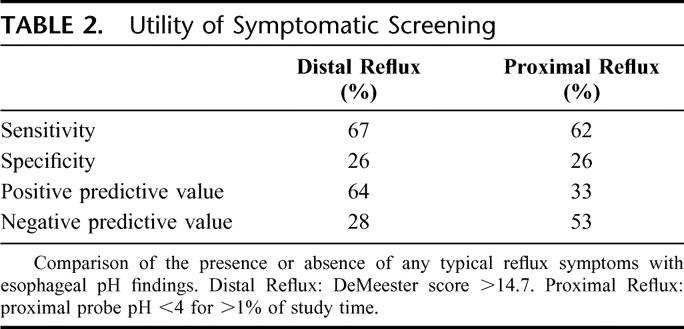
To determine the utility of symptom assessment as a screening test for reflux, we compared typical reflux symptoms with the esophageal pH findings. The presence of one or more typical reflux symptoms was compared with the esophageal pH findings for distal and proximal reflux (Table 2). The sensitivity and specificity for distal reflux were 67% and 26%, respectively. The sensitivity and specificity for proximal reflux were 62% and 26%, respectively.
TABLE 2. Utility of Symptomatic Screening
Esophageal Manometry
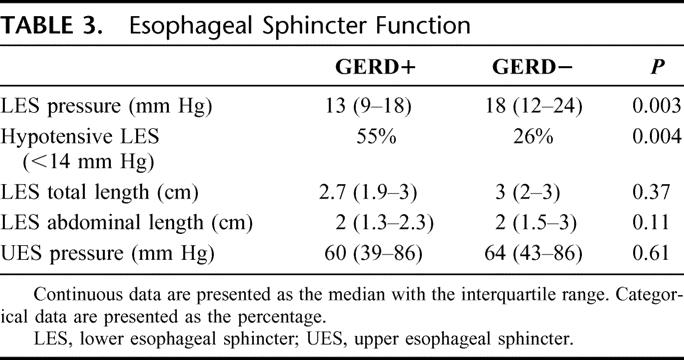
GERD+ patients had lower LES pressures. Separating the LES pressures into normal and abnormal (ie, above or below 14 mm Hg) demonstrated that 55% of subjects with reflux had a hypotensive LES compared with 26% of those without reflux (P = 0.004). The groups had similar total and abdominal LES lengths (Table 3).
TABLE 3. Esophageal Sphincter Function
The amplitude of peristalsis in the distal esophagus was lower in GERD+ patients, and ineffective esophageal motility was more common. There was no difference in amplitude of peristalsis in the proximal esophagus (Table 4).
TABLE 4. Esophageal Body Function
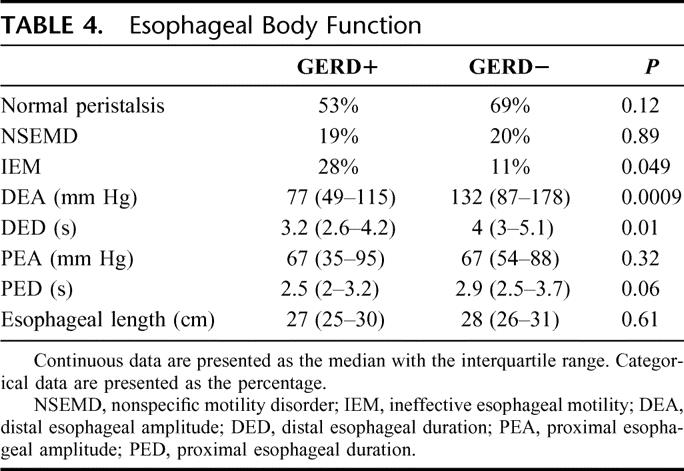
Analysis by lung disease subgroup found that distal esophageal amplitude was lower in the subjects with scleroderma (P = 0.0002) and higher in those with COPD and LAM (P = 0.02 and 0.01). When a correction for multiple comparisons was made, only the lower distal esophageal amplitude among subjects with scleroderma remained statistically significant.
The upper esophageal sphincter pressure was similar in the 2 groups.
The ratio of esophageal length to patient height correlated with type of lung disease (Kruskal-Wallis, P = 0.0047). The esophagus was shorter in patients with IPF and cystic fibrosis and longer in those with COPD and bronchiectasis. Esophageal length was not different between those with and without GERD.
Ambulatory pH Monitoring
The prevalence of distal and proximal reflux was 68% and 37%, respectively. The reflux profile of GERD+ and GERD− groups is given in Table 5.
TABLE 5. Esophageal pH Findings
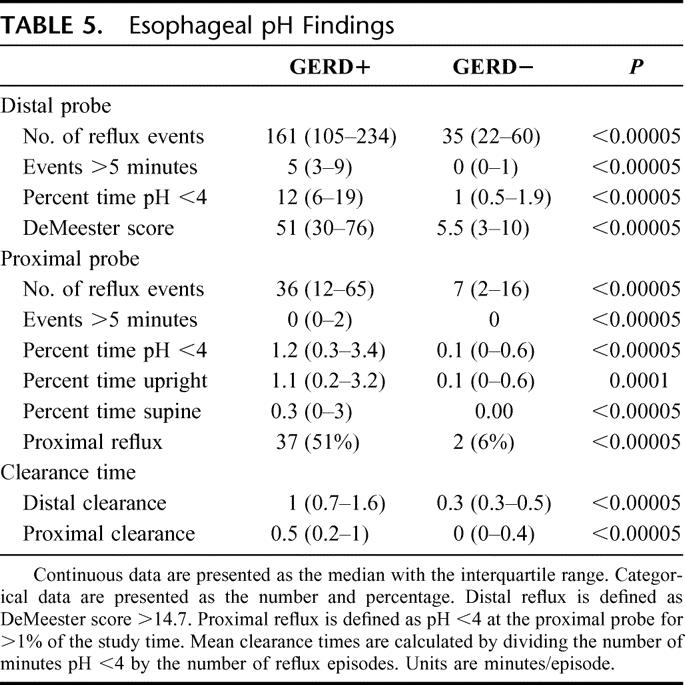
The DeMeester scores separated the 2 groups, as did the rest of reflux data (Table 5). In addition to more reflux episodes and more time with pH <4.0 in the supine and upright positions, GERD+ patients had slower acid clearance in the lower and upper esophagus.
GERD+ subjects had more proximal reflux, with a median percentage of time pH <4 of 1.2 versus 0 (P < 0.00005). Proximal reflux was more common when the patients were upright.
Proximal reflux occurred in 2 patients with normal distal esophageal acid exposure. One patient had IPF and one had COPD. Both had normal LES and upper esophageal sphincter pressures and normal esophageal body motility.
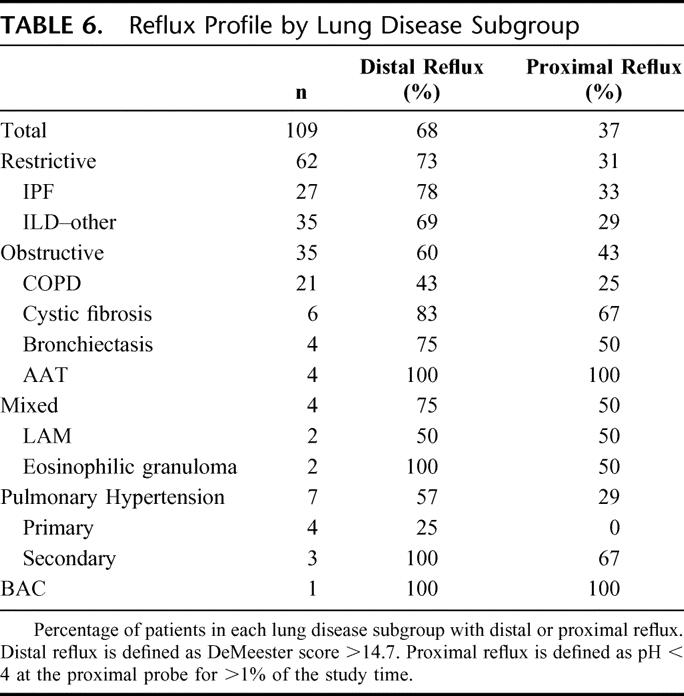
Reflux among lung disease subgroups is presented in Table 6.
TABLE 6. Reflux Profile by Lung Disease Subgroup
Other Tests
Seventeen patients with symptoms suggestive of delayed gastric emptying (postprandial bloating and fullness, nausea, vomiting) had gastric emptying studies. Sixteen of them had abnormal distal acid exposure. Among these 16 patients, 4 had delayed emptying of liquids and 10 had delayed emptying of solids.
Ten patients had upper endoscopy. Six patients were found to have a hiatal hernia. All patients with a hiatal hernia had abnormal distal reflux. Four had irregular z-lines suggestive of chronic inflammation and 1 patient had linear erosions. Of the 4 patients without hiatal hernia, 2 had reflux.
DISCUSSION
These results show the following in patients with end-stage lung disease (ESLD) awaiting lung transplantation: 1) the prevalence of GERD was 68%, and in half of those with distal reflux, acid refluxed into the proximal esophagus; 2) GERD was associated with a hypotensive LES and abnormal esophageal peristalsis; and 3) symptoms did not distinguish between those with and without GERD.
Prevalence of GERD in Patients With ESLD Awaiting Lung Transplantation
In this study, GERD was detected in 68% of patients with ESLD awaiting lung transplantation. Our data confirm the observation of a high prevalence of GERD similar to cohorts of patients studied at other centers. For instance, Cantu et al found GERD in 63% of patients who were candidates for lung transplant,5 whereas a prevalence of 32% was found by D'Ovidio et al.16 The lower prevalence found in the latter study may be explained by the fact that PPIs were stopped for only 5 days before the pH monitoring. Because these medications affect the acid secretion by the parietal cells for up to 10 days, the prevalence of GERD might have been underestimated.
In half of the patients with GERD, reflux reached 20 cm above the LES, similar to the findings of D'Ovidio et al.16 Proximal reflux is more dangerous than distal reflux, for it predisposes to microaspiration, which in time can permanently damage the lungs.6,7,8,17 Because ambulatory pH monitoring is a surrogate marker of aspiration, detecting bile acids or pepsin in bronchoalveolar fluid would provide stronger evidence that gastric contents are entering the tracheobronchial tree.6,11 Most proximal reflux occurred during the day (upright reflux), as reported by others.3 Two patients with normal distal acid exposure had abnormal proximal reflux, suggesting that most of the “physiologic” reflux occurring in the distal esophagus eventually reached 20 cm above the LES. The meaning of this finding, as noted by others,16,18 is still unclear, but it underscores the importance of measuring both distal and proximal reflux.
Reflux was common among all subgroups. Among 27 patients with IPF, distal and proximal reflux was found in 78% and 33%, respectively. In another report, the prevalence was 87% among 65 patients with IPF.18 The high prevalence of GERD in this group of patients with idiopathic ESLD raises the possibility that reflux plays a role in pathogenesis of IPF.
Some patients develop reflux after lung transplantation,2,3 possibly as a consequence of vagal nerve injury and delayed gastric emptying, or immunosuppressive medications.19
Clinical Presentation of GERD in Patients With ESLD Awaiting Lung Transplantation
This study confirms that the clinical history, even using a standardized questionnaire, cannot distinguish between patients with and without GERD. Neither the presence nor the severity of reflux symptoms predicted the presence of reflux, which is consistent with other reports on this subject.10,18,20 For example, among 822 patients with a clinical diagnosis of GERD, manometry and pH monitoring showed that only 575 patients (70%) actually had reflux.20 Furthermore, we found reflux in 33% of patients who had none of the typical symptoms (ie, heartburn, regurgitation) of GERD. A high prevalence (53%) of asymptomatic reflux was also found by Raghu et al in patients with IPF.18
We think that every patient with ESLD should be screened by esophageal manometry and ambulatory pH monitoring to learn if abnormal reflux is present.
Pathophysiology of GERD in Patients With ESLD Awaiting Lung Transplantation
Patients with GERD more frequently had a hypotensive LES and low amplitude and abnormally propagating peristaltic waves in the distal esophagus. Almost one third of GERD+ patients had ineffective esophageal motility, a disorder often associated with respiratory symptoms.13,21 Since peristalsis is the main determinant of esophageal acid clearance, esophageal clearance in both the distal and proximal esophagus was probably delayed in the patients with GERD. A higher volume of refluxate may also produce this finding. D'Ovidio et al reported that the LES was hypotensive and motility of the body of the esophagus was abnormal in 63% and 33% of patients with ESLD, respectively.16
In the few patients who had upper endoscopy, a hiatal hernia was common. This last finding, which needs confirmation, is important because the size of a hiatal hernia correlates with the magnitude of the reflux and with respiratory symptoms.22 In addition, gastric emptying was frequently delayed among the patients who were tested, and it was associated with abnormal reflux. Gastric emptying usually slows after transplantation, which may contribute to the development of BOS.19
CONCLUSION
We recognize that this study has limitations. The study population comprised selected patients with end-stage lung disease who were candidates for transplantation. They were younger and had fewer comorbidities than the general population of patients with end-stage lung disease.
We recommend that esophageal manometry and pH monitoring be performed in every patient with end-stage lung disease to check for GERD and proximal reflux. Future studies should include the use of multichannel impedance technology to search for acid and nonacid reflux23 and determination of bile acids or pepsin in bronchoalveolar fluid.6,11 The same diagnostic approach should also be used for patients with less severe lung disease, particularly in patients with IPF. Controlling reflux at an earlier stage may improve the lung function or stop the progression of the disease, avoiding the need for transplantation.
Although a cause-and-effect relationship between GERD and lung injury is not definitively proved, we recommend antireflux surgery for the patients with reflux. A total fundoplication is the treatment of choice as it is the only way to reestablish the competence of the gastroesophageal junction and stop any type of gastric reflux.24 Treatment with proton pump inhibitors should be second-line therapy for reflux persists in most patients even though symptoms improve as the pH of the refluxate rises.18,23,25
Discussions
Dr. Tom R. DeMeester (Los Angeles, California): Dr. Sweet's paper focuses on the relationship of esophageal disease to pulmonary disease, a fascinating development in our understanding of pulmonary disease. It is the second paper in the literature that documents this very high prevalence of reflux in patients with end-stage lung disease. In fact, the percentages are almost identical in the 2 papers, this one and the previous one from Duke.
Now, that opens up a very exciting area of new dimensions in pulmonary disease, as was pointed out, our president pointed out, the relationship between reflux disease and pulmonary fibrosis, idiopathic pulmonary fibrosis. When one thinks about 80% of asthmatics have reflux disease and then when one thinks about pulmonary hypertension and the association with reflux disease, this is a very fruitful area for study.
I have a couple of questions. First of all, the problem is, which comes first? Is it the reflux disease that induces the pulmonary disease? Or is it the exaggerated dynamics of breathing with pulmonary disease that induces the reflux disease? Did you have anybody who had a positive 24-hour but had normal manometry? That would kind of suggest that maybe it was the pulmonary disease encouraging it. Give us some insight into that. And how sure are we that reflux does really cause bronchiolitis obliterans syndrome? Could you comment on that a little bit?
Now, the real advantage of all of this would be that if indeed reflux causes pulmonary disease, can we detect it earlier and prevent end-stage pulmonary disease? And that is going to be a problem when I look at your relationship of symptoms to something to apply to the score. So how would you anticipate picking up these patients earlier? Would you extend the criterion to more cases of pulmonary problems or have you got a way of grappling that? Obviously we don't want to wait until end-stage lung disease to pick them up.
It is a very good paper and opens up a big, wide area. I congratulate you for presenting it.
Dr. Marco G. Patti (San Francisco, California): I want to thank Dr. DeMeester for reviewing the manuscript and for his questions. It was about 3 decades ago that Dr. DeMeester postulated that aspiration secondary to gastroesophageal reflux disease could cause lung fibrosis. Our work is a tribute to his ideas.
It is very difficult to know whether or not the reflux precedes the lung disease. A theory that has been around for a long time is that the altered respiratory mechanics in patients with end-stage lung disease may cause the reflux. Specifically, the increased negative pressure in the chest during inspiration could cause a gradient favoring reflux. We examined this issue in our patients and found very contrasting data that did not support this hypothesis. It appears as though transplantation increases the percentage of patients who reflux. If reflux was indeed due to the altered respiratory mechanics, we should see a decrease after transplantation.
In some patients reflux occurred despite the presence of a mechanically competent lower esophageal sphincter and normal esophageal peristalsis. It is possible that reflux occurred because of transient relaxations of the LES and a higher volume of refluxate, which were not measured in our study.
There are data in the literature that suggests that aspiration of gastric contents can cause the bronchiolitis obliterans syndrome (BOS). The lung transplant group from Toronto examined the bronchoalveolar lavage fluid from 120 posttransplant patients. They found that in those patients with BOS, levels of bile acids were high compared with specimens from patients without BOS. In addition, the group from Duke University has shown that in lung transplant patients with GERD early fundoplication may improve allograft function and prevent BOS.
Based on these data and on the findings of our study, we think that every patient awaiting lung transplant should undergo esophageal manometry and ambulatory pH monitoring, regardless of the presence of symptoms. We recommend a total fundoplication for the patients with reflux, as it is the only way to re-establish the competence of the gastroesophageal junction and stop any type of gastric reflux. We think that the same protocol should be used in any patient with IPF. The high prevalence of reflux in patients with IPF suggests a possible cause and effect relationship, and early intervention might alter the natural history of this disease. Further studies are required to elucidate this association.
Dr. Richard J. Finley (Vancouver, British Columbia, Canada): Great paper. I have 2 questions.
Do you have any objective information such as tracheitis or laryngitis to suggest these patients are aspirating?
It appears that some of these patients have decreased peristalsis. Do you have any other objective information such as a nuclear medicine esophageal transit or a timed barium swallow to suggest that these patients have decreased transits?
Dr. Marco G. Patti (San Francisco, California): Some of these patients had laryngitis but they were not uniformly studied. We plan to study pepsin in the bronchoalveolar lavage for evidence of microaspiration. We did not perform nuclear medicine studies.
Dr. Jeffrey H. Peters (Rochester, New York): Very nice study, Dr. Patti. I have a quick question about PPI therapy and how its use or not may relate to the problem of recognizing reflux-pulmonary disease associations. You didn't give us data on how many patients were receiving PPI therapy pre-op. If patients were on it, it suggests they still have reflux and continue to destroy their lungs. And if they are not on PPI therapy, it suggests we have a fundamental problem relating reflux and respiratory disease. Can you comment regarding PPI use?
Dr. Marco G. Patti (San Francisco, California): Most patients were on proton pump inhibitors because they were treated with steroids. They were taken off proton pump inhibitors for 2 weeks before the 24-hour pH monitoring study. Most patients were asymptomatic before and they remained asymptomatic after. We believe that proton pump inhibitors should not be the first line of therapy because reflux persists in most patients.
Dr. Lee L. Swanstrom (Portland, Oregon): Very nice study, Dr. Patti. Could you comment briefly on the use of impedance pH and whether that might contribute in evaluating these patients? Also, there is a lot of other information on 24-hour pH testing aside from pure acid exposure such as correlation with coughing episodes, supine versus upright reflux, and issues like that. Is there data to be gained by looking at that as well?
Dr. Marco G. Patti (San Francisco, California): We did not perform impedance. This test is very important as it clearly shows that reflux still occurs during treatment with proton pump inhibitors. As shown by Drs. Peters and DeMeester, proton pump inhibitors just change the pH of the gastric refluxate so that the number of the acid reflux episodes decreases while the number of nonacid episodes increases. It was difficult to establish a correlation with symptoms. For instance, coughing was very common in these patients. We felt that sometimes reflux caused the cough and sometimes the cough actually caused the reflux because of the increase in intra-abdominal pressure. As other groups have shown, most of the reflux to the proximal esophagus occurred in the upright position.
Footnotes
Reprints: Marco G. Patti, MD, Department of Surgery, University of California, San Francisco, 521 Parnassus Avenue, Room C-341, San Francisco, CA 94143-0790. E-mail: pattim@surgery.ucsf.edu.
REFERENCES
- 1.Reid KR, McKenzie FN, Menkis AH, et al. Importance of chronic aspiration in recipients of heart-lung transplants. Lancet. 1990;336:206–208. [DOI] [PubMed] [Google Scholar]
- 2.Young LR, Hadjiliadis D, Davis RD, et al. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124:1689–1693. [DOI] [PubMed] [Google Scholar]
- 3.Hadjiliadis D, Davis RD, Steele MP, et al. Gastroesophageal reflux disease in lung transplant recipients. Clin Transplant. 2003;17:363–368. [DOI] [PubMed] [Google Scholar]
- 4.Davis RD, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125:533–542. [DOI] [PubMed] [Google Scholar]
- 5.Cantu E, Appel JZ, Hartwig MG, et al. Early fundoplication prevents chronic allograft dysfunction in patients with gastroesophageal reflux disease. Ann Thorac Surg. 2004;78:1142–1151. [DOI] [PubMed] [Google Scholar]
- 6.D'Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–1152. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DA, Drane WE, Curran J, et al. Pulmonary disease in progressive systemic sclerosis: a complication of gastroesophageal reflux and occult aspiration? Arch Intern Med. 1989;149:589–593. [PubMed] [Google Scholar]
- 8.Patti MG, Debas HT, Pellegrini CA. Clinical and functional characterization of high gastroesophageal reflux. Am J Surg. 1993;165:163–168. [DOI] [PubMed] [Google Scholar]
- 9.Patti MG, Debas HT, Pellegrini CA. Esophageal manometry and 24-hour pH monitoring in the diagnosis of pulmonary aspiration secondary to gastroesophageal reflux. Am J Surg. 1992;163:401–406. [DOI] [PubMed] [Google Scholar]
- 10.Oelschlager BK, Chang L, Pope CE, et al. Typical GERD symptoms and esophageal pH monitoring are not enough to diagnose pharyngeal reflux. J Surg Res. 2005;128:55–60. [DOI] [PubMed] [Google Scholar]
- 11.Farrell S, McMaster C, Gibson D, et al. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastroesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41:289–293. [DOI] [PubMed] [Google Scholar]
- 12.Patti MG, Fisichella PM, Perretta S. Preoperative evaluation of patients with gastroesophageal reflux disease. J Laparoendosc Adv Surg Tech. 2001;11:327–331. [DOI] [PubMed] [Google Scholar]
- 13.Diener U, Patti MG, Molena D, et al. Esophageal dysmotility and gastroesophageal reflux disease. J Gastrointest Surg. 2001;5:260–265. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson JR, Stein HJ, DeMeester TR, et al. Ambulatory pH monitoring: normal values, optimal thresholds, specificity, sensitivity and reproducibility. Am J Gastroenterol. 1992;87:1102–1111. [PubMed] [Google Scholar]
- 15.Dobhan R, Castell DO. Normal and abnormal proximal esophageal acid exposure: results of ambulatory dual-probe pH monitoring. Am J Gastroenterol. 1993;88:25–29. [PubMed] [Google Scholar]
- 16.D'Ovidio F, Singer LG, Hadjiliadis D, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80:1254–1261. [DOI] [PubMed] [Google Scholar]
- 17.Patti MG, Arcerito M, Tamburini A, et al. Effect of laparoscopic fundoplication on gastroesophageal reflux-induced respiratory symptoms. J Gastrointest Surg. 2000;4:143–149. [DOI] [PubMed] [Google Scholar]
- 18.Raghu G, Fredenberger TD, Yang S, et al. High prevalence of abnormal acid gastroesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. [DOI] [PubMed] [Google Scholar]
- 19.Berkowitz N, Schulman LL, McGregory C, et al. Gastroparesis after lung transplantation: potential role in postoperative respiratory complications. Chest. 1995;108:1602–1607. [DOI] [PubMed] [Google Scholar]
- 20.Patti MG, Diener U, Tamburini A, et al. Role of esophageal function tests in diagnosis of gastroesophageal reflux disease. Dig Dis Sci. 2001;46:597–602. [DOI] [PubMed] [Google Scholar]
- 21.Fouad YM, Katz PO, Hatlebakk JG, et al. Ineffective esophageal motility: the most common abnormality in patients with GERD-associated respiratory symptoms. Am J Gastroenterol. 1999;94:1464–1467. [DOI] [PubMed] [Google Scholar]
- 22.Patti MG, Goldberg H, Arcerito M, et al. Hiatal hernia size affects lower esophageal sphincter function, esophageal acid exposure, and the degree of mucosal injury. Am J Surg. 1996;171:182–186. [DOI] [PubMed] [Google Scholar]
- 23.Tamhankar AP, Peters JH, Portale G, et al. Omeprazole does not reduce gastroesophageal reflux: new insights using multichannel intraluminal technology. J Gastrointest Surg. 2004;8:888–896. [DOI] [PubMed] [Google Scholar]
- 24.Patti MG, Robinson T, Galvani C, et al. Total fundoplication is superior to partial fundoplication even when peristalsis is weak. J Am Coll Surg. 2004;198:863–870. [DOI] [PubMed] [Google Scholar]
- 25.Milkes D, Gerson LB, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal and intragastric pH in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2004;99:991–996. [DOI] [PubMed] [Google Scholar]


